Abstract
BACKGROUND
Diabetic patients with hypertension and dyslipidemia are at a high risk of cardiovascular complications.
OBJECTIVES
To determine the effect of Nigella sativa supplementation on the lipid profile, mean arterial pressure, and heart rate in persons with type 2 diabetes on oral hypoglycemic agents (OHA).
DESIGN
Single-blind, nonrandomized.
SETTING
Diabetes clinic of a university hospital in Saudi Arabia.
PATIENTS AND METHODS
Type-2 diabetic patients were recruited by purposive sampling and assigned to treatment or control at the discretion of the investigator with the patient blinded to treatment. Before the intervention and every 3 months thereafter until the end of the treatment period, the following parameters were measured: triglycerides (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), heart rate (HR), and body mass index (BMI). Results at the baseline and each subsequent visit were compared between the two groups.
MAIN OUTCOME MEASURE(S)
Lipid and cardiovascular parameters, and BMI.
RESULTS
Fifty-seven patients were assigned to receive N sativa 2 g daily for one year and 57 were assigned to receive an identical regimen of placebo, along with OHA. A significant decrease in HDL-C and increase in the TC/HDL-C and LDL-C/HDL-C ratios were seen in the control group. The N sativa group had a significant decline in TC, LDL-C, TC/HDL-C and LDL-C/HDL-C ratios, compared with the respective baseline data and the control group. HDL-C was significantly elevated in the N sativa group. The control group showed a significant elevation in MAP. The N sativa group had a significant reduction in SBP, DBP, MAP and HR and a significant decrease in DBP, MAP and HR as compared with the control group.
CONCLUSION
N sativa supplementation improves total cholesterol, mean arterial pressure and heart rate in type 2 diabetes patients on oral hypoglycemic agents.
LIMITATIONS
There were 9 subjects in each group lost to follow up; thus the sample size could not be maintained as per the sample size calculation. The study was nonrandomized and thus there was a possibility of allocation bias. (Clinical trial registration number: CTRI/2013/06/003781, Clinical Trial Registry of India).
Diabetes mellitus is a major and growing public health issue worldwide, but especially in Saudi Arabia.1 Diabetes is associated with cardiovascular disease (CVD) risk factors such as dyslipidemia, hypertension and obesity, among others, which are established predictors of adverse cardiovascular outcome.2,3 The altered composition of lipoproteins and lipids in type 2 diabetic patients, called diabetic dyslipidemia, is characterized by elevated levels of triglyceride and cholesterol-rich very low density lipoprotein with reduced levels of high-density lipoprotein cholesterol (HDL-C).4 It has been suggested that lowering lipid levels intensively might immediately reduce the likelihood of CVD events arising in diabetic patients.5
Remarkably, hypertension occurs approximately twice as often in patients with diabetes compared with non-diabetic persons. In addition, up to 75% of CVD in patients with diabetes may be attributed to hypertension.6 Moreover, diastolic heart failure has been recognized as a major adverse manifestation of hypertension and diabetes,7 leading to recommendations for more aggressive blood pressure control in diabetic persons with coexistent hypertension.6 The use of herbal drugs as complementary medicine is prevalent worldwide and is gaining popularity. Nigella sativa is a widely used medicinal plant throughout the world with a long history of use of the raw seeds as well as oil in medicines and food.8
The lipid lowering effect of N sativa powdered seeds9 and its extract10 has been observed in normal rats. Moreover, the protective effects of thymoquinone (TQ), the active ingredient of N sativa, on development of atherosclerosis have been reported in cholesterol-fed rabbits.11,12 Likewise, strong evidence supports the usefulness of TQ in the prevention of CVD risk parameters in rats fed an atherogenic suspension.13 Other studies explored the hypotensive effect of N sativa seed extract in spontaneously hypertensive rats14 and in humans with mild hypertension.15 N sativa also has a potential to reduce elevated heart rate induced by the toxic effect of alloxan-induced diabetes in rabbits16 and cadmium-treated rats.17
Despite the evidence in favor of N sativa effects on major cardiovascular risk factors in different animal models, convincing therapeutic strategy and strict risk factor control by N sativa are still lacking in human diabetics. A recent systematic review and meta-analysis of 17 randomized controlled trials concluded that N sativa significantly decreases plasma lipid concentration (total cholesterol, LDL, and TG) whereas HDL-C increased with the powdered form only.18 The same effect on lipids was observed in centrally obese men treated with N sativa for 3 months.19 In the past our group has found a significant decrease in lipids20 and hemodynamic parameters21 after three months of treatment with N sativa seeds in type 2 diabetic patients. We concluded from that study that a prolonged study was required to establish the effect and safety of N sativa supplementation. Therefore, we planned this study to evaluate the effect of long-term (one year) N sativa supplementation, as adjuvant therapy, on the lipid profile, blood pressure and heart rate in type 2 diabetic patients on oral hypoglycemic agents (OHA).
PATIENTS AND METHODS
This study was conducted at the Department of Physiology, College of Medicine, University of Dammam while the patients were recruited from the diabetes clinic of King Fahd Hospital of the University of Dammam, Saudi Arabia. The study was approved by the Research and Ethical committee of the University of Dammam and registered as a clinical trial in the clinical trial registry of India (Clinical trial registration number: CTRI/2013/06/003781, Clinical Trial Registry of India, Website URL: http://ctri.nic.in).
Sample size was calculated by software G*Power (University of Dusselldorf, Germany, version 3.1.9.2, http://www.gpower.hhu.de/en.html)22 to be 57 for each of the two the groups (N=114). The parameters used for calculation were α error probability=0.05, power 1-β, error probability=0.95, effect size=0.62 (based on the closely matching contemporary studies) and allocation ratio (N2/N1)=1, computed for independent sample t test for two groups.
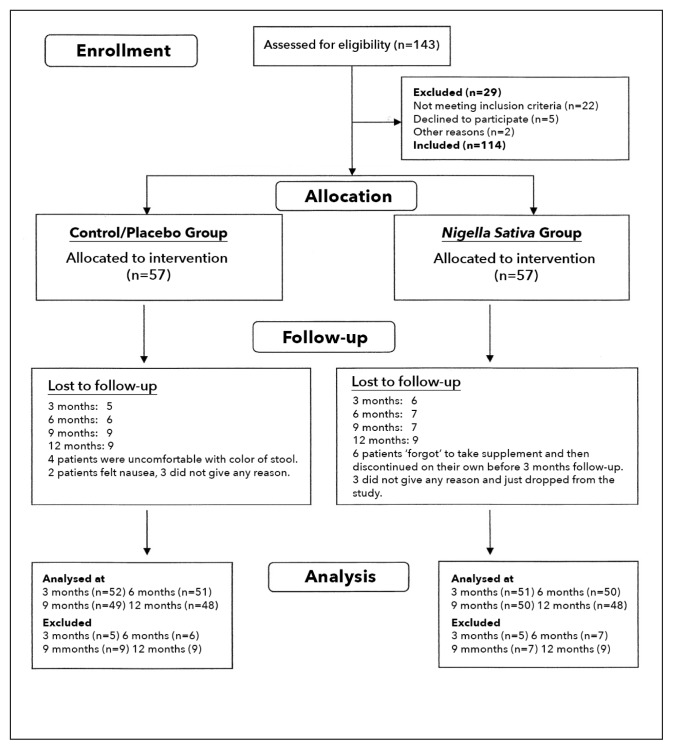
Patients with type 2 diabetes were recruited by purposive sampling. Allocation to the groups was done at the discretion of the investigator who matched the male-to-female ratio, age, duration of diabetes and body mass index (BMI) in the two groups (Figure 1).
Figure 1.
Flow diagram for allocation of patients.
The criteria for inclusion was poorly controlled type 2 diabetes (determined by two readings of HbA1c of more than 7% taken three months apart, 18–60 years of age, and regular use standardized oral medications [glibenclamide, metformin or rosiglitazone]). Criteria for exclusion were HbA1c in excess of 9%, insulin therapy, BMI in excess of 40kg/m2, triglyceride (TG) more than 400 mg/dL, any other reason for deranged lipids, major cardiovascular disease, hepatic disorder, renal disorder, pregnancy/lactation and compliance <90% or change in medications during the study period. Informed consent, which included full information about the purpose and the duration of the study, was taken from all the subjects in writing. The subjects were told that they could leave the study at any time.
Intervention
Nigella sativa seeds (Bioextract [Pvt] Ltd, Sri Lanka) were provided in the form of 500 mg oral capsules. A dose of 2 grams/day was used. This dose was already determined to be effective in reducing blood glucose in a previous study published by our department.23 The placebo was an activated charcoal capsule (260 mg), supplied by Arkopharma Pharmaceutical Laboratories Carros, France that closely matched the color and size of the N sativa capsules. The supplementation in two divided doses daily, along with the regular standardized treatment continued for one year.
The baseline data including age, sex and duration of disease was recorded before the start of treatment.
Clinical and analytical methods
Lipid parameters; triglycerides (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C), as well as BMI and hemodynamic parameters; heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) were measured before intervention (baseline) and every three months thereafter until the end of the study period. Also, TC/HDL-C ratio and LDL-C/HDL-C were calculated. In addition, fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), renal and liver function tests and complete blood count were also carried out at baseline and at all the follow up visits. Patients were asked not to smoke or engage in physical activity for 30 minutes before the above measurements and drawing of blood. Patients were asked to report any adverse sign or symptom via phone (numbers were provided) to make decisions about discontinuation of medicine.
Blood samples were collected, after at least 12 hours of fasting, into plain tubes (without anticoagulant) and allowed to clot. They were then centrifuged at 3000 rpm for 8 min for separation of the serum. Serum was stored and kept frozen at −20°C for up to 1 week until used for determination of TG, TC, and HDL-C. The kits were supplied by Dade Behring, Germany. Assays were performed according to the manufacturer’s instructions, using the automated assay analyzer (Dimension Clinical Chemistry System, Germany). LDL-C was calculated using the Friedewald formula.24
Blood pressure was measured in the sitting position with the arm supported at the level of the heart, after 10 minutes of rest, with a mercury sphygmomanometer by the auscultatory method. Blood pressure readings were based on the average of two independent measurements taken 5 minutes apart. HR at rest was determined by counting the radial pulse for one minute. The mean arterial pressure (MAP) was calculated by formula (MAP=diastolic pressure + 1/3 pulse pressure).
Statistical analysis
Statistical analyses were performed with SPSS version 19 (IBM, Armonk, NY, USA). Means and standard deviations (SD) were calculated. Comparison between the two groups (control and NS) was done using an independent samples t test at the baseline, 3 months, 6 months, 9 months and 12 months. P value was considered significant if P≤.05. The TREND statement (Transparent Reporting of Evaluations with Nonrandomized Designs) was used in the preparation of the manuscript.
RESULTS
Subjects (n=114, 63 males and 51 females) were nonrandomly assigned to a placebo (control) (n=57, 30 males and 27 females) or N sativa (n=57, 33 males and 24 females) (Figure 1). The two groups were of similar age, duration of disease, BMI, fasting blood glucose (FBG) and hemoglobin A1C (HbA1c) at baseline (Table 1). Other pretreatment baseline data did not differ significantly (Tables 2 and 3). Differences in TG and HDL-C were nonsignificant between the two groups at all timepoints (Table 2). TC was significantly lower in the N sativa group at 6, 9 and 12 months. Likewise, LDL-C was significantly less in the N sativa group as compared with the control group at 3 and 6 months. TC/HDL-C ratio was significantly less in the N sativa group at 6 and 9 months as compared with the control group. Likewise, LDL-C/HDL-C was significantly less in the N sativa group at 6 months as compared with the control group. BMI was not significantly different in any of the two groups, therefore the intergroup and intragroup differences were non-significant (Table 3). HR was significantly (P<.01) less in N sativa group as compared with the control group at 3, 6, 9 and 12 months. SBP was not significantly different between the control and N sativa groups. DBP showed a significant difference between the control and N sativa groups at the 9th and 12th month. MAP was significantly different at the 12th month between the two groups. All patients tolerated the treatment well and reported no adverse effects throughout the study period. Furthermore, all blood tests related to renal and liver functions and complete blood count were normal in all readings taken during the one-year treatment with N sativa.
Table 1.
Baseline demographic and clinical data.
| Variables | Control (n=57) | N sativa (n=57) | P |
|---|---|---|---|
|
| |||
| Age (years) | 46.12 (6.41) | 46.82 (8.63) | .62 |
| Duration of disease (years) | 5.95 (4.33) | 7.24 (3.81) | .09 |
| Body mass index (kg/m2) | 31.83 (3.93) | 30.47 (3.99) | .07 |
| Fasting blood glucose (mg/dL) | 180.9 (41.07) | 195.9 (47.41) | .07 |
| Hemoglobin A1C (%) | 8.23 (0.90) | 8.56 (0.90) | .06 |
Values are mean and standard deviation. Statistical method was the independent sample ‘t’ test.
Table 2.
Lipid profile at different treatment durations, between the control and Nigella sativa groups.
| Variables (Mean ± SD) | Treatment duration | Control | N sativa | P value |
|---|---|---|---|---|
|
| ||||
| TG (mg/dL) | Baseline | 180.82 (124.14) (57) | 170.87 (102.19) (57) | .64 |
| 3 months | 185.82 (111.09) (52) | 168.35 (92.38) (51) | .38 | |
| 6 months | 193.49 (110.28) (51) | 167.06 (108.72) (50) | .22 | |
| 9 months | 184.02 (115.19) (48) | 162.34 (85.02) (50) | .29 | |
| 12 months | 189.72 (114.99) (48) | 169.62 (102.60) (48) | .36 | |
|
| ||||
| TC (mg/dL) | Baseline | 195.63 (46.30) (57) | 194.19 (40.58) (57) | .86 |
| 3 months | 199.30 (45.84) (52) | 185.56 (40.94) (51) | .11 | |
| 6 months | 200.94 (43.31) (51) | 177.64 (41.24) (50) | .007 | |
| 9 months | 195.64 (34.54) (48) | 180.18 (42.99) (50) | .05 | |
| 12 months | 199.27 (39.29) (48) | 180.66 (41.91) (48) | .02 | |
|
| ||||
| LDL-C (mg/dL) | Baseline | 122.98 (33.12) (57) | 126.49 (33.91) (57) | .57 |
| 3 months | 127.98 (30.02) (52) | 114.23 (35.29) (51) | .03 | |
| 6 months | 128.62 (35.93) (51) | 107.14 (36.48) (50) | .004 | |
| 9 months | 120.58 (27.73) (48) | 115.34 (38.50) (50) | .44 | |
| 12 months | 120.72 (26.96) (48) | 114.25 (34.62) (48) | .30 | |
|
| ||||
| HDL-C (mg/dL) | Baseline | 42.45 (10.05) (57) | 42.54 (43.01 (57) | .96 |
| 3 months | 42.21 (9.83) (52) | 43.01 (10.36) (51) | .68 | |
| 6 months | 41.70 (8.41) (51) | 42.68 (10.66) (50) | .61 | |
| 9 months | 43.25 (11.13) (48) | 45.02 (10.67) (50) | .42 | |
| 12 months | 43.79 (9.81) (48) | 44.02 (10.45) (48) | .91 | |
|
| ||||
| TC/HDL-C | Baseline | 4.72 (1.21) (57) | 4.76 (1.31) (57) | .88 |
| 3 months | 4.88 (1.32) (52) | 4.53 (1.39) (51) | .19 | |
| 6 months | 4.95 (1.26) (51) | 4.38 (1.33) (50) | .02 | |
| 9 months | 4.70 (1.07) (48) | 4.16 (1.22) (50) | .02 | |
| 12 months | 4.75 (1.23) (48) | 4.27 (1.40) (48) | .07 | |
|
| ||||
| LDL-C/HDL-C | Baseline | 3.00 (0.94) (57) | 3.12 (1.07) (57) | .53 |
| 3 months | 3.16 (0.94) (52) | 2.80 (1.12) (51) | .07 | |
| 6 months | 3.19 (1.01) (51) | 2.66 (1.08) (50) | .01 | |
| 9 months | 2.92 (0.88) (48) | 2.70 (1.10) (50) | .28 | |
| 12 months | 2.89 (0.80) (48) | 2.77 (0.98) (48) | .51 | |
Values are mean and standard deviation. Number of patients at each visit is given in parenthesis. The numbers vary because of drop-outs (Figure 1). Missing values were replaced by linear interpolation in SPSS. Comparisons between control and NS groups were done by independent sample t test. Statistically significant differences are bolded. TG: Triglycerides; TC: Total cholesterol; LDL-C: Low density lipoprotein cholesterol; HDL-C: High density lipoprotein cholesterol
Table 3.
Comparison of body mass index, heart rate, systolic blood pressure, diastolic blood pressure and mean arterial pressure at different treatment durations, between the control and Nigella sativa groups.
| Variables (Mean ± SD) | Treatment duration | Control | N sativa | P value |
|---|---|---|---|---|
|
| ||||
| BMI (kg/m2) | Baseline | 31.83 (3.93) (57) | 30.47 (3.99) (57) | .071 |
| 3 months | 32.09 (3.76) (52) | 30.68 (3.83) (51) | .064 | |
| 6 months | 32.15 (3.88) (51) | 30.76 (3.74) (50) | .071 | |
| 9 months | 32.06 (4.11) (48) | 30.69 (3.66) (50) | .085 | |
| 12 months | 32.14 (4.12) (48) | 30.84 (3.51) (48) | .101 | |
|
| ||||
| HR (beats/minute) | Baseline | 87.40 (8.83) (57) | 85.80 (7.64) (57) | .305 |
| 3 months | 87.57 (7.73) (52) | 84.09 ( 6.13) (51) | .013 | |
| 6 months | 88.19 (7.65) (51) | 84.04 (7.31) (50) | .006 | |
| 9 months | 88.45 (9.17) (48) | 84.28 (6.86) (50) | .013 | |
| 12 months | 88.16 (7.76) (48) | 82.68 (7.16) (48) | .001 | |
|
| ||||
| SBP (mmHg) | Baseline | 133.77 (14.37) (57) | 139.03 (15.68) (57) | .053 |
| 3 months | 131.53 (14.70) (52) | 133.92 (18.82) (51) | .475 | |
| 6 months | 131.76 (13.25) (51) | 132.90 (20.07) (50) | .738 | |
| 9 months | 134.68 (14.34) (48) | 132.00 (16.22) (50) | .388 | |
| 12 months | 134.37 (15.32) (48) | 129.37 (17.43) (48) | .139 | |
|
| ||||
| DBP (mmHg) | Baseline | 78.59 (7.60) (57) | 80.17 (8.34) (57) | .293 |
| 3 months | 78.26 (5.41) (52) | 76.27 (8.47) (51) | .157 | |
| 6 months | 78.52 (7.02) (51) | 76.10 (8.70) (50) | .126 | |
| 9 months | 80.41 (7.13) (48) | 77.20 (6.71) (50) | .024 | |
| 12 months | 79.89 (7.88) (48) | 76.66 (7.80) (48) | .47 | |
|
| ||||
| MAP (mmHg) | Baseline | 96.92 (7.89) (57) | 99.75 (9.26) (57) | .083 |
| 3 months | 96.23 (7.59) (52) | 95.31 (10.31) (51) | .607 | |
| 6 months | 95.67 (7.91) (51) | 95.11 (10.54) (50) | .607 | |
| 9 months | 98.77 (8.37) (48) | 95.69 (8.73) (50) | .078 | |
| 12 months | 98.15 (9.43) (48) | 94.01 (9.78) (48) | .038 | |
Values are mean and standard deviation. Number of patients at each visit is given in parenthesis. Number of patients varies because of drop outs. Missing values replaced by linear interpolation in SPSS. Comparisons between control and NS groups were done by independent sample t test. Statistically significant differences are bolded. BMI: body mass index; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure.
DISCUSSION
In the present study, N sativa supplementation for one year in patients with type 2 diabetes taking OHA was effective in lowering total cholesterol, LDL, blood pressure and heart rate. This reflects a potential protective role against CVD risks associated with diabetes. A recent study reported that immediate management of lipid levels after diagnosis of an abnormality correlated significantly with reduction in CVD events arising in diabetic patients with moderate or severe myocardial ischemia.5 In addition, it is known that a reduction in blood pressure by antihypertensive drugs is associated with a major decrease in cardiovascular morbidity and mortality.25
In the present investigation, patients receiving N sativa seeds displayed a significant decrease in TC, LDL-C and the calculated lipid ratios (TC/HDL-C and LDL-C/HDL-C) (compared with the control group). In addition, HDL-C was higher than baseline values in the N sativa group. These results confirm the lipid lowering and antiatherogenic lipoprotein pattern activities of N sativa seeds previously observed in patients with stable coronary artery disease26 and in type 2 diabetic patients.20 Another study was conducted in patients with hypercholesterolemia. They received 2 g N sativa per day for 4 weeks. This resulted in a significant decrease in TG, TC, LDL-C; however, it indicated that N sativa has no beneficial effects on fasting blood sugar and HDL-C.27 Moreover, a nonsignificant reduction in lipids has been reported in adults who received powdered N sativa seeds18 and in men with central obesity treated with N sativa for 3 months.19
HDL-C levels are known to be inversely associated with prevalence of coronary artery disease.28 However, not all therapies that increase HDL-C reduce CVD events, leading to the conclusion that targeting both HDL-C and LDL-C leads to greater benefit for CVD and clinical events.29 Therefore, the increase in HDL-C with pronounced reduction in LDL-C, TC/HDL-C ratio and LDL-C/HDL-C ratios, seen in the present study, points to improvement in the lipoprotein components towards a non-atherogenic pattern. This highlights the efficacy of N sativa seeds as an ameliorating adjuvant for the CVD risks in type 2 diabetics on OHA. In support of these results is an animal study, in which feeding hypercholesterolemic rabbits with N sativa either in powder or oil forms was shown to significantly reduce TC and LDL-C levels and enhance HDL-C levels, indicating hypocholesterolemic and antiatherogenic cardioprotective properties of N sativa.30
The significant decline in SBP, DBP, MAP and HR, demonstrated in this study is similar to results in two previous studies, one on rats and the other on humans.14,15 MAP was reduced by 22% in spontaneously hypertensive rats treated with N sativa extract.14 Oral supplementation with N sativa seed extract in patients with mild hypertension reduced both SBP and DBP in a dose-dependent manner.15 However, nonsignificant reductions in SBP and DBP have been reported in males with central obesity receiving N sativa for 3 months compared to the controls.19 Differences in methodology and the type of subjects under study could account for this discrepancy.
Earlier results are consistent with our results, as indicated by a decrease in the elevated heart rate induced by a toxic effect in (alloxan-induced) diabetic rabbits16 and in cadmium-treated rats17 treated with extract of N sativa. An in vitro study also showed a potent inhibitory effect of aqueous and macerated extracts from N sativa on both HR and contractility of isolated guinea pig heart.31 In contrast, a rise in HR and cardiac contractility was induced in isolated perfused rat hearts treated with N sativa.32
BMI in the present study did not differ significantly in either the placebo or N sativa groups when compared to their baseline values or to each other. In contrast to our result, a study in males with central obesity found a significant reduction in body weight and waist circumference with N sativa.19 Additionally, a nonsignificant reduction in abdominal circumference has been reported in patients with insulin resistance syndrome treated with N sativa oil.33 However, results reported by Le et al10 suggested that petroleum ether extract of N sativa caused a 25% reduction in food intake that translated into a transient weight loss in normal rats.
The relationship between dyslipidemia and hypertension has been well recognized.34 Therefore, the prominent lipid lowering and antiatherogenic effects of N sativa demonstrated in the current study might, in part, play a role in reducing blood pressure. A study in Turkish hypertensive patients reported that poor BP control despite appropriate treatment was associated with metabolic syndrome, diabetes, and undertreatment of atherogenic dyslipidemia.35 Tan et al indicated that prehypertension is associated with atherosclerosis, independent of conventional cardiovascular risk factors in type 2 diabetic patients, speculating that maintenance of SBP and DBP within a normal range may reduce the risk of atherosclerosis in type 2 diabetes.36
The precise mechanism by which N sativa may induce its favorable potential on lipids and blood pressure is not fully elucidated. However, several lines of evidence are accumulating denoting a pivotal role for oxidative stress in the pathogenesis of dyslipidemia37 and hypertension.38 The beneficial effects of N sativa on lipid levels and blood pressure demonstrated in the current study may be explained by the high antioxidant potential of N sativa, reported previously.39,40
Studies on TQ have shown a significant reduction in (elevated) lipid levels and improvement in (altered) levels of lipid peroxidation products as well as antioxidant enzymes. These data suggested a high antioxidant potential for TQ in rats with doxorubicin-induced nephropathy41 and for ethanol extract of N sativa seeds in streptozotocin-induced diabetic rats.42
Basal superoxide anion production was reported to increase in spontaneously hypertensive rats, compared to normotensive rats.43 Consequently, superoxide anion scavenging by the use of the TQ was shown to lower blood pressure in nitric oxide deficient hypertensive rats.44 Recently, N sativa and its active constituents have exhibited antioxidant, hypotensive, calcium channel blockade and diuretic properties, which may contribute to reduced blood pressure, suggesting a potential role for N sativa in the management of hypertension.45
Our study adds to the reliability of a growing number of studies that have contributed evidence on the beneficial effects of N sativa on serum lipids as reflected in a recent systematic review based upon 17 clinical trials.18 Now there is enough evidence from animal as well as human studies to test the use of N sativa in larger populations who have or who are at risk of lipid derangements.
Our study has quite a few limitations. We initially planned to include patients with elevated lipids and group them into three (namely placebo, statins and N sativa); however the clinicians on our team disagreed on the ground that it would be unethical to not give recognized treatment (statins) to diabetic patients with lipid derangements. Random allocation was not feasible due to limitation of informed consent and rigorous exclusion criteria. However, we feel that despite utmost care and observance of a well defined and restricted selection criteria there is possibility of allocation bias.
Patients were generally found the suggestion of a long follow up unappealing. Although sample size was calculated and the number of subjects inducted accordingly, there were subjects lost to follow up. Missing subjects were not necessarily the same in the subsequent follow ups as some of the subjects missed one follow up visit and reappeared at the next visit. This made adherence to sample size impossible. This would definitely reduce the significance of the results.
In conclusion, N sativa supplementation over a one-year period effectively reduced TC, MAP and HR in type 2 diabetic patients receiving OHA. This suggests that N sativa seeds might be useful as a complementary therapy with other antiatherogenic and antihypertensive drugs for the management of diabetic complications. This may open the field for a novel preventive therapeutic strategy tailored to reduce CVD in this large population at risk.
Acknowledgment
This study was carried out with the support of a grant from KACST (King Abdul Aziz City for Science & Technology).
Footnotes
Conflict of interest
Authors declare no conflict of interests.
REFERENCES
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes research and clinical practice. 2010 Jan 31;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Bauters C, Lamblin N, Mc Fadden EP, Van Belle E, Millaire A, de Groote P. Influence of diabetes mellitus on heart failure risk and outcome. Cardiovas Diabetol. 2003;2:1. doi: 10.1186/1475-2840-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel WB. Framingham study insights on diabetes and cardiovascular disease. Clinical chemistry. 2011 Feb 1;57(2):338–9. doi: 10.1373/clinchem.2010.149740. [DOI] [PubMed] [Google Scholar]
- 4.Erkelens DW. Insulin resistance syndrome and type 2 diabetes mellitus. Am J Cardiol. 2001;88(7B):38J–42J. doi: 10.1016/s0002-9149(01)01883-5. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki Y, Katakami N, Kaneto H, Nakajima K, Kusuoka H, Kashiwagi A, Nishimura T. Improved lipid profiles are associated with reduced incidence of coronary vascular events in asymptomatic patients with type 2 diabetes and impaired myocardial perfusion. J Atheroscler Thromb. 2013;20(4):330–5. doi: 10.5551/jat.13474. [DOI] [PubMed] [Google Scholar]
- 6.Sowers JR, Frohlich ED. Insulin and insulin resistance: impact on blood pressure and cardiovascular disease. Med Clin North Am. 2004;88(1):63–82. doi: 10.1016/s0025-7125(03)00128-7. [DOI] [PubMed] [Google Scholar]
- 7.Pieske B, Wachter R. Impact of diabetes and hypertension on the heart. Curr Opin Cardiol. 2008;23(4):340–9. doi: 10.1097/HCO.0b013e3283031ab3. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, et al. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed. 2013;3(5):337–52. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocyigit Y, Atamer Y, Uysal E. The effect of dietary supplementation of Nigella sativa L. on serum lipid profile in rats. Saudi Med J. 2009;30(7):893–6. [PubMed] [Google Scholar]
- 10.Le PM, Benhaddou-Andaloussi A, Elimadi A, Settaf A, Cherrah Y, Haddad PS. The petroleum ether extract of Nigella sativa exerts lipid-lowering and insulin-sensitizing actions in the rat. J Ethnopharmacol. 2004;94(2–3):251–9. doi: 10.1016/j.jep.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Ragheb A, Elbarbry F, Prasad K, Mohamed A, Ahmed MS, Shoker A. Attenuation of the development of hypercholesterolemic atherosclerosis by thymoquinone. Int J Angiol. 2008;17(4):186–92. doi: 10.1055/s-0031-1278307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nader MA, el-Agamy DS, Suddek GM. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res. 2010;33(4):637–43. doi: 10.1007/s12272-010-0420-1. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad S, Beg ZH. Hypolipidemic and antioxidant activities of thymoquinone and limonene in atherogenic suspension fed rats. Food Chem. 2013;138(2–3):1116–24. doi: 10.1016/j.foodchem.2012.11.109. [DOI] [PubMed] [Google Scholar]
- 14.Zaoui A, Cherrah Y, Lacaille Dubois MA, Settaf A, Amarouch H, Hassar M. Diuretic and hypotensive effects of Nigella sativa on the spontaneously hypertensive rat. Therapie. 2000;55:379–82. [PubMed] [Google Scholar]
- 15.Dehkordi FR, Kamkhah AF. Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam Clin Pharmacol. 2008;22:447–52. doi: 10.1111/j.1472-8206.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 16.Meral I, Donmez N, Baydas B, Belge F, Kanter M. Effect of Nigella sativa L. on heart rate and some haematological values of alloxan-induced diabetic rabbits. Scand J Lab Anim Sci. 2004;31(1):49–53. [Google Scholar]
- 17.Demir H, Kanter M, Coskun O, Uz YH, Koc A, Yildiz A. Effect of black cumin (Nigella sativa) on heart rate, some hematological values, and pancreatic beta-cell damage in cadmium treated rats. Biol Trace Elem Res. 2006;110(2):151–62. doi: 10.1385/BTER:110:2:151. [DOI] [PubMed] [Google Scholar]
- 18.Sahebkar A, Beccuti G, Simental-Mendía LE, Nobili V, Bo S. Nigella sativa (black seed) effects on plasma lipid concentrations in humans: A systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol Res. 2016 Apr;106:37–50. doi: 10.1016/j.phrs.2016.02.008. Review. [DOI] [PubMed] [Google Scholar]
- 19.Datau EA, Wardhana, Surachmanto EE, Pandelaki K, Langi JA, Fias Efficacy of Nigella sativa on serum free testosterone and metabolic disturbances in central obese male. Acta Med Indones. 2010;42(3):130–4. [PubMed] [Google Scholar]
- 20.Kaatabi H, Bamosa AO, Lebda FM, Al Elq AH, Al-Sultan AI. Favorable impact of Nigella sativa seeds on lipid profile in type 2 diabetic patients. J Family Community Med. 2012;19(3):155–61. doi: 10.4103/2230-8229.102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebda FM, Bamosa AO, Kaatabi H, Al Elq A, Al-Sultan A. Effect of Nigella sativa on hemodynamics, hemoglobin, and blood coagulation in patients with type 2 diabetes. Egypt J Haematol. 2012;37:73–80. [Google Scholar]
- 22.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 23.Bamosa AO, Kaatabi H, Lebda FM, Al Elq AM, Al Sultan A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. 2010;54:344–54. [PubMed] [Google Scholar]
- 24.Tremblay AJ, Morrissette H, Gagné JM, Bergeron J, Gagné C, Couture P. Validation of the Friedewald formula for the determination of low-density lipoprotein cholesterol compared with beta-quantification in a large population. Clin Biochem. 2004;37:785–90. doi: 10.1016/j.clinbiochem.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Neal B, MacMahon S, Chapman N Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration Lancet. 2000;356:1955–64. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 26.Tasawar Z, Seraj Z, Ahmad N, Lashari HM. The effect of Nigella sativa(Kalonji) on lipid profile in patients with stable coronary artery disease in Multan, Pakistan. Pak J Nutr. 2011;10(2):162–7. [Google Scholar]
- 27.Sabzghabaee AM, Dianatkhah M, Sarrafzadegan N, Asgary S, Ghannadi A. Clinical evaluation of Nigella sativa seeds for the treatment of hyperlipidemia: a randomized, placebo controlled clinical trial. Med Arh. 2012;66(3):198–200. doi: 10.5455/medarh.2012.66.198-200. [DOI] [PubMed] [Google Scholar]
- 28.Schaffer A, Verdoia M, Barbieri L, Aprami TM, Suryapranata H, Marino P, et al. High-Density Lipoproteins and Coronary Artery Disease: A Single-Center Cohort Study. Angiology 2013. 2014;65(8):696–702. doi: 10.1177/0003319713502253. [DOI] [PubMed] [Google Scholar]
- 29.Negi S, Ballantyne CM. Insights from recent meta-analysis: role of high-density lipoprotein cholesterol in reducing cardiovascular events and rates of atherosclerotic disease progression. J Clin Lipidol. 2010;4(5):365–70. doi: 10.1016/j.jacl.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Al-Naqeep G, Al-Zubairi AS, Ismail M, Amom ZH, Esa NM. Antiatherogenic Potential of Nigella sativa Seeds and Oil in Diet-Induced Hypercholesterolemia in Rabbits. Evid Based Complement Alternat Med. 2011;2011:213628. doi: 10.1093/ecam/neq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boskabady MH, Shafei MN, Parsaee H. Effects of aqueous and macerated extracts from Nigella sativa on guinea pig isolated heart activity. Pharmazie. 2005;60(12):943–8. [PubMed] [Google Scholar]
- 32.El Bahai MN, Al Hariri MT, Yar T, Bamosa AO. Cardiac inotropic and hypertrophic effects of Nigella sativa supplementation in rats. Int J Cardiol. 2009;131:e115–e7. doi: 10.1016/j.ijcard.2007.07.075. [DOI] [PubMed] [Google Scholar]
- 33.Najmi A, Nasiruddin M, Khan RA, Haque SF. Effect of Nigella sativa oil on various clinical and biochemical parameters of insulin resistance syndrome. Int J Diabetes Dev Ctries. 2008;28(1):11–4. doi: 10.4103/0973-3930.41980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biswas UK, Kumar A. A study on lipid profile, oxidation stress and carbonic anhydrase activity in patients with essential hypertension. J Clin Diag Res. 2010;4(6):3414–20. [Google Scholar]
- 35.Kabakcı G, Aydın M, Demir I, Kırma C, Özerkan F. Global cardiometabolic risk profile in patients with hypertension: results from the Turkish arm of the pan-European GOOD survey. Turk Kardiyol Dern Ars. 2010;38(5):313–20. [PubMed] [Google Scholar]
- 36.Tan JR, Chen YH, Bi YF, Xu M, Huang Y, Dai M, et al. Prehypertension is associated with atherosclerosis in Type 2 diabetes. J Diabetes. 2010;2(1):56–63. doi: 10.1111/j.1753-0407.2009.00062.x. [DOI] [PubMed] [Google Scholar]
- 37.Afolabi OK, Oyewo EB, Adekunle AS, Adedosu OT, Adedeji AL. Oxidative Indices Correlate with Dyslipidemia and Pro-Inflammatory Cytokine Levels in Fluoride-Exposed Rats. Arh Hig Rada Toksikol. 2013;64(4):521–9. doi: 10.2478/10004-1254-64-2013-2351. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigo R, Passalacqua W, Araya J, Orellana M, Rivera G. Implications of oxidative stress and homocysteine in the pathophysiology of essential hypertension. J Cardiovasc Pharmacol. 2003;42(4):453–61. doi: 10.1097/00005344-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Al-Naqeeb G, Ismail M, Al-Zubairi AS. Fatty acid profile, α-tocopherol content and total antioxidant activity of oil extracted from Nigella sativa seeds. Int J Pharmacol. 2009;5(4):244–50. [Google Scholar]
- 40.Kaatabi H, Bamosa AO, Badar A, Al Elq A, Abou-Hozaifa B, Lebda F, et al. Nigella sativa Improves Glycemic Control and Ameliorates Oxidative Stress in Patients with Type 2 Diabetes Mellitus: Placebo Controlled Participant Blinded Clinical Trial. PloS one. 2015;10(2):e0113486. doi: 10.1371/journal.pone.0113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badary OA, Abdel-Naim AB, Abdel-Wahab MH, Hamada FM. The influence of thymoquinone on doxorubicin-induced hyperlipidemic nephropathy in rats. Toxicology. 2000;143(3):219–26. doi: 10.1016/s0300-483x(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 42.Kaleem M, Kirmani D, Asif M, Ahmed Q, Bano B. Biochemical effects of Nigella sativa L seeds in diabetic rats. Indian J Exp Biol. 2006;44(9):745–8. [PubMed] [Google Scholar]
- 43.Nabha L, Garbern JC, Buller CL, Charpie JR. Vascular oxidative stress precedes high blood pressure in spontaneousl yhypertensive rats. Clin Exp Hypertens. 2005;27:71–82. doi: 10.1081/ceh-200044267. [DOI] [PubMed] [Google Scholar]
- 44.Khattab MM, Nagi MN. Thymoquinone supplementation attenuates hypertension and renal damage in nitric oxide deficient hypertensive rats. Phytother Res. 2007;21(5):410–4. doi: 10.1002/ptr.2083. [DOI] [PubMed] [Google Scholar]
- 45.Leong X, Mustafa MR, Jaarin K. Nigella sativa and Its Protective Role in Oxidative Stress and Hypertension. Evid Based Complement Alternat Med. 2013;2013:120732. doi: 10.1155/2013/120732. [DOI] [PMC free article] [PubMed] [Google Scholar]