Abstract
BACKGROUND
The prevalence of metabolic syndrome varies widely by ethnicity and by the criteria used in its definition.
OBJECTIVE
To identify the optimal cutoff values for waist circumference (WC), waist-to-hip ratio (WHR) and body mass index (BMI) for identifying metabolic syndrome among the Saudi population.
DESIGN
Nationwide household cross-sectional population-based survey.
SETTING
Thirteen health sectors in Saudi Arabia.
SUBJECTS AND METHODS
We used data for subjects in the Saudi Abnormal Glucose Metabolism and Diabetes Impact Study (SAUDI-DM), which was conducted from 2007 to 2009. Using International Diabetes Federation (IDF) criteria, metabolic syndrome and its different components were assessed using anthropometric measurements, blood pressure, fasting plasma glucose, triglycerides and HDL cholesterol. Receiver operating characteristic (ROC) curves were generated to assess sensitivity and specificity for different cutoff values of WC, WHR, and BMI. The Youden index was used to calculate the optimal cutoff value for each anthropometric measurement.
MAIN OUTCOME MEASURE(S)
Optimal cutoff value for WC, WHR, and BMI for identifying the risk of metabolic syndrome.
RESULTS
The prevalence of two or more risk factors for metabolic syndrome was observed in 43.42% of the total cohort of 12 126 study participants ≥18 years of age. The presence of two or more risk factors were significantly higher among men (46.81%) than women (40.53%) (P<.001). The optimal cutoff values for WC, WHR, and BMI were 92 cm, 0.89, and 25 kg/m2 for men and 87 cm, 0.81 and 28 kg/m2 for women for identifying the risk of metabolic syndrome. The prevalence of elevated triglycerides, blood pressure, and fasting plasma glucose significantly increased with age for both genders.
CONCLUSIONS
The proposed WC cutoff values were better than WHR and BMI in predicting metabolic syndrome and could be used for screening people at high risk for metabolic syndrome in the Saudi population.
LIMITATIONS
No direct measure of body fatness and fat distribution, cross-sectional design.
Saudi Arabia has the highest prevalence of diabetes in the Middle East and North Africa.1 The country is also known for having a high prevalence of other components related to metabolic syndrome, including hypertension, dyslipidemia, and central obesity.2–4 The prevalence of metabolic syndrome worldwide varies widely depending on ethnicity and criteria applied to define metabolic syndrome.5 The main difference between the two widely accepted set of criteria: (the National Cholesterol Education Program, Third Adult Treatment Panel III (ATPIII)6,7 and the International Diabetes Federation (IDF) criteria8 is the definition of central obesity. In the IDF definition, the cutoff values for waist circumference (WC) are lower and are ethnic-specific. Waist circumference is affected by factors like gender, age, and ethnicity.9–11 This ethnicity related variation has recently pressured many national and international organizations to propose their own specific cutoff points, especially when the currently proposed ATP III and IDF cutoff values for WC and other anthropometric measurements including waist-hip ratio (WHR) and body mass index (BMI) have been based on studies in Western populations that may not be appropriate for other ethnic groups.12
There is currently enough evidence that Asians should have lower waist circumference cutoff values for defining metabolic syndrome than Americans and Europeans since they have an increased risk of morbidity and mortality associated with lower BMI and WC cutoff values.13–16 The same could be true for the Arab ethnic group. Although there are limited data for assessing the optimal cutoff values for WC, WHR, and BMI among Arabs, the available data from different Middle Eastern and North African countries including Iraq,17 Oman,18 Qatar,19 Jordan,20 Egypt21 and Tunisia22 unexpectedly have shown wide variations in these anthropometric cutoff values. This inter-ethnicity variation warrants the determination of the optimal cutoff values for WC, as an integral component of metabolic syndrome, based on population specific data that can be used in defining this medical condition. This would be in line with the WHO recommendation for establishing country specific cutoff points for WC when defining metabolic syndrome.23
Other anthropometric measurements including WHR and BMI are used in the definition of metabolic syndrome based on WHO 1999 criteria;24 however, each measurement has its own limitations. Even though WHR is more consistent across different ethnicities and would provide a better prediction for disease risk, the measurement of WC is much easier and more convenient than hip circumference measurement for practical considerations.23 BMI is less sensitive compared to WC and WHR in assessing abnormal body fat distribution.25–27 Despite these limitations, determining the country specific optimal cutoff points for those two measurements would help researchers in their respective countries in reporting a more appropriate prevalence of metabolic syndrome.
Since there are no previous studies that have looked into the cutoff values for WC, WHR and BMI to identify risk for metabolic syndrome in the Saudi population, the main aim of this study was to determine the optimal cutoff values for those three anthropometric measurements in this population using data from The Saudi Abnormal Glucose Metabolism and Diabetes Impact Study (SAUDI-DM).28
PATIENTS AND METHODS
Study population
SAUDI-DM was a nationwide household cross-sectional population-based survey using a multistage stratified cluster sampling technique conducted from 2007 to 2009. Sampling took into consideration the urban to rural ratio in the 13 administrative regions in Saudi Arabia: Bahah, Aljouf, Northern border, Asir, Hail, Jizan, Madinah, Makkah, Najran, Qassim, Riyadh, Eastern, and Tabuk.28 The study was conducted through primary healthcare centers by trained physicians and nurses by recruiting family members of Saudi nationals from every third house in the selected areas. All family members who were available during the visit of the survey team were recruited regardless of their age, gender or diabetes status, excluding participants who refused to participate or were not present during the recruitment visit.28 A total of 87 417 participants were recruited. Adjustment for age, area of residency and gender distribution led to the exclusion of 34 047 (38.95%) participants. Of the remaining 53 370 (61.05%), the current analysis further excluded 23 523 (44.08%) subjects who were <18 years of age, 549 (1.09%) pregnant females, and 17 172 (32.18%) subjects who did not have a lipid assessment, leaving 12 126 subjects aged ≥18 years.
Anthropomorphic data collection
After obtaining consent from the eligible subjects, the data were collected using a pre-designed questionnaire that consisted of general demographic information including age and gender, in addition to diabetes history, hypertension and dyslipidemia. Each subject was assessed for weight and height in a standing position without shoes and wearing light clothing using an Adam’s weighing scale (model MDW-250L, with capacity of 250 kg and reliability of 0.1 kg, Oxford CT USA) wherein the height was expressed in centimeters (cm) and weight in kilograms (kg). WC was measured at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest based on the WHO STEP wise approach to surveillance (STEPS) protocol29 while the hip circumference was measured around the widest portion of the buttocks in a standing position wearing light clothing and looking straight ahead with arms at the sides and feet adherent to each other.3 The measuring tape was kept horizontal during the measurement of the hip and waist circumference. All measurements were rounded to the nearest 1 decimal point. BMI was calculated by dividing weight (kg) to height in square meters (m2). A mercury sphygmomanometer (Baumanometer, Model 0320, W.A. Baum Co., Inc. USA) was used to measure the blood pressure for each subject in a sitting position. Measurements were repeated twice at least 5 minutes apart, and the average of the two readings was used for data analysis.
Biochemical assessment
All subjects were asked to report to the nearest primary care center after more than 10 hours of overnight fasting and after which venous blood sampling was collected using a sodium fluoride tube. Plasma was stored at –20 degrees centigrade. All blood samples were sent to the central laboratory at the Strategic Center for Diabetes Research in the capital city of Riyadh. The blood glucose assessment was done using glucose oxidase/peroxidase method while blood lipids were measured using the esterase oxidase/peroxidase method for serum cholesterol, and the glycerokinase oxidase/peroxidase method for HDL, LDL and triglycerides, which were performed in an RX Daytona clinical chemistry analyzer by Randox (UK).
Definition of metabolic risk factors
Metabolic risk factors were defined using the 2006 IDF criteria30 that define elevated triglyceride (TG) as ≥150 mg/dL (≥1.7 mmol/L) and reduced HDL cholesterol as <40 mg/dL (<1.03 mmol/L) for men and as <50 mg/dL (<1.29 mmol/L) for women. Elevated blood pressure was defined when the systolic blood pressure (SBP) was ≥130 mm Hg and/or diastolic blood pressure (DBP) was ≥85 mm Hg in addition to receiving any medication for hypertension. Abnormal glucose metabolism was considered when fasting plasma glucose (FPG) was ≥100 mg/dL (≥5.6 mmol/L) or when patients were known to have type 2 diabetes. A combination of two or more of these risk factors was used to assess cutoff values for WC, WHR and BMI. This study was approved by the Institutional Review Board (IRB) at the College of Medicine, King Saud University.
Statistical analysis
Data were analyzed using SPSS statistical package version 21. Continuous variables were expressed as mean and standard deviation (SD), and categorical variables were expressed as percentages. The t test was used for continuous variables and the chi-square test for categorical variables. Imputation, based on a regression model, was used to estimate missing data. Receiver operating characteristic (ROC) curves were used to plot true positive (sensitivity) against false positive (1-specificity) rates. ROC analysis was used to quantify how accurately medical diagnostic tests (or systems) might discriminate between a diseased and “nondiseased” patient state. The area under the curve (AUC) is an indication of how well a parameter distinguishes a diagnostic state (presence of absence of metabolic syndrome in this case). The Youden index, a commonly used measure of overall diagnostic effectiveness which is the maximum vertical distance or difference between the ROC curve and the diagonal or chance line, was calculated to determine the optimal cutoff values for WC, WHR, and, BMI. A P value of less than .05 was used as the level of significance.
RESULTS
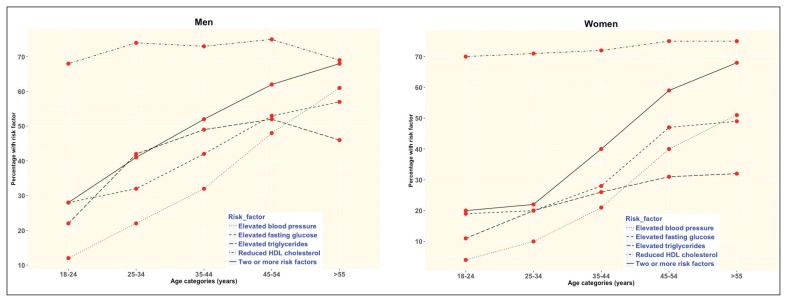
In the 12 126 subjects from the SAUDI-DM study, men were significantly older and had higher mean body weight than women (Table 1). The mean values for weight, height, WC, WHR, TG, FPG, SBP and DBP were significantly greater in men than those found in women, while only the mean values of BMI, hip circumference and HDL cholesterol were lower in men than women. Additionally, the prevalence of all metabolic syndrome risk factors (elevated triglycerides, reduced HDL cholesterol, elevated blood pressure, elevated fasting glucose) was higher in men compared with women, except for reduced HDL cholesterol. Men had a higher prevalence of two or more risk factors for metabolic syndrome than women (46.81% versus 40.53%, P<.001). The proportion of subjects with metabolic risk factors also increased with age in both the genders except for reduced HDL cholesterol, which was similar across age groups (Figure 1). The prevalence of two or more metabolic syndrome risk factors in both genders increased in a linear pattern by age from around 20% to more than 70% (Figure 1).
Table 1.
Characteristics of the population of the The Saudi Abnormal Glucose Metabolism and Diabetes Impact Study by sex.
| Clinical and demographic variables | Total 12 126 |
Men 5571 (45.9%) |
Women 6555 (54.1%) |
P |
|---|---|---|---|---|
|
| ||||
| Age (years) | 35.74 (15.00) | 36.05 (15.20) | 35.47 (14.82) | .035 |
| Weight (kg) | 71.38 (18.31) | 76.07 (18.56) | 67.40 (17.11) | <.001 |
| Height (cm) | 160.31 (10.24) | 166.86 (8.88) | 154.74 (7.71) | <.001 |
| Body mass index (kg/m2) | 27.81 (7.06) | 27.35 (6.77) | 28.20 (7.28) | <.001 |
| Waist circumference (cm) | 87.02 (16.72) | 89.71 (17.11) | 84.75 (16.03) | <.001 |
| Hip circumference (cm) | 99.07 (16.81) | 98.66 (16.81) | 99.41 (16.8) | .014 |
| Waist:hip ratio | 0.88 (0.12) | 0.91 (0.11) | 0.86 (.12) | <.001 |
| Triglyceride (mmol/L) | 1.62 (1.15) | 1.83 (1.30) | 1.45 (.98) | <.001 |
| HDL cholesterol (mmol/L) | 0.97 (0.32) | 0.89 (0.32) | 1.03 (.32) | <.001 |
| Fasting plasma glucose (mmol/L) | 5.68 (2.44) | 5.83 (2.63) | 5.55 (2.27) | <.001 |
| Systolic blood pressure (mm Hg) | 117.72 (13.76) | 119.69 (13.08) | 116.04 (14.09) | <.001 |
| Diastolic blood pressure (mm Hg) | 76.13 (8.64) | 77.29 (8.38) | 75.15 (8.73) | <.001 |
| Risk factors for metabolic syndrome | ||||
| Elevated triglyceride, n (%) | 3959 (32.65) | 2263 (40.62) | 1696 (25.87) | <.001 |
| Reduced HDL cholesterol, n (%) | 9531 (78.6) | 4155 (74.58) | 5376 (82.01) | <.001 |
| Elevated blood pressure, n (%) | 3344 (27.58) | 1784 (32.02) | 1560 (23.8) | <.001 |
| Elevated fasting plasma glucose, n (%) | 4439 (36.61) | 2250 (40.39) | 2189(33.39) | <.001 |
| ≥2 risk factors, n (%) | 5265 (43.42) | 2608 (46.81) | 2657 (40.53) | <.001 |
Values are mean (standard deviation) unless indicated otherwise. The t test was used for continuous variables and the chi-square test for categorical variables. HDL: high-density lipoprotein.
Figure 1.
The prevalence of metabolic syndrome risk factors according to age groups for men and women.
Metabolic parameters by anthropometric category
In both genders, mean values of all metabolic parameters increased in a linear pattern with increasing BMI values, except for HDL cholesterol, which decreased with increasing BMI (Appendix 1). A similar relationship was seen with WC and WHR except for WHR values >0.95, in which the prevalence of metabolic syndrome risk factors decreased compared with the preceding WHR value of 0.9–0.95. The mean values for triglycerides increased linearly, but mean HDL cholesterol decreased with increasing values of WC or WHR.
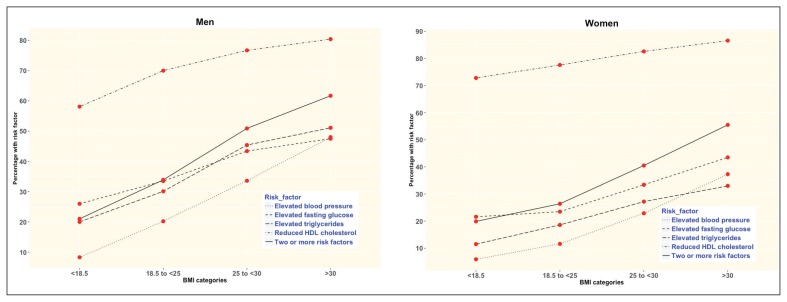
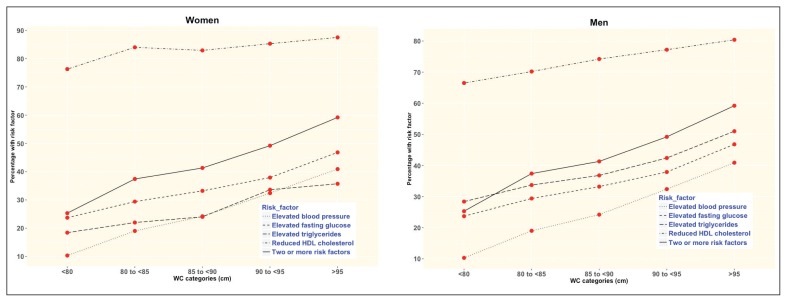
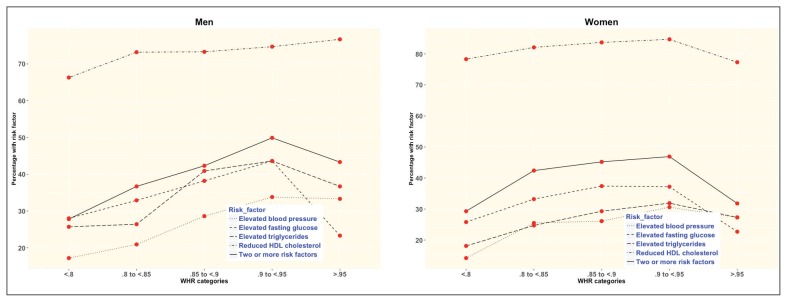
The proportion of both men and women with two or more risk factors increased about threefold from the lowest to the highest categories of BMI (Figure 2), and about twofold from the lowest to the highest categories of WC (Figure 3). For WHR, the proportion also doubled although it declined for women with WHR >0.95 (Figure 4 and Appendix 1).
Figure 2.
The prevalence of metabolic syndrome risk factors according to body mass index categories for men and women.
Figure 3.
The prevalence of metabolic syndrome risk factors according to waist circumference categories for men and women.
Figure 4.
The prevalence of metabolic syndrome risk factors according to waist-to-hip ratio categories for men and women.
Optimal cutoff values for metabolic risk factors
The optimal cutoff values for two or more risk factors are shown in Table 2, along with the sensitivity and specificity, and the Youdin index. The optimal cutoffs and other parameters for each metabolic risk factor are in Appendices 2–4.
Table 2.
Optimal cutoff values for identifying metabolic syndrome by the presence of two or more risk factors among Saudi men and women.
| Risk factor | Cut-off value | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|
|
| ||||
| Men | ||||
| Body mass index | 25 | 73.7 | 48.9 | 22.6 |
| Waist circumference | 92 | 57.3 | 67.4 | 24.65 |
| Waist:hip ratio | .89 | 69.4 | 48.7 | 18.1 |
| Women | ||||
| Body mass index | 28 | 62.1 | 62.9 | 24.9 |
| Waist circumference | 87 | 61.0 | 65.6 | 26.62 |
| Waist:hip ratio | .81 | 75.5 | 40.4 | 15.9 |
Body mass index in kg/m2; waist circumference in centimeters.
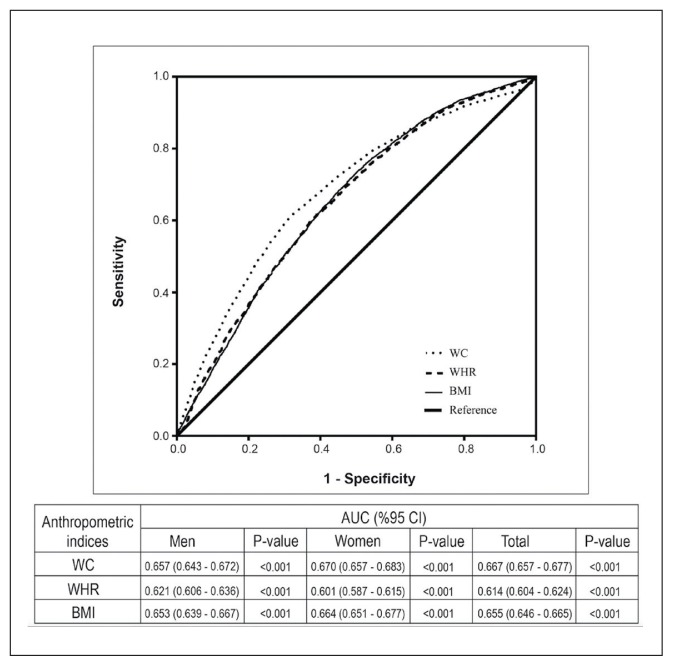
In the area under the curve corresponding to the optimal cutoff points for anthropometric parameters in both genders, WC had the highest AUC value compared with WHR and BMI, especially in women as shown in Figure 5.
Figure 5.
Area under the receiver operating characteristic curves (AUC) for waist circumference (WC), waist to hip ratio (WR), and body mass index (BMI) for total subjects and both genders.
DISCUSSION
Waist circumference is an important component of metabolic syndrome definition. Numerous studies have demonstrated different cutoff values for waist circumference that warrant establishing country- or region-specific cutoff points.23 The Saudi cohort in our study was used to determine the optimal gender-specific cutoff points of WC, WHR, and BMI for predicting two or more risk factors of metabolic syndrome. These cut-off points yielded the highest Youden index values. The optimal cutoff value of WC for identifying two or more risk factors of metabolic syndrome in the Saudi population was 92 cm for men and 87 cm for women, which lies within the WHO recommended range.23 This proposed cutoff value for the Saudi population is similar to data reported in other studies from individuals of the same ethnicity (Arabs) (Table 3), Using the Youden index methodology as in the current study, Qataris reported a WC cutoff value of 99.5 cm in men and 91 cm in women,14 while Iraqis reported WC cutoff values at 99 cm for men and at 97 cm for women.12 The discrepancies could be due to either different statistical approaches or to different clinical methods used in measuring WC. Another factor may be the definition of cardiovascular risk. In Oman, for instance, two or more risk factors are used in the definition,18 while in Qatar three or more risk factors are used;19 however, all the proposed cutoff values lie in the reported WHO range for Middle Eastern countries.23
Table 3.
Comparison between the optimal cutoff values for waist circumference for identifying metabolic syndrome among different Arab countries.
| Author | Year | Country | Statistical approach | WC cutoff values (cm) | WHR cutoff values | BMI cutoff values (kg/m2) | |||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | ||||
|
| |||||||||
| Al-Rubeaan et al | Current | Saudi Arabia | Youden index | 92 | 87 | 0.89 | 0.81 | 25 | 28 |
| Al-Lawati et al18 | 2001 | Oman | Shortest distance | 80 | 84.5 | 0.91 | 0.91 | 23.2 | 26.8 |
| Mansour et al17 | 2006 | Iraq | Youden index | 99 | 97 | - | - | - | - |
| Khader et al20 | 2009 | Jordan | Youden index | 88.5–91.8 | 84.5–88.5 | 0.88–0.90 | 0.80–0.83 | 26.2–27.2 | 27.2–30.0 |
| Bener et al19 | 2012 | Qatar | Youden index | 99.5 | 91.0 | 0.90 | 0.88 | 28.0 | 28.4 |
The WC cutoff values for the Saudi population are similar to those for Caucasians. In Latin America, 94 cm in men and 91 cm in women have been reported.31 However, these values were higher than the WC cutoff values reported from South and South East Asia that ranged between 71.5 cm for Chinese women and 95.1 cm for South Asian men.23 This difference in the WC cutoff values between different ethnic groups could be explained by ethnic differences in socioeconomic status, physical activities, lifestyle and cultural factors that could affect the body composition of each population that consequently uses the WC as a surrogate for abdominal fat.20
The current study highlights that using the proposed WC cutoff values of NECP ATP III criteria6,7,32 will underestimate the prevalence of metabolic syndrome in the Saudi population. At the same time, if IDF criteria for Asians33 were used in this society, it would underestimate the prevalence of metabolic syndrome among men and overestimate this prevalence among women. Therefore, the current study shall serve as a reference for providing accurate cutoff values for anthropometric measurements that could be used in future studies that aim to define metabolic syndrome in the Saudi population.
The cutoff values for WHR in the Saudi cohort were 0.89 and 0.81 for men and women respectively, which is lower than what has been reported among Qataris19 at 0.90 and 0.88 for men and women respectively, and Omanis18 at 0.91 for both men and women, but higher than what has been proposed for Jordanians at 0.88 for men and 0.82 for women.20 When comparing our results with Caucasians, we had a lower WHR for men (0.89 versus 0.95) but a higher WHR for women (0.84 versus 0.80).34 The proposed cutoff points for WHR in the current study are close to the proposed cutoff values used in the WHO 1998 definition of metabolic syndrome,35 at 0.89 versus 0.90 for men and 0.84 versus 0.85 for women. Although WHR was claimed by Welborn et al,36 to be a good predictor for morbidity and mortality, it is difficult to interpret biologically and any change in body fat distribution may produce little or no change in the ratio.37 Additionally, a reduction in body weight is usually associated with the reduction in both waist and hip circumferences, but not necessarily a change in WHR. A decrease in WHR might not be related to a reduction in cardiovascular risk factors.38
Based on the ROC curve analysis, the optimal cutoff values for BMI among our subjects was ≥25 kg/m2 for men and ≥28 kg/m2 for women. The proposed values for BMI in the current study are lower than that for Qataris,19 where they reported BMI cutoff values of 28 kg/m2 and 28.4 kg/m2 for men and women, respectively, and for Jordanians,20 with reported BMI cutoff values of 27.2 and 30 kg/m2 for men and women, respectively. However, our proposed cutoff points were higher than what has been reported among Omanis at 23.2 kg/m2 and 26.8 kg/m2 for men and women, respectively.18 It is noteworthy that the proposed cutoff for BMI among men at 25 kg/m2 in this study is less than the increased risk category according to WHO recommendations for appropriate BMI for an Asian population, while the BMI cutoff for women at 28 kg/m2 is under the very high risk category for an Asian population.39 The current study reported significantly higher BMI among women while reporting a significantly lower WC than men. This observation is similar to the findings among Jordanians20 and could be explained by the fact that men are mostly taller than women, especially when the BMI calculation is mainly dependent on the net body weight and height, regardless of the distribution of muscle and bone mass.20
The best anthropometric index for identifying metabolic syndrome is still controversial and varied widely depending on the studied population40 and ethnicity.41 In some populations including Australia, Canada, and Japan, the WHR was the best indicator for cardiovascular risk factors compared to BMI,42–44 while in another Iranian study, WC was superior to BMI and WHR in predicting cardiovascular risk factors.45 On the other hand, among a Chinese population, the three indicators were equally useful for detecting metabolic syndrome.46 In the current study WC yielded the highest AUC followed by BMI, and then WHR. This finding indicates that WC is the best anthropometric indicator for identifying metabolic syndrome among the Saudi population. This finding is also in consistent with data reported from cross-sectional studies among an Iranian population,45 white and African American populations,47 and a Qatari population where the WC was reported as the most powerful indicator for predicting metabolic syndrome.19
The superiority of WC in identifying metabolic syndrome compared with BMI could be due to the fact that WC is better correlated with abdominal fat and strongly associated with cardiovascular risk factors than BMI.48 This is in addition to the reportedly poor ability of BMI to discriminate between excess adipose tissue and high lean muscle mass, and therefore failing to account for body fat distribution.49 In addition, aging results in a decreased standing posture that can inaccurately increase the BMI by 1.5 kg/m2 in men and 2.5 kg/m2 in women, despite a minimal change in body weight.50,51 In addition to its superiority, WC has been acknowledged by WHO as the easiest and most efficient anthropometric measurement for fatness and fat location.52
The current study also shows that the mean values for cardiovascular risk factors as well as the prevalence increase with higher cutoff values for the three anthropometric measurements (BMI, WC, and WHR). This linear association of BMI and WC with cardiovascular risk factors is consistent with data reported from China and Korea.53,54
A strength of the current study is that it is the only source of optimal cutoff values for WC, WHR, and BMI for identifying metabolic syndrome among the Saudi population. The study is also nationwide, representing adults aged ≥18 years from all 13 administrative rural and urban regions of Saudi Arabia. Thus, the results of the current study could be generalized to the full adult population of Saudi Arabia.
Since WC, BMI and WHR are surrogates for body fatness and fat distribution, the present study is limited primarily by not having a direct measure of the body fatness and fat distribution. However, those direct measures are complicated and might not be cost-effective in population-based studies. A second limitation of this study is the cross-sectional design that might have affected the temporality and therefore limited the ability to draw causal inferences. Therefore, prospective studies are needed to provide stronger evidence and predictive power from anthropometric measures for metabolic syndrome.
We conclude that WC is the best predictor for metabolic syndrome in the Saudi population. The proposed cutoff values for the three anthropometric measures are lower than the values in the ATP III or IDF criteria. Therefore, the proposed cutoff values from the current study could be used for screening people at high risk for metabolic syndrome. Future studies should be conducted to assess the prevalence of metabolic syndrome based on the newly proposed cutoff values for central obesity.
Supplementary Information
Footnotes
Conflict of interest
The authors declared no competing interests.
Funding source
This study was funded by the University Diabetes Center at King Saud University, Ministry of Health and the Tawuniya Company for health insurance.
REFERENCES
- 1.IDF Diabetes Atlas. International Diabetes Federation 2015. 7th edition. ( http://www.diabetesatlas.org/)
- 2.Bahijri SM, Al Raddadi RM. The importance of local criteria in the diagnosis of metabolic syndrome in Saudi Arabia. Ther Adv Endocrinol Metab. 2013;4(2):51–59. doi: 10.1177/2042018813483165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Nozha M, Al-Khadra A, Arafah MR, Al-Maatouq MA, Khalil MZ, Khan NB, et al. Metabolic syndrome in Saudi Arabia. Saudi Med J. 2005;26(12):1918–1925. [PubMed] [Google Scholar]
- 4.Alzahrani AM, Karawagh AM, Alshahrani FM, Naser TA, Ahmed AA, Alsharef EH. Prevalence and predictors of metabolic syndrome among healthy Saudi Adults. Br J Diab Vasc Dis. 2012;12(2):78–80. [Google Scholar]
- 5.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 6.National Cholesterol Education Program (NCEP): Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 7.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 9.Stevens J, Katz EG, Huxley RR. Associations between gender, age and waist circumference. Eur J Clin Nutr. 2010;64(1):6–15. doi: 10.1038/ejcn.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (MCHAT) Am J Clin Nutr. 2007;86(2):353–359. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 11.Kagawa M, Binns CB, Hills AP. Body composition and anthropometry in Japanese and Australian Caucasian males and Japanese females. Asia Pac J Clin Nutr. 2007;16(Suppl 1):31–36. [PubMed] [Google Scholar]
- 12.Lear SA, James PT, Ko GT, Kumanyika S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur J Clin Nutr. 2010;64(1):42–61. doi: 10.1038/ejcn.2009.70. [DOI] [PubMed] [Google Scholar]
- 13.Deurenberg-Yap M, Chew SK, Lin VF, Tan BY, van Staveren WA, Deurenberg P. Relationships between indices of obesity and its co-morbidities in multi-ethnic Singapore. Int J Obes Relat Metab Disord. 2001;25:1554–1562. doi: 10.1038/sj.ijo.0801739. [DOI] [PubMed] [Google Scholar]
- 14.Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P. The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes Relat Metab Disord. 2000;24(8):1011–1017. doi: 10.1038/sj.ijo.0801353. [DOI] [PubMed] [Google Scholar]
- 15.Koster A, Leitzmann MF, Schatzkin A, Mouw T, Adams KF, van Eijk JT, et al. Waist circumference and mortality. Am J Epidemiol. 2008;167(12):1465–1475. doi: 10.1093/aje/kwn079. [DOI] [PubMed] [Google Scholar]
- 16.Wu CH, Heshka S, Wang J, Pierson Rn, Heymsfield SB, Laferrère B, et al. Truncal fat in relation to total body fat: influences of age, sex, ethnicity and fatness. Int J Obes (Lond) 2007;31(9):1384–1391. doi: 10.1038/sj.ijo.0803624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansour AA, Al-Hassan AA, Al-Jazairi MI. Cut-off values for waist circumference in rural Iraqi adults for the diagnosis of metabolic syndrome. Rural Remote Health. 2007;7(4):765. [PubMed] [Google Scholar]
- 18.Al-Lawati JA, Jousilahti P. Body mass index, waist circumference and waist-to-hip ratio cut-off points for categorisation of obesity among Omani Arabs. Public Health Nutr. 2008;11(1):102–108. doi: 10.1017/S1368980007000183. [DOI] [PubMed] [Google Scholar]
- 19.Bener A, Yousafzai MT, Darwish S, Al-Hamaq AO, Nasralla EA, Abdul-Ghani M. Obesity index that better predict metabolic syndrome: body mass index, waist circumference, waist hip ratio, or waist height ratio. J Obes. 2013;2013:269038. doi: 10.1155/2013/269038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khader YS, Batieha A, Jaddou H, Batieha Z, El-Khateeb M, Ajlouni K. Anthropometric cutoff values for detecting metabolic abnormalities in Jordanian adults. Diabetes Metab Syndr Obes. 2010;3:395–402. doi: 10.2147/DMSOTT.S15154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assaad-Khalil SH, Mikhail MM, Aati TA, Zaki A, Helmy MA, Meqallaa MH, et al. Optimal waist circumference cutoff points for the determination of abdominal obesity and detection of cardiovascular risk factors among adult Egyptian population. Indian J Endocrinol Metab. 2015;19(6):804–810. doi: 10.4103/2230-8210.167556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouguerra R, Alberti H, Smida H, Salem LB, Rayana CB, El Atti J, et al. Waist circumference cut-off points for identification of abdominal obesity among the Tunisian adult population. Diabetes Obes Metab. 2007;9(6):859–868. doi: 10.1111/j.1463-1326.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization (WHO) Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. Geneva: World Health Organization; 2008. [Google Scholar]
- 24.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO Consultation. Part 1: diagnosis and classification of diabetes mellitus. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 25.Frankenfield DC, Rowe WA, Cooney RN, Smith JS, Becker D. Limits of body mass index to detect obesity and predict body composition. Nutrition. 2001;17(1):26–30. doi: 10.1016/s0899-9007(00)00471-8. [DOI] [PubMed] [Google Scholar]
- 26.Aekplakorn W, Pakpeankitwatana V, Lee CM, Woodward M, Barzi F, Yamwong S, et al. Abdominal obesity and coronary heart disease in Thai men. Obesity (Silver Spring) 2007;15:1036–1042. doi: 10.1038/oby.2007.604. [DOI] [PubMed] [Google Scholar]
- 27.Zhu S, Heymsfield SB, Toyoshima H, Wang Z, Pietrobelli A, Heshka S. Race-ethnicity-specific waist circumference cutoffs for identifying cardiovascular disease risk factors. Am J Clin Nutr. 2005;81(2):409–415. doi: 10.1093/ajcn.81.2.409. [DOI] [PubMed] [Google Scholar]
- 28.Al-Rubeaan K, Al-Manaa H, Khoja T, Ahmad N, Al-Sharqawi A, Siddiqui K, et al. The Saudi Abnormal Glucose Metabolism and Diabetes Impact Study (SAUDI-DM) Ann Saudi Med. 2014;34(6):465–475. doi: 10.5144/0256-4947.2014.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO STEPwise approach to surveillance (STEPS) Geneva: World Health Organization (WHO); 2008b. [Google Scholar]
- 30.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 31.Aschner P, Buendía R, Brajkovich I, Gonzalez A, Figueredo R, Juarez XE, et al. Determination of the cutoff point for waist circumference that establishes the presence of abdominal obesity in Latin American men and women. Diabetes Res Clin Pract. 2011;93(2):243–247. doi: 10.1016/j.diabres.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 33.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Department of Agriculture. U.S. Department of Health and Human Services. Dietary Guidelines for Americans. 3rd ed. Government Printing Office; Washington, DC, US: 1990. [Google Scholar]
- 35.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. (1998) [DOI] [PubMed] [Google Scholar]
- 36.Welborn TA, Dhaliwal SS. Preferred clinical measures of central obesity for predicting mortality. European Journal of Clinical Nutrition. 2007;61:1373–1379. doi: 10.1038/sj.ejcn.1602656. [DOI] [PubMed] [Google Scholar]
- 37.Molarius A, Seidell JC. Selection of anthropometric indicators for classification of abdominal fatness--a critical review. Int J Obes Relat Metab Disord. 1998;22(8):719–727. doi: 10.1038/sj.ijo.0800660. [DOI] [PubMed] [Google Scholar]
- 38.Wing RR, Jeffery RW, Burton LR, Thorson C, Kuller LH, Folsom AR. Change in waist-hip ratio with weight loss and its association with change in cardiovascular risk factors. Am J Clin Nutr. 1992;55(6):1086–1092. doi: 10.1093/ajcn/55.6.1086. [DOI] [PubMed] [Google Scholar]
- 39.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 40.Molarius A, Seidell JC. Selection of anthropometric indicators for classification of abdominal fatness--a critical review. Int J Obes Relat Metab Disord. 1998;22(8):719–727. doi: 10.1038/sj.ijo.0800660. [DOI] [PubMed] [Google Scholar]
- 41.Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143(3):228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 42.Welborn TA, Dhaliwal SS, Bennett SA. Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust. 2003;179:580–585. doi: 10.5694/j.1326-5377.2003.tb05704.x. [DOI] [PubMed] [Google Scholar]
- 43.Brenner DR, Tepylo K, Eny KM, Cahill LE, El-Sohemy A. Comparison of body mass index and waist circumference as predictors of cardiometabolic health in a population of young Canadian adults. Diabetol Metab Syndr. 2010;2(1):28. doi: 10.1186/1758-5996-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito H, Nakasuga K, Ohshima A, Maruyama T, Kaji Y, Harada M, et al. Detection of cardiovascular risk factors by indices of obesity obtained from anthropometry and dual-energy X-ray absorptiometry in Japanese individuals. Int J Obes Relat Metab Disord. 2003;27(2):232–237. doi: 10.1038/sj.ijo.802226. [DOI] [PubMed] [Google Scholar]
- 45.Gharipour M, Sarrafzadegan N, Sadeghi M, Andalib E, Talaie M, Shafie D, et al. Predictors of metabolic syndrome in the Iranian population: waist circumference, body mass index, or waist to hip ratio? Cholesterol. 2013;2013:198384. doi: 10.1155/2013/198384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Tong G, Tong W, Lu L, Qin X. Can body mass index, waist circumference, waist-hip ratio and waist-height ratio predict the presence of multiple metabolic risk factors in Chinese subjects? BMC Public Health. 2011;11:35. doi: 10.1186/1471-2458-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beydoun MA, Kuczmarski MT, Wang Y, Mason MA, Evans MK, Zonderman AB. Receiver-operating characteristics of adiposity for metabolic syndrome: the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. Public Health Nutr. 2011;14(1):77–92. doi: 10.1017/S1368980010002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu S, Heymsfield SB, Toyoshima H, Wang Z, Pietrobelli A, Heshka S. Race-ethnicity-specific waist circumference cutoffs for identifying cardiovascular disease risk factors. Am J Clin Nutr. 2005;81(2):409–415. doi: 10.1093/ajcn.81.2.409. [DOI] [PubMed] [Google Scholar]
- 49.Grundy SM, Neeland IJ, Turer AT, Vega GL. Waist circumference as measure of abdominal fat compartments. J Obes. 2013;2013:454285. doi: 10.1155/2013/454285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ness-Abramof R, Apovian CM. Waist circumference measurement in clinical practice. Nutr Clin Pract. 2008;23(4):397–404. doi: 10.1177/0884533608321700. [DOI] [PubMed] [Google Scholar]
- 51.Srikanthan P, Seeman TE, Karlamangla AS. Waist-hip-ratio as a predictor of all-cause mortality in high-functioning older adults. Ann Epidemiol. 2009;19:724–731. doi: 10.1016/j.annepidem.2009.05.003. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 53.Wildman RP, Gu D, Reynolds K, Duan X, He J. Appropriate body mass index and waist circumference cutoffs for categorization of overweight and central adiposity among Chinese adults. Am J Clin Nutr. 2004;80(5):1129–36. doi: 10.1093/ajcn/80.5.1129. [DOI] [PubMed] [Google Scholar]
- 54.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75(1):72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.