Abstract
Neural plasticity—the ability to change and adapt in response to stimuli—is an essential aspect of healthy brain function and, in principle, can be harnessed to promote recovery from a wide variety of brain disorders. Many neuropsychiatric diseases including mood, anxiety, and substance use disorders arise from an inability to weaken and/or strengthen pathologic and beneficial circuits, respectively, ultimately leading to maladaptive behavioral responses. Thus, compounds capable of facilitating the structural and functional reorganization of neural circuits to produce positive behavioral effects have broad therapeutic potential. Several known drugs and experimental therapeutics have been shown to promote plasticity, but most rely on indirect mechanisms and are slow-acting. Here, I describe psychoplastogens—a relatively new class of fast-acting therapeutics, capable of rapidly promoting structural and functional neural plasticity. Psychoplastogenic compounds include psychedelics, ketamine, and several other recently discovered fast-acting antidepressants. Their use in psychiatry represents a paradigm shift in our approach to treating brain disorders as we focus less on rectifying “chemical imbalances” and place more emphasis on achieving selective modulation of neural circuits.
Keywords: Psychoplastogen, psychedelic, neural plasticity, induced plasticity, ketamine, DMT, LSD, MDMA, depression, PTSD
Comment on: Ly C, Greb AC, Cameron LP, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170–3182. doi:10.1016/j.celrep.2018.05.022. PubMed PMID: 29898390. https://www.ncbi.nlm.nih.gov/pubmed/29898390
Behavior is ultimately controlled by a combination of activity in a variety of neural circuits distributed across the brain. In several disease states, circuits that drive maladaptive behaviors are potentiated, whereas those that are more constructive become weakened. Juvenile brains are remarkably plastic and given an appropriate stimulus can often rebalance these circuits. However, after the closure of critical periods, adult brains become far less plastic making it necessary to artificially promote plasticity to repair damaged circuits. In principle, interventions that promote plasticity and enable the rebalancing of neural circuits can be used to treat a variety of brain diseases. Stress-related mood and anxiety disorders are particularly good examples of diseases resulting from circuit imbalances and thus are ideally suited to highlight plasticity-related strategies for improving brain health.
The prefrontal cortex (PFC) plays a critical role in the top-down control of fear and reward and thus it is of central importance to the treatment of neuropsychiatric diseases such as posttraumatic stress disorder (PTSD) and depression. In fact, one of the hallmarks of depression is the retraction of dendrites and loss of dendritic spines and synapses in the PFC. These structural phenotypes are thought to underlie circuit-level changes leading to behaviors characteristic of the disease. The neurotrophic hypothesis of depression posits that loss of trophic support in areas of the brain such as the PFC and the hippocampus leads to atrophy of these brain regions, which ultimately disrupts critical mood-regulating circuits. Direct infusion of brain-derived neurotrophic factor (BDNF) into the PFC or hippocampus is known to produce antidepressant/anxiolytic effects in rodents. Unfortunately, the proteinaceous nature of BDNF imparts poor pharmacokinetic properties and renders it completely ineffective as a systemically administered central nervous system (CNS) therapeutic. Therefore, small molecules capable of crossing the blood-brain barrier and activating plasticity mechanisms possess great medicinal value.
Compound-induced neural plasticity, sometimes referred to as iPlasticity, is a well-established phenomenon occurring after treatment with several classes of small molecules.1 However, most of these compounds act through slow, indirect processes typically relying on the regulation of neurotrophic factors and other proteins critical for plasticity. Traditional antidepressants, such as selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors, and tricyclics, are some of the most efficacious plasticity-promoting compounds known. For example, traditional antidepressants increase the expression of BDNF and promote the growth of critical mood-regulating neurons in the PFC and hippocampus. In addition, fluoxetine can promote cortical remapping of ocular dominance columns and facilitate fear extinction learning.1 However, their effects on plasticity parallel their behavioral effects, which are quite slow and require chronic administration. Compounds that rapidly promote plasticity and produce beneficial, long-lasting behavioral changes represent an exciting advance over current plasticity-promoting medicines.
The discovery that ketamine—a dissociative anesthetic—produces fast-acting and relatively long-lasting antidepressant effects has had a profound impact on psychiatry and represents one of the field’s most important findings in recent years. Ketamine promotes the growth of dendritic spines and the formation of synapses in the PFC within 24 hours of administration,2 a period of time that correlates with its antidepressant effects. Moreover, it has long-lasting effects, implicating positive neural adaptations in the circuits critical for regulating mood. Although extremely promising, ketamine is far from an ideal therapeutic as it has the potential for abuse. Therefore, a substantial amount of effort has been directed toward the identification of compounds that mimic the beneficial effects of ketamine. To classify compounds like ketamine capable of altering neural circuits by rapidly promoting plasticity (Figure 1), and to distinguish them from other slow-acting molecules that induce plasticity, we have recently introduced the term “psychoplastogen,” from the Greek roots psych- (mind), -plast (molded), and -gen (producing).3
Figure 1.
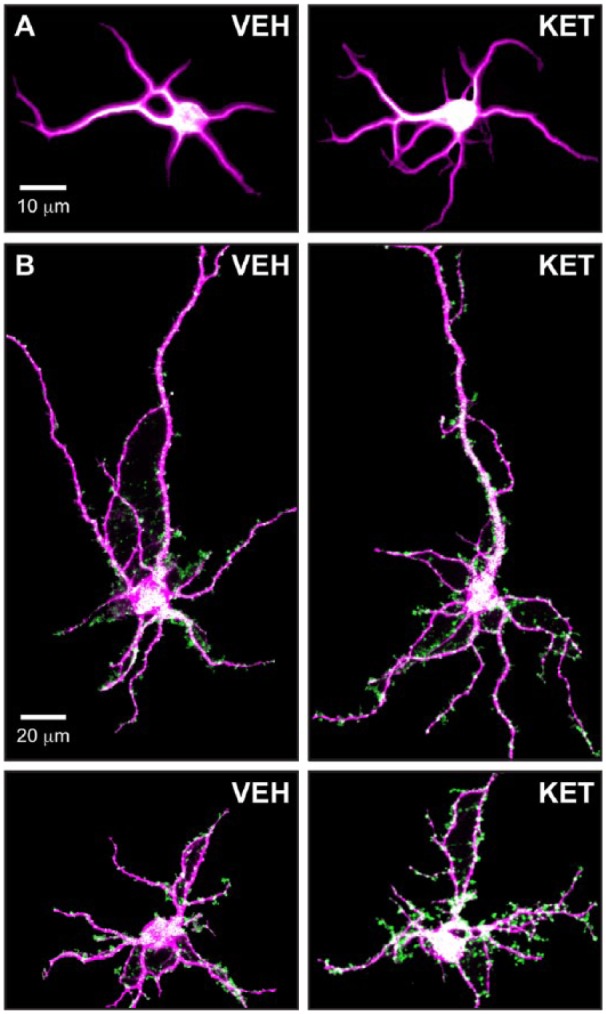
Ketamine is the prototypical psychoplastogen. (A) Immature cultured cortical neurons (DIV6) treated with ketamine display increased dendritic branching compared to vehicle-treated neurons. (B) Mature cultured cortical neurons (DIV20) treated with ketamine display increased synapse formation relative to vehicle-treated neurons. VEH, vehicle; KET, ketamine; magenta, MAP2 staining; green, synapses determined by colocalization of pre- (VGLUT1) and postsynaptic (PSD-95) puncta.
By definition, psychoplastogens are small molecules and thus plasticity-promoting proteins like BDNF do not fall into this category. To be classified as a psychoplastogen, a compound should produce a measurable change in plasticity (eg, changes in neurite growth, dendritic spine density, synapse number, intrinsic excitability, etc.) within a short period of time (typically 24-72 hours) following a single administration. Because their impact on neural plasticity enables subsequent stimuli to reshape neural circuits, they should produce relatively long-lasting changes in behavior that extend beyond the acute effects of the drug. In addition to ketamine, several other psychoplastogens have been identified, all of which produce fast-acting antidepressant effects in humans.4 These include the muscarinic receptor antagonist scopolamine,5 the NMDA receptor partial agonist GLYX-13 (ie, rapastinel),6 and 5-HT2A receptor agonists such as psychedelics (Table 1).
Table 1.
Examples of psychoplastogens with demonstrated in vivo activity in rodents and antidepressant effects in humans.
| Name | Structure | References |
|---|---|---|
| Ketamine |
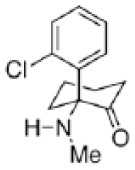
|
Li et al2 |
| GLYX-13 (rapastinel) |
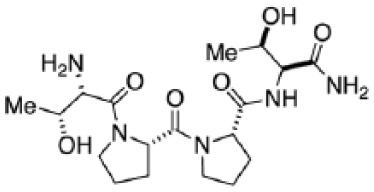
|
Liu et al6 |
| Scopolamine |
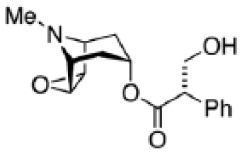
|
Voleti et al5 |
| DMT |
|
Ly et al3 |
DMT, N,N-dimethyltryptamine.
Recently, our group has demonstrated that psychedelic compounds such as lysergic acid diethylamide (LSD), N,N-dimethyltryptamine (DMT), and 2,5-dimethoxy-4-iodoamphetamine promote dendritic branching and/or increase spine/synapse number both in cultured cortical neurons and in vivo.3 These results provide a potential explanation for the known ability of these compounds to produce long-lasting changes in personality and positively impact circuits relevant to the treatment of mood, anxiety, and substance use disorders. Although our cellular studies have shown that a wide variety of psychedelic compounds produce psychoplastogenic effects similar to ketamine, our in vivo work thus far has primarily focused on the effects of DMT—the archetype for all tryptamine-containing psychedelics.
Our initial efforts investigating the plasticity-promoting properties of psychedelics focused on DMT for a variety of reasons. First, the simple structure of DMT represents the minimal pharmacophore for all tryptamine-containing psychedelics as others such as LSD, ibogaine, psilocybin, and 5-MeO-DMT can be considered either conformationally restricted or substituted analogues of DMT.7 Like ketamine,2 a single intraperitoneal injection of DMT increases dendritic spine density as well as the frequency and amplitude of spontaneous excitatory postsynaptic currents in the PFC of rats 24 hours after administration. As the half-life of DMT in rats is on the order of 15 minutes, the compound is cleared from the body within 24 hours and thus these changes in neuronal structure and function must reflect plasticity and not simply the acute effects of the drug. Moreover, DMT produces behavioral effects in rodents that mirror those of ketamine such as promoting fear extinction learning and reducing immobility in the forced swim test.8 In humans, a DMT-containing tisane known as ayahuasca has been shown to produce rapid and sustained antidepressant effects.7
The use of a psychoplastogen to promote fear extinction learning is an excellent example of how small molecules can be used to rewire neural circuits. The PFC plays a critical role in encoding extinction memories and thus the administration of a psychoplastogenic compound shortly before extinction training can strengthen fear extinction memories more effectively than training alone. Like ketamine and DMT, the psychedelic 3,4-methylenedioxymethamphetamine (MDMA) has been shown to promote fear extinction learning in rodents. Furthermore, MDMA has demonstrated great promise in the clinic for treating PTSD via a treatment paradigm that can best be described as pharmaceutical-enhanced psychotherapy.9 The use of psychoplastogens to enhance fear extinction is gaining traction and in principle, the same strategy could be used to extinguish drug-cue memories. Perhaps, this is why psychedelic compounds such as DMT, ibogaine, and LSD have shown promise for treating substance use disorders. The true therapeutic potential of psychoplastogens is not known, but the possibilities are incredibly exciting and include the treatment of stroke, brain trauma, and neurodegenerative diseases. In fact, slow-acting plasticity-promoting compounds such as fluoxetine have already been used to promote functional recovery after brain insults.1
Mechanistically, psychoplastogens appear to induce changes in neuronal structure by activating the mammalian target of rapamycin (mTOR)—a key protein involved in cell growth, autophagy, and the production of proteins necessary for synapse formation.10 Many psychoplastogens including ketamine, psychedelics, and scopolamine increase the secretion of glutamate, which stimulates mTOR by activating AMPA receptors. However, many psychoplastogens alter perception or produce other undesired effects. A better mechanistic understanding of mTOR signaling in the brain could lead to the development of compounds with psychoplastogenic properties but better safety profiles. A number of companies and academic scientists are currently pursuing this strategy.
The most useful psychoplastogens will be the ones capable of promoting plasticity in a circuit-specific manner. While brain-penetrant small molecule TrkB agonists solved the pharmacokinetic problem of using BDNF as a therapeutic, they do not offer any selectivity as TrkB is widely distributed throughout the brain and the periphery. Promoting plasticity indiscriminately is not likely to be beneficial. For example, increased plasticity in the mesolimbic pathway could lead to addiction, whereas activation of TrkB on nociceptors could result in chronic pain. In terms of developing circuit-specific psychoplastogens, psychedelics are extremely attractive starting points because they activate mTOR by stimulating 5-HT2A receptors—receptors that are highly expressed on layer V pyramidal neurons of the cortex. This genetic localization ensures that psychoplastogenic effects are localized to cortical regions like the PFC and could possibly explain why psychedelic compounds are not generally considered to be addictive (ie, they do not promote plasticity in the mesolimbic pathway).
Although psychoplastogens offer many exciting possibilities for therapeutic interventions, it is important to consider the potential risks associated with promoting plasticity through the activation of mTOR. Excessive stimulation of mTOR has been associated with autism spectrum disorder and Alzheimer’s disease,10 and therefore, more research needs to be done to firmly establish the risks and benefits associated with using these compounds. Many psychoplastogens cause the release of glutamate leading to trophic effects; however, excessive glutamate can result in excitotoxicity and neuronal atrophy. Therefore, the dose and frequency of administration will likely be critical to maximize efficacy while minimizing deleterious effects.
The advent of psychoplastogenic compounds has enabled us to move beyond simplistic therapeutic strategies aimed at controlling monoamine levels toward the selective modulation of neural circuits—a fundamental shift in our approach to treating CNS disorders. Significant progress has been made in recent years and provides hope that modern research on ketamine, psychedelics, and other psychoplastogens will lead to safe and effective strategies for harnessing neural plasticity to treat mood and anxiety disorders such as depression and PTSD. As the number of psychoplastogenic compounds continues to grow, so do our chances of identifying the next generation of medicines for treating neuropsychiatric and neurodegenerative diseases. Regardless, it is clear that psychoplastogens can serve as powerful tools for understanding the basic biology of neural plasticity.
Acknowledgments
The author would like to thank Calvin Ly and Whitney Duim for providing the images shown in Figure 1, Calvin Ly and Lindsay Cameron for helpful discussions about the manuscript, and Valentina Popescu in the Department of Classics at UC Davis for assistance with coining the term “psychoplastogen.”
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funds from the National Institute of General Medical Sciences (R01 GM128997), a UC Davis STAIR grant, and the National Institute of Child Health and Human Development (U54 HD079125). The content of this manuscript is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: DEO wrote the manuscript.
ORCID iD: David E Olson  https://orcid.org/0000-0002-4517-0543
https://orcid.org/0000-0002-4517-0543
References
- 1. Castrén E, Antila H. Neuronal plasticity and neurotrophic factors in drug responses. Mol Psychiatry. 2015;22:1085-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ly C, Greb AC, Cameron LP, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duman RS. Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. doi: 10.12688/f1000research.14344.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voleti B, Navarria A, Liu RJ, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74:742-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu RJ, Duman C, Kato T, et al. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology. 2017;42:1231-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cameron LP, Olson DE. Dark classics in chemical neuroscience: N,N-Dimethyltryptamine (DMT) [published online ahead of print July 23, 2018]. ACS Chem Neurosci. doi: 10.1021/acschemneuro.8b00101. [DOI] [PubMed] [Google Scholar]
- 8. Cameron LP, Benson CJ, Dunlap LE, Olson DE. Effects of N,N-Dimethyltryptamine on rat behaviors relevant to anxiety and depression. ACS Chem Neurosci. 2018;9:1582-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dunlap LE, Andrews AM, Olson DE. Dark classics in chemical neuroscience: 3,4-Methylenedioxymethamphetamine [published online ahead of print July 12, 2018]. ACS Chem Neurosci. doi: 10.1021/acschemneuro.8b00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]


