Short abstract
Fragile X syndrome (FXS) is a neurodevelopmental disorder that causes intellectual disability. It is a leading known genetic cause of autism. In addition to cognitive, social, and communication deficits, humans with FXS demonstrate abnormal sensory processing including sensory hypersensitivity. Sensory hypersensitivity commonly manifests as auditory, tactile, or visual defensiveness or avoidance. Clinical, behavioral, and electrophysiological studies consistently show auditory hypersensitivity, impaired habituation to repeated sounds, and reduced auditory attention in humans with FXS. Children with FXS also exhibit significant visuospatial impairments. Studies in infants and toddlers with FXS have documented impairments in processing texture-defined motion stimuli, temporal flicker, perceiving ordinal numerical sequence, and the ability to maintain the identity of dynamic object information during occlusion. Consistent with the observations in humans with FXS, fragile X mental retardation 1 (Fmr1) gene knockout (KO) rodent models of FXS also show seizures, abnormal visual-evoked responses, auditory hypersensitivity, and abnormal processing at multiple levels of the auditory system, including altered acoustic startle responses. Among other sensory symptoms, individuals with FXS exhibit tactile defensiveness. Fmr1 KO mice also show impaired encoding of tactile stimulation frequency and larger size of receptive fields in the somatosensory cortex. Since sensory deficits are relatively more tractable from circuit mechanisms and developmental perspectives than more complex social behaviors, the focus of this review is on clinical, functional, and structural studies that outline the auditory, visual, and somatosensory processing deficits in FXS. The similarities in sensory phenotypes between humans with FXS and animal models suggest a likely conservation of basic sensory processing circuits across species and may provide a translational platform to not just develop biomarkers but also to understand underlying mechanisms. We argue that preclinical studies in animal models of FXS can facilitate the ongoing search for new therapeutic approaches in FXS by understanding mechanisms of basic sensory processing circuits and behaviors that are conserved across species.
Keywords: autism spectrum disorders, neurodevelopmental disorders, sensory hypersensitivity, fragile X syndrome
Introduction
Fragile X syndrome (FXS) is the most prevalent cause of inherited intellectual disability and is a leading genetic cause of autism (Crawford et al., 2001). FXS affects 1 in 4,000 boys and 1 in 8,000 girls and is caused by the silencing, deletion, or loss-of-function mutation of the fragile X mental retardation 1 (FMR1) gene. As a result, its protein product, fragile X mental retardation protein (FMRP), is either not expressed or is nonfunctional (Verkerk et al., 1991; Sutcliffe et al., 1992; Okray et al., 2015). FMRP is a messenger RNA (mRNA)-binding protein (Ashley et al., 1993b) that regulates several aspects of mRNA metabolism such as nuclear export, transport to synaptic terminals, activity-dependent ribosome stalling, and protein translation (Laggerbauer et al., 2001; Bagni & Greenough, 2005; Bassell & Warren, 2008; Darnell et al., 2011; Santoro et al., 2012).
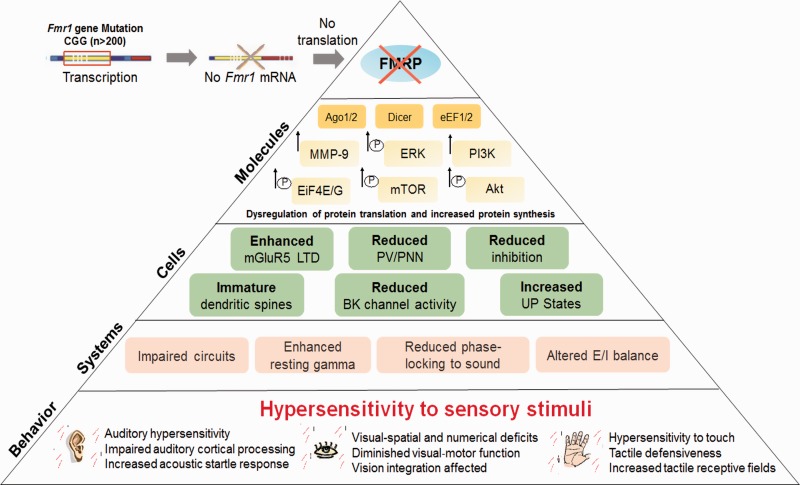
FMRP regulates translation of mRNAs at synapses, some of which encode proteins involved in synaptic plasticity (Brown et al., 2001; Zalfa et al., 2003). The absence of FMRP leads to the dysregulation of protein translation and increased protein synthesis (Bear et al., 2004; Darnell & Klann, 2013), which may contribute to altered metabotropic glutamate receptor 5 (mGluR5) signaling resulting in exaggerated long-term depression (LTD) in the hippocampus (Huber et al., 2002). FMRP also negatively regulates matrix metalloproteinase-9 (MMP-9) translation in neurons (Dziembowska & Wlodarczyk, 2012; Janusz et al., 2013; Dziembowska et al., 2013), and MMP-9 levels are elevated in FXS (Bilousova et al., 2009; Gkogkas et al., 2014; Sidhu et al., 2014). mGluR5 and MMP-9 may mediate changes in synaptic functions by signaling through the phosphatidylinositide-3-kinase (PI3K)/mammalian target of rapamycin complex 1 (mTORC1) and extracellular signal-regulated kinase pathways to increase cap-dependent translation (Ferraguti et al., 1999; Gallagher et al., 2004; Hou & Klann, 2004; Klann & Dever, 2004; Banko et al., 2006; Hou et al., 2006; Antion et al., 2008; Ronesi & Huber, 2008; Sharma et al., 2010). Recent data suggest that FMRP may also directly regulate PI3K and mTORC1 signaling through other signaling proteins, such as phosphatidylinositide-3-kinase enhancer, phosphatase and tensin homolog, neurofibromin 1, and tuberous sclerosis 2 (Sharma et al., 2010; Enriquez-Barreto & Morales, 2016; Sato, 2016). In addition, all three isoforms of eukaryotic translation initiation factor 4 G, eukaryotic translation elongation factor 1 and 2, argonaute proteins, and Dicer are FMRP targets, and their dysregulation may also contribute to enhanced neuronal translation in FXS (Figure 1; Cheever & Ceman, 2009; Darnell et al., 2011; Muddashetty et al., 2011).
Figure 1.
Mechanisms of sensory phenotypes associated with FXS. Fragile X syndrome is associated with an expansion of CGG repeats in 5′ untranslated area of the fragile X mental retardation 1 (Fmr1) gene, which leads to silencing Fmr1 gene and a partial or full loss of the fragile X mental retardation protein (FMRP). FMRP is an RNA-binding protein that regulates translation of mRNAs at synapses, some of which encode proteins involved in protein synthesis and synaptic plasticity. FMRP is known to regulate protein translation through eukaryotic translation elongation factor 1/2 (eEF1/2), argonaute proteins (Ago1/2), eukaryotic translation initiation factor 4 E/G (eIF4E/G), and Dicer. FMRP may also directly regulate phosphatidylinositide-3-kinase (PI3K), Akt, mammalian target of rapamycin (mTOR), and extracellular signal-regulated kinase (ERK) signaling. Lack of FMRP also leads to enhanced metabotropic glutamate receptor 5 (mGluR5)-mediated long-term depression (LTD), reduced voltage and Ca2+ activated K+ (BK) channel activity, and increased matrix metalloproteinase-9 (MMP-9) activity, which affect cellular responses resulting in reduced inhibition, impaired development of parvalbumin (PV) interneurons and perineuronal nets (PNN), increased UP states, and abnormal dendritic spine development. These molecular and cellular alterations can contribute to system-level changes, such as impaired development of neural circuits, enhanced resting gamma, and altered excitatory/inhibitory (E/I) balance, which may underlie sensory hypersensitivity and altered behaviors observed in FXS.
Dysregulated PI3K/mTOR signaling, enhanced mGluR5-dependent LTD, increased MMP-9 activity, and reduced activity of the voltage and Ca2+ activated K+ (BKCa or BK) channel may contribute to the immature dendritic spine morphology in rodent models of FXS (Figure 1; Huber et al., 2000; Nimchinsky et al., 2001; Vanderklish & Edelman, 2002; Hou & Klann, 2004; Hu et al., 2008; Sidhu et al., 2014). In mice, FMRP may also regulate neuronal branching (Galvez et al., 2003) as well as dendritic spine development (Nimchinsky et al., 2001). Consistent with animal work, clinical studies revealed alterations in dendritic spine number and morphology in the cortex of FXS humans, with a prevalence of immature dendritic spines (Hinton et al., 1991; Irwin et al., 2001). In fact, dendritic abnormalities are consistent anatomical correlates of intellectual disability (Kaufmann & Moser, 2000). Although most of FMRP activity is considered to be related to the regulation of synaptic functions (Zhang et al., 2001; Edbauer et al., 2010; Darnell et al., 2011), little is known about how the synaptic alterations in the absence of FMRP may lead to deficits in neurophysiology and behavior in humans with FXS. Abnormal dendritic spine development alone cannot explain increased cortical excitability observed in FXS.
FMRP loss increases network-level hyperexcitability in the rodent cortex through impaired inhibition and altered neural synchrony (Figure 1; Gonçalves et al., 2013; Zhang et al., 2014). The Fmr1 KO mouse shows decreased gamma-aminobutyric acid (GABA) receptor levels, decreased GABA synthesis, increased GABA catabolism, and overall decreased GABAergic input in many regions of the brain (El Idrissi et al., 2005; D'Hulst et al., 2006; Selby et al., 2007; Curia et al., 2009; D'Hulst et al., 2009; Adusei et al., 2010; Liu et al., 2013). FMRP was also shown to regulate neuronal excitability through the direct interactions with several ion channels, such as sodium‐activated potassium channel Slack, presynaptic N‐type voltage‐gated calcium channels, and calcium‐activated potassium BK channels (Brown et al., 2010; Zhang et al., 2012; Deng et al., 2013; Ferron et al., 2014; Hébert et al., 2014; Myrick et al., 2015). The enhanced excitability is associated with neurological symptoms observed in FXS, such as hypersensitivity, hyperarousal, hyperactivity, anxiety, and seizures (Figure 1; Penagarikano et al., 2007; Braat & Kooy, 2015). However, limited knowledge of the neuronal circuits underlying complex behaviors, such as anxiety and communication deficits, has hindered the progress in translating the results of the mouse studies into successful clinical trials. Potential mechanisms of altered neuronal circuit excitability and how these changes might impact sensory perception and behavior in FXS are beginning to be understood. In this review, we bring together clinical, functional, and neuroanatomical studies that outline auditory, visual, and somatosensory processing deficits in FXS and how understanding these mechanisms using preclinical studies in animal models can help our search for new therapeutic applications in FXS.
Animal Models of FXS
To understand the molecular and cellular pathogenesis of FXS, the disease has been successfully modeled in rodents (Bakker et al., 1994; Eadie et al., 2009; Hamilton et al., 2014), Drosophila (Pan et al., 2008), and zebrafish (den Broeder et al., 2009). The mouse Fmr1 gene product shows 97% homology to human FMRP (Ashley et al., 1993a). The Fmr1 knockout (KO) mouse model was generated with phenotypes similar to those observed in human FXS patients, such as progressive macroorchidism (Bakker et al., 1994) and abnormal dendritic spine development (Nimchinsky et al., 2001). Fmr1 KO mice also demonstrate impaired cognitive functions and aberrant behaviors (Yan et al., 2005; Hayashi et al., 2007). More robust cognitive deficits have been identified in studies of memory extinction that include inhibitory avoidance paradigm, trace fear conditioning, and lever-press escape/avoidance tasks (Zhao et al., 2005; Brennan et al., 2006; Dölen et al., 2007; Hayashi et al., 2007; Eadie et al., 2009). Susceptibility to age-dependent audiogenic seizures is another reproducible phenotype observed in Fmr1 KO mice (Chen & Toth, 2001; Yan et al., 2005; Dölen et al., 2007). The mouse FXS model is also tractable for electrophysiology experiments to define the synaptic alterations associated with FXS. Fmr1 KO hippocampal neurons show enhanced metabotropic glutamate receptor (mGluR5)-dependent LTD (Huber et al., 2002). Other studies have shown deficits in long-term potentiation (LTP) in the hippocampus, cortex, and the lateral amygdala (Li et al., 2002; Larson et al., 2005; Zhao et al., 2005; Desai et al., 2006; Volk et al., 2007; Wilson & Cox, 2007), suggesting alterations in synaptic plasticity that may underlie deficits in experience-dependent brain functions in FXS.
The development of the Fmr1 KO rat model allows for modeling more complex cognitive and social behaviors associated with FXS (Hamilton et al., 2014). It also provides an opportunity for comparison of phenotypes across mammalian species that result from FMRP deletion. Similar to mouse studies, mGluR-LTD was enhanced in Fmr1 KO rats, whereas mGluR LTP was significantly decreased at both cortical and thalamic inputs to the lateral amygdala (Jackson, 2017). Adult Fmr1 KO rats also showed disrupted cortical processing of auditory stimuli (Engineer et al., 2014), recapitulated spine density and synaptic plasticity defects observed in mouse models, and displayed deficits in hippocampal forms of associative recognition memory (Till et al., 2015) and novel social interaction phenotypes (Hamilton et al., 2014). Juvenile Fmr1 KO rats exhibit abnormal cortical state regulation that begins at ages equivalent to human birth (Berzhanskaya et al., 2017). Despite largely normal patterns of spontaneous activity during the first two postnatal weeks before eye opening, Fmr1 KO rats exhibit signs of mild hyperexcitability during the third and fourth postnatal weeks, including an increase in the visually evoked firing of excitatory neurons and reduced firing of inhibitory neurons (Berzhanskaya et al., 2017).
Similar to the rodent models of FXS, the fruit fly (Drosophila) is a powerful genetic model organism for study of FXS. The single FMRP homolog, dFMRP, is well conserved to human FMRP with respect to its functional amino acid motifs (Wan et al., 2000) and RNA-binding properties (Darnell et al., 2005). The fly FXS model system has collectively yielded much insight into the cognitive, behavioral, and morphological phenotypes associated with FXS. Morphological analyses of fly neurons have identified defects in axons and dendrites of specific neuronal subsets, in particular in the neuromuscular junction and the mushroom body. In the absence of dFmr1 activity, the axons within the neuromuscular junction display significant increase in synaptic boutons and branching (Zhang et al., 2001). Neurons in the mushroom body, a brain area that is required for short-term and long-term memory, are also affected in dFmr1 mutants (McBride et al., 1999; Zars et al., 2000; Pascual & Préat, 2001). Moreover, long-term memory defects have been reported in dFmr1 mutants using olfactory-based assays (Bolduc et al., 2008). dFMRP has also been shown to be necessary for long-term, but not short-term olfactory habituation, as indicated by an olfactory avoidance task (Sudhakaran et al., 2014). Electrophysiology analysis also shows defects in synaptic transmission in the optic lobe (Zhang et al., 2001). In addition to axonal, dendritic, and synaptic transmission defects, male dFmr1-null flies also have enlarged testes, a phenotype that is observed in both FXS humans and Fmr1 KO mice (Zhang et al., 2004). Similar to Fmr1 KO mice (Zhang et al., 2008), dFmr1-null flies lack the ability to maintain a normal circadian rhythm when placed in total darkness and exhibit erratic patterns of locomotor activity (Dockendorff et al., 2002; Inoue et al., 2002; Morales et al., 2002; Xu et al., 2012). They also lack interest in courtship (Dockendorff et al., 2002), a social impairment similar to that found in autism. Furthermore, FXS flies exhibit impaired olfactory behaviors. The absence of dFMRP results in reduced olfactory attraction and aversion. Calcium imaging data show that antennal lobe projection neurons have broader odor tuning in dFmr1 flies, leading to reduced specificity in odor coding and alterations in olfactory representations. Consistent with these results, lateral inhibition across olfactory glomeruli, as well as the inhibitory connections between local interneurons and projection neurons are impaired in dFmr1 flies (Franco et al., 2017). This suggests that absence of dFMRP leads to abnormal lateral inhibition across olfactory glomeruli, which, in turn, results in impaired odor coding and olfactory behaviors. Thus, the fly FXS model displays significant social, cognitive, and sensory deficits that can be used to examine the underlying mechanisms.
Zebrafish is a more recent animal model that has shown potential as a complementary vertebrate model in studying the pathophysiology of FXS. The adult zebrafish FMRP shares 72% amino acid identity with human FMRP and is highly expressed in the brain, including in the telencephalon, diencephalon, metencephalon, and cerebellum, and spinal cord (van 't Padje et al., 2005). In adult zebrafish, Fmr1 KO produces the anxiolytic-like responses of increased exploratory behavior in light/dark and open-field tests and avoidance learning impairment, indicating that hyperactivity and anxiety can be also tested in Fmr1 KO zebrafish. Furthermore, electrophysiological recordings from telencephalic slice preparations of Fmr1 KO zebrafish displayed markedly reduced LTP and enhanced LTD compared to wild-type (WT) counterparts (Ng et al., 2013). Animal models of FXS have a great potential for elucidating mechanisms underlying cognitive, behavioral, and morphological phenotypes associated with FXS, as well as preclinical studies.
Auditory Hypersensitivity and Underlying Mechanisms
Auditory hypersensitivity is common in humans with FXS and mouse models of FXS (Rotschafer & Razak, 2014; Sinclair et al., 2017b). Notably, studies indicate abnormalities in auditory processing in people with FXS (St Clair et al., 1987; Rojas et al., 2001; Castrén et al., 2003; Van der Molen et al., 2012a, 2012b; Schneider et al., 2013). Tone-evoked responses measured using magnetic fields are higher in the auditory cortex of humans with FXS (Rojas et al., 2001). Increased activation of left hemispheric circuitry, including superior temporal gyrus, was observed in FXS subjects during auditory temporal discrimination task (Hall et al., 2009). To assess sensory-cognitive processing in humans with FXS, various event-related brain potential (ERP) techniques have been employed. ERPs reflect the activity of neuronal populations in response to specific sensory-cognitive processes and can be detected using electroencephalograms (EEG) and magnetoencephalograms (MEG; Luck, 2014). A relatively simple auditory stimulus can elicit a N1 wave in the auditory cortex. Auditory ERP studies report abnormally high amplitude of the N1 wave in response to tones and reduced habituation to repeated sound in FXS (Rojas et al., 2001; Castrén et al., 2003; Van der Molen et al., 2012a, 2012b; Schneider et al., 2013; Ethridge et al., 2016). FXS patients also exhibit increased gamma frequency band power during resting state. This increased gamma activity is believed to be linked to increased neural excitability, and examining the relationship of alpha and theta band activity with gamma band activity might provide system-level understanding about the altered balance between excitatory and inhibitory activity (Wang et al., 2017). Furthermore, a recent study shows that humans with FXS demonstrate a marked reduction in the ability to synchronize evoked high-frequency neural activity to time-varying signals, suggesting impairments in underlying neural generators involved in sensory processing (Ethridge et al., 2017). These data indicate a noisy resting state of sensory cortex in people with FXS that may lead to abnormal synchronization of evoked responses. Auditory cortex processing abnormalities that arise early in development may contribute to higher order auditory functional deficits such as language deficits seen in FXS and autism (Roberts et al., 2001; Nieto Del Rincón, 2008; Barnes et al., 2009; Finestack et al., 2009; Roberts et al., 2011). However, very little is known about development of EEG/MEG abnormalities and correlations with language development in humans.
Fmr1 KO mice also exhibit abnormal prepulse inhibition and auditory startle responses, with greater startle responses than WT mice to low-intensity (80 dB) white noise bursts and decreased responses to high-intensity (120 dB) white noise bursts (Nielsen et al., 2002). Fmr1 KO mice are also acoustically hypersensitive and are prone to audiogenic seizures (Miller et al., 1999; Chen & Toth, 2001; Nielsen et al., 2002; Frankland et al., 2004), suggesting enhanced excitability in the auditory system. Intense auditory stimuli (>100 dB SPL) induces a period of wild running, clonic–tonic seizing, and can result in the death of the animal (Musumeci et al., 2000; Chen & Toth, 2001; Musumeci et al., 2007; Dansie et al., 2013). Reintroduction of FMRP to Fmr1 KO mice significantly reduces audiogenic seizure susceptibility (Musumeci et al., 2007). In addition, the audiogenic seizure phenotype of Fmr1 KO mice is prevented by the systemic administration of the mGluR5 receptor antagonist, MPEP (Yan et al., 2005). Enhanced susceptibility to audiogenic seizures is a robust phenomenon in Fmr1 KO mice and is one of the most widely used outcome measures in preclinical drug discovery studies. The auditory brainstem expresses high FMRP levels (Wang et al., 2014), and abnormal sensory processing at the level of the auditory brainstem may underlie the enhanced susceptibility to audiogenic seizures. FMRP interactions with sodium‐activated potassium channel Slack in the auditory brainstem and its ability to regulate Slack activity may also explain increased excitability in the auditory brainstem of Fmr1 KO mice (Brown et al., 2010). In addition, Fmr1 KO mice show enhanced acoustic startle responses (Chen & Toth, 2001; Nielsen et al., 2002; Frankland et al., 2004; Yun et al., 2006). Abnormal habituation of acoustic startle responses, which is accompanied with hypersensitivity or hyposensitivity to sensory stimuli, was also shown to be dependent on BK channel functions (Zaman et al., 2017). BK channels can directly interact with FMRP, and their functions are affected by the loss of FMRP (Deng et al., 2013), whereas the upregulation of BK channel activity in a mouse model of FXS was shown to normalize the enhanced glutamate release and excessive epileptiform activity (Deng & Klyachko, 2016). However, the mechanisms by which the absence of FMRP in the specific brain areas, such as brainstem or cortex, leads to the enhanced excitability need to be further studied to better understand the epileptic phenotype of FXS.
In vivo recordings from the auditory cortex show that the abnormal cortical processing may underlie auditory hypersensitivity in Fmr1 KO mouse (Rotschafer & Razak, 2013). First, single-unit recordings show that cortical neurons respond to tones with more action potentials in Fmr1 KO mice than WT neurons in both adults (Rotschafer & Razak, 2013) and P21 mice (Wen et al., 2017). The increased responses are due to prolonged firing of action potentials well after stimulus offset. Second, there is also increased variability of spike timing, broader frequency receptive fields, and reduced spectrotemporal selectivity in the Fmr1 KO cortex. The broader receptive fields mean that more neurons will be activated synchronously for any given sound in the KO cortex. Third, recordings from KO mice cortex to repeated sound presentation shows reduced habituation of response amplitudes. Together these findings suggest that hypersensitivity arises due to a triple hit—increased response per neuron, more number of responsive neurons, and reduced habituation of responses.
Remarkably similar EEG phenotypes are also present in Fmr1 KO mice and humans with FXS (Sinclair et al., 2017a, 2017b; Lovelace et al., 2018). Lovelace et al. recorded EEG signals from both auditory and frontal cortex of awake, freely moving mice and compared the WT and Fmr1 KO genotypes. They identified increased gamma power in baseline EEG, reduced evoked phase synchronization to auditory stimuli in the gamma band, and larger ERP N1 component amplitudes in the KO mice (Lovelace et al., 2018). These data are essentially identical to findings in humans with FXS (Ethridge et al., 2017; Wang et al., 2017). Together these data support the notion that there is a milieu of noisy resting state in the auditory cortex in FXS in addition to the triple hit mentioned earlier giving rise to auditory hypersensitivity.
Most studies on humans with FXS have focused on older children or adolescents. However, abnormalities in auditory processing may arise from altered critical period plasticity during development. In the auditory cortex, Fmr1 KO mice show abnormal critical period plasticity in response to developmental tone exposure (Kim et al., 2013), which effectively reduces activity- or experience-evoked responses of neuronal networks. The impaired sound exposure-induced cortical map plasticity in the Fmr1 KO mice may extend into adulthood affecting stability of auditory circuits and may underlie the abnormalities found in the adult auditory cortical responses (Rotschafer & Razak, 2013). We have proposed a specific mechanism for development of auditory hypersensitivity in the Fmr1 KO mice (Wen et al., 2017). Impaired development of parvalbumin (PV)-expressing inhibitory interneurons may underlie abnormal auditory processing in Fmr1 KO mice via MMP-9-dependent regulation of perineuronal nets (Wen et al., 2017). In normal brain, the development of PV interneurons is implicated in shaping critical period plasticity, stabilization of synaptic networks, and network synchronization (Hensch, 2005; Jeevakumar & Kroener, 2016), whereas perineuronal net loss around PV cells is associated with abnormal critical period plasticity and reduced excitability of PV cells (Pizzorusso et al., 2002; Balmer, 2016; Lensjø et al., 2017). The formation of perineuronal nets, which consists of extracellular matrix proteins, coincides with the closure of critical period plasticity window creating a nonpermissive environment for new synapse growth and structural plasticity. A disruption of extracellular matrix affects the stability of existing circuits and opens critical period plasticity window, which may underlie auditory hyperexcitability in FXS (Happel & Frischknecht, 2016). Studies have reported higher MMP-9 activity in Fmr1 KO mouse brains and humans with FXS, suggesting that MMP-9 dysregulation may contribute to FXS-associated deficits (Bilousova et al., 2009; Gkogkas et al., 2014; Sidhu et al., 2014). The increased MMP-9 activity may delay the maturation of cortical circuits and extend critical period plasticity past the normal developmental window affecting the maturation of functional circuits.
The role of MMP-9 upregulation in FXS symptoms is supported by the fact that the genetic reduction of MMP-9 activity in the brain of Fmr1 KO mice restored auditory responses and the formation of perineuronal nets around PV cells in the Fmr1 KO mice to WT levels (Wen et al., 2017). MMP-9 deletion in the Fmr1 KO mice also reversed ERP N1 amplitude habituation deficits (Lovelace et al., 2016). As genetic deletion of MMP-9 can also reverse FXS-associated behaviors in Fmr1 KO mice (Bilousova et al., 2009; Sidhu et al., 2014), MMP-9 is an attractive therapeutic target to reduce sensory deficits in FXS and potentially other FXS-associated behaviors. Indeed, minocycline, which beside its antibiotic effects inhibits MMP-9, has emerged as a potential treatment for FXS (Bilousova et al., 2009; Paribello et al., 2010; Dansie et al., 2013; Dziembowska et al., 2013; Leigh et al., 2013; Schneider et al., 2013; Rotschafer & Razak, 2014; Yau et al., 2018). In humans with FXS, minocycline can reduce MMP-9 levels, reverse auditory ERP deficits, and improve FXS-associated behaviors (Paribello et al., 2010; Dziembowska et al., 2013; Leigh et al., 2013; Schneider et al., 2013). However, several adverse effects of minocycline, such as stained teeth, skin pigmentation, gastrointestinal disturbance, drug-induced lupus, and autoimmune hepatitis, are associated with its antibiotic properties, limiting its chronic use in humans (Edition, 1994; Akin et al., 1998; Eisen & Hakim, 1998; Teitelbaum et al., 1998; Tournigand et al., 1999; Schlienger et al., 2000; Lawson et al., 2001; Ang et al., 2002; Shepherd, 2002; Shetty, 2002; Abe et al., 2003; Porter & Harrison, 2003; Cascio et al., 2004; Sánchez et al., 2004; LaPorta et al., 2005; Smith & Leyden, 2005). Therefore, there is an unmet need in developing novel, potent, and selective MMP-9 inhibitors to treat auditory hypersensitivity associated with FXS and potentially other neurodevelopmental disorders associated with sensory hypersensitivity, such as autism.
Taken together, studies of auditory processing and sensitivity in humans with FXS and Fmr1 KO mice show remarkable overlap in phenotypes, providing a translation relevant framework for both mechanism and drug discovery. It must be noted that FMRP is expressed in multiple nuclei of the auditory system (Zorio et al., 2017), and cortical processing deficits may be intrinsic to cortical changes or inherited from subcortical sites (Strumbos et al., 2010; Rotschafer et al., 2015; Garcia-Pino et al., 2017; Rotschafer & Cramer, 2017). How multiple regions of the auditory system contribute to symptoms that range from hypersensitivity to language and communication deficits is not understood and is an important direction for future studies. The availability of mouse models in which the protein can be removed from specific neuron types, regions, and time points will aid such future studies.
Visual-Motor Deficits
A prominent feature of the FXS neurobehavioral phenotype is diminished performance on neuropsychological tasks that assess visual‐motor function. Visuomotor dysfunction have been described for tasks that require drawing skills (Crowe & Hay, 1990; Freund & Reiss, 1991), tasks that involve manipulation of blocks to construct abstract designs (Crowe & Hay, 1990; Cornish et al., 1999), and tasks requiring psychomotor coordination (Cornish et al., 1999). Although these tasks are multifactorial in nature and the performance affected by many causes, visual‐motor ability is a common feature. This led to the hypothesis that the visual‐motor deficiencies observed in FXS may reflect underlying neuroanatomical and functional abnormalities specific to the thalamic component of one of the two main parallel visual pathways called the magnocellular pathway (Kogan et al., 2004b). Dysfunctions of the pathway may lead to impaired visually guided actions requiring the manipulation of objects, further explaining why individuals with FXS perform poorly on a variety of neuropsychological tasks that have a visual‐motor component.
Additional behavioral studies in infants and toddlers with FXS have documented impairments in processing texture-defined motion stimuli (Farzin et al., 2008), temporal flicker (Farzin et al., 2011), perceiving the ordinality of sequences of numerical displays (Owen et al., 2013), and the ability to maintain the identity of dynamic object information during occlusion (Farzin & Rivera, 2010). Impaired performance has also been demonstrated on tasks requiring inhibitory control (Scerif et al., 2007) as well as numerical reasoning (Rivera et al., 2002; Murphy et al., 2006). One possible reason behind the visual-spatial and numerical deficits seen in FXS is disruption of the so-called dorsal stream (occipitoparietal visual pathway, projecting to the posterior-lateral parietal cortex, which processes information involved in guiding actions, including spatial location and motion) with relative sparing of the ventral stream (occipitotemporal visual pathway, projecting to the inferior temporal cortex, which processes object features such as form and color; Ungerleider, 1982; Milner & Goodale, 2006). Because of its relatively prolonged time course of development (Atkinson, 2002), the dorsal stream is thought to be particularly vulnerable to atypical development in a number of disorders, including FXS (Kogan et al., 2004a; Farzin & Rivera, 2010).
Vision integration is affected in humans with FXS with alteration of spatiotemporal visual processing, reduction of contrast sensitivity for visual stimuli presented at high temporal frequencies, and visual sensitivity for both static and moving images (Kogan et al., 2004b; Farzin et al., 2011). These deficits may be associated with a delayed development in the primary visual cortex as seen in the model of FXS premutation (Berman et al., 2012). However, before being integrated at the cortex level, the visual signals are detected, processed, and transmitted by the retina. Fmr1 deficiency has been shown to affect retinal function, with abnormal wiring of neuronal connections and synaptic destabilization in the retina leading to similar cellular and functional phenotypes as seen in the brain (Rossignol et al., 2014). Since animal behaviors rely on sensory processing (which allows mice to integrate environmental stimuli and to adapt their action); this makes one wonder how far retinal defects, as opposed to cortical processing defects, are involved in the recorded behavioral impairments seen in Fmr1 KO mice, such as visuospatial deficits, diminished performance on neuropsychological tasks that assess visuomotor function, and impairments in processing texture-defined motion stimuli.
Enhanced mGluR5 signaling may contribute to sensory impairments seen in Fmr1 KO mice as mGluR5 signaling is downregulated during normal maturation and synaptic stabilization in the postnatal brain (Dudek & Bear, 1989). Indeed, using genetic approach, Dölen et al. (2007) have shown the importance of mGluR5, as well as FMRP, in the regulation of ocular dominance plasticity during the development of visual cortex. A 50% reduction in mGluR5 expression prevents ocular dominance plasticity induced by a 3-day monocular deprivation, suggesting that this receptor normally serves to enable plasticity in the visual cortex. In contrast, in the absence of FMRP, Fmr1 KO mice show altered ocular dominance plasticity (Dölen et al., 2007). The response to monocular deprivation is characterized by both deprived-eye response depression and open-eye response potentiation, suggesting that FMRP normally serves to restrict plasticity in the visual cortex (Dölen & Bear, 2008). Interestingly, since ocular dominance plasticity is protein synthesis dependent (Taha & Stryker, 2002), it is a possibility that excessive protein synthesis is responsible for altered plasticity in the visual cortex of Fmr1 KO mice.
Last, by examining the visual cortices in Fmr1 KO mice as well as those in the individuals with FXS, multiple studies have shown that FMRP is critical to the pruning and maturation of dendritic spines (Greenough et al., 2001; Irwin et al., 2001; Churchill et al., 2002). Neurons lacking FMRP retain characteristically immature dendritic spines within the visual cortex (Kogan et al., 2004b). Furthermore, the density of immature spines is elevated in FXS humans compared with normal control brains. Interestingly, this immature spine phenotype is also induced by the activation of Gp1 mGluRs in the visual cortical pyramidal neurons (Vanderklish & Edelman, 2002). Spine density is significantly increased in Fmr1 KO mice, and the phenotype can be rescued by 50% reduction in mGluR5 expression (Dölen et al., 2007). This indicates that the absence of FMRP and the upregulation of mGluR5 may therefore lead to abnormal development of visual circuits and potentially impaired processing of visual stimuli.
Somatosensory Processing Deficits and Tactile Defensiveness
Impaired processing of tactile information is seen in individuals with FXS, with hypersensitivity to touch being common (Cascio, 2010). The Fmr1 KO mouse model has phenotypes similar to those observed in humans with FXS (van den Ouweland et al., 1994). In mice, tactile information received through deflections of whiskers is processed in the somatosensory barrel cortex (Diamond et al., 2008; Diamond & Arabzadeh, 2013; Feldmeyer et al., 2013). Correct processing of whisker-mediated touch information requires the formation of receptive fields in the somatosensory cortex (Simons, 1978; Simons & Carvell, 1989). Development of intracortical connections plays a key role in the formation of the receptive fields and depends on sensory experience (Allen et al., 2003; Bender et al., 2006). Exposure of juvenile animals to patterned sensory input refined the balance of excitation and inhibition (Dorrn et al., 2010; Sun et al., 2010), resulting in receptive field and sensory map reorganization and a long-lasting impact on sound perception (Han et al., 2007). Therefore, the enlarged receptive fields in Fmr1 KO mice may be a consequence of altered sensory integration during the early postnatal development.
In vivo recordings from barrel cortex revealed that Fmr1 KO mice show an enlargement in the cortical area activated by whisker deflections, that is, an expansion of the somatosensory maps in L2/3. Furthermore, the encoding of tactile stimuli at different frequencies was severely impaired in Layer 2/3 as well (Juczewski et al., 2016). These findings highlight neuronal mechanisms that could contribute to the different exploratory behavior such as tactile defensiveness or tactile sensitivity (Reiss & Freund, 1990; Baranek et al., 1997; Miller et al., 1999; Baranek et al., 2008), which is observed in Fmr1 KO mice (Arnett et al., 2014; Santos et al., 2014). Furthermore, a decrease in the whisker selectivity index is evident in Fmr1 KO mice over a range of stimulation parameters, indicating that the specificity with which deflection of a given whisker activates cortex has decreased (Juczewski et al., 2016).
Moreover, there are profound alterations in the neuronal excitability in Layer 4 of somatosensory barrel cortex in the Fmr1 KO mouse at the synaptic, cellular, and network levels. Gibson et al.’s work on Fmr1 KO mice somatosensory cortex has shown that there is a decrease in connectivity frequency and strength resulting in an approximate 50% reduction in excitatory drive onto fast-spiking (FS) inhibitory interneurons. In addition, excitatory neurons become intrinsically more excitable in the KO mice. These changes can lead to hyperexcitable circuits, a hypothesis that was supported by observed increase in UP state duration in somatosensory cortex of Fmr1 KO mice (Gibson et al., 2008). Consistent with impaired FS inhibitory circuitry, network synchrony within a single cortical column during the UP state is decreased as well (Gibson et al., 2008). Similar to our findings in the auditory cortex (Wen et al., 2017), there is a significant reduction in PV immunoreactivity in the somatosensory cortex of adult Fmr1 KO mice (Selby et al., 2007). PV interneurons receive both intracortical and thalamic excitatory inputs, which develop during the cortical critical period (Daw et al., 2007a; Chittajallu & Isaac, 2010). A recent study has shown that there is a significant delay in the formation of excitatory contacts onto FS interneurons, which likely has a large impact on the integration of feedforward inhibitory circuits in the developing somatosensory cortex of Fmr1 KO mice (Nomura et al., 2017).
Gonçalves et al. showed that Fmr1 KO mice exhibit abnormally high synchrony of neocortical network activity in mouse somatosensory cortex, especially during development. Neuronal firing rates are significantly higher in Fmr1 KO mice compared with WT mice during whole-cell recordings manifesting UP/Down states (slow-wave sleep, quiet wakefulness), probably due to the higher firing probability during UP states. Combined electroencephalography and calcium imaging experiments confirmed that neurons in KO mice have abnormally high firing and synchrony during sleep, leading to the conclusion that cortical networks in FXS are hyperexcitable in a brain state-dependent manner during a critical period for experience-dependent plasticity (Gonçalves et al., 2013). Several studies have also shown both molecular and functional disruption in GABA signaling in FXS (El Idrissi et al., 2005; D'Hulst et al., 2006; Gantois et al., 2006; Paluszkiewicz et al., 2011). The timing of the switch from depolarizing to hyperpolarizing GABA is delayed in the somatosensory cortex of Fmr1 KO mice, and there is a concurrent alteration in the expression of the neuronal chloride cotransporter NKCC1 that promotes the accumulation of intracellular chloride (He et al., 2014). While the actual mechanisms that control the developmental expression of the NKCC1 are not known, it is significant that NKCC1 is predominantly found in astrocytes. With the discovery of FMRP in astrocytes (Pacey & Doering, 2007), coupled with a role of astrocytes in synaptic function and glutamate metabolism (Ullian et al., 2004; Ethell & Pasquale, 2005; Paixão & Klein, 2010), it is possible that astrocytes contribute, in some capacity, to the abnormal dendritic spine and synapse development, as well as circuit hyperexcitability seen in FXS (Jacobs & Doering, 2010; Jacobs et al., 2010; Higashimori et al., 2013; Higashimori et al., 2016). Abnormal trophic effects of GABA during cortical development may also disrupt the normal trophic function of GABA and contribute to the delayed maturation of glutamatergic synapses in FXS.
Cellular deficits have also been observed in the somatosensory cortex of Fmr1 KO mice. As in the auditory and visual cortices, an abundance of abnormally long, thin dendritic spines have been reported in pyramidal neurons during early development in the somatosensory cortex (Nimchinsky et al., 2001; Galvez & Greenough, 2005), and abnormal developmental pruning of the Layer 4 spiny stellate cell dendrites has been described in Fmr1 KO mice (Galvez et al., 2003). Despite the clear alterations in cellular morphology in Fmr1 KO mice, it is not known whether the anatomical deficits have an impact on the functional development of excitatory glutamatergic synapses in somatosensory cortex. During perinatal development in rodents, activity-dependent refinement of excitatory thalamocortical synapses in the somatosensory cortex leads to a stereotypical maturation of glutamatergic signaling (Crair & Malenka, 1995; Barth & Malenka, 2001). Thalamocortical synapses exhibit LTP and LTD throughout the critical plasticity period in Layer 4 (Crair & Malenka, 1995; Feldman et al., 1998). Activity-dependent maturation of excitatory thalamocortical synapses during the critical period results in rapid changes in the synaptic composition of glutamate receptors (Crair & Malenka, 1995; Kidd & Isaac, 1999; Daw et al., 2007b). The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor contribution increases relative to the N-methyl-D-aspartate (NMDA) receptors (Crair & Malenka, 1995), and the proportion of NMDA-only silent synapses is reduced (Isaac et al., 1997).
Cortical glutamate receptors have been implicated in the development of the barrel cortex maps and the refinement of cortical sensory circuits that underlie sensory processing (Schlaggar et al., 1993). Harlow et al. (2010) showed that early postnatal development of excitatory connections from the thalamus to Layer 4 spiny stellate neurons is critically disrupted during a critical plasticity period in Fmr1 KO mice. Moreover, the progressive development of excitatory ionotropic glutamate receptor signaling, which normally occurs over the first postnatal week, is delayed as well (Harlow et al., 2010; Till et al., 2012). There is also an altered NMDAR-to-AMPAR ratio observed in the somatosensory cortex of Fmr1 KO mice manifesting as an increase in the fraction of silent synapses (NMDA-only thalamocortical synapses) at the closure of the critical plasticity period (Harlow et al., 2010). Synaptic plasticity and experience-dependent refinement of sensory circuits are inextricably linked, and NMDA receptors play a central role in both processes. Therefore, alteration in NMDA receptor signaling and the developmental maturation of silent synapses during the critical plasticity period will most likely affect the development of cortical circuits.
Just like in the auditory and visual cortices, FMRP is required for the normal developmental progression of synaptic maturation in the somatosensory cortex. Loss of FMRP impacts the development of cortical synapses and results in dysregulation of glutamatergic maturation in the somatosensory cortex during the early postnatal critical plasticity period. Moreover, increased proportion of silent synapses persists into late postnatal development, which coincides with a temporal delay in the window for synaptic plasticity (Harlow et al., 2010; Till et al., 2012).
Conclusions and Future Studies
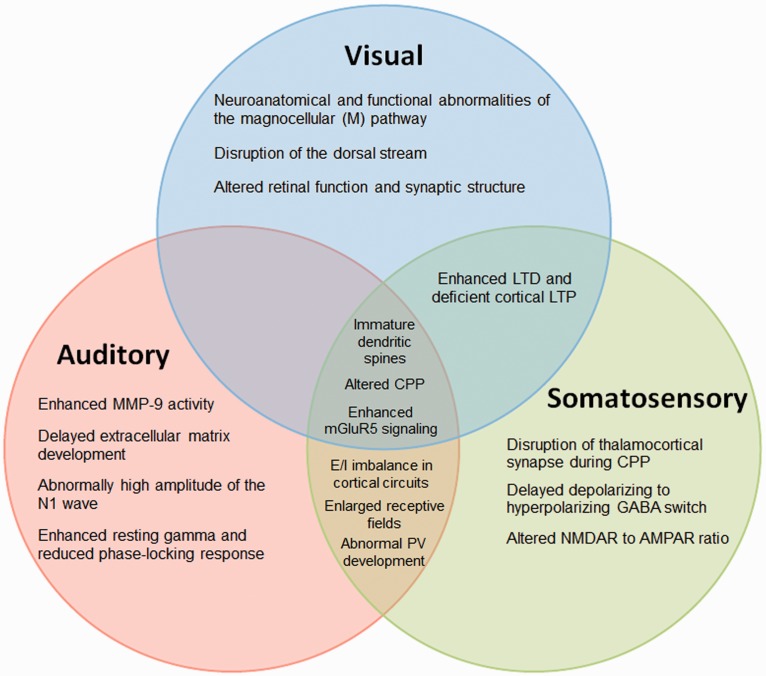
Hyperarousal and anxiety in humans with FXS may be linked to strong reactions to sensory stimuli. There is an abundance of evidence describing sensory cortical dysfunctions in the Fmr1 KO mice and in humans with FXS (Table 1). The common underlying phenotype is sensory hypersensitivity, including hypersensitivity to visual, auditory, or tactile stimuli that may lead to behavioral alterations such as poor eye contact, avoidance of noisy places, anxiety, and impaired social reciprocity. These alterations in sensory processing appear to be a universal problem in individuals with FXS, as they cause impairment in processing and encoding of many types of sensory information, which may affect more complex social behaviors. Moreover, sensory processing disorders could occur because of dysfunction at multiple levels of each sensory system. The Fmr1 KO mice also display deficiencies in sensory processing that may help to understand the mechanism of sensory hypersensitivity in FXS (Figure 2). Mechanisms underlying the sensory hypersensitivity may be relatively more tractable compared with more complex social behaviors typically studied in FXS. Therefore, it is of critical importance to use sensory hypersensitivity as a robust, reliable, and translatable phenotype to integrate preclinical and clinical investigations at multiple levels of analysis to facilitate drug discovery in FXS.
Table 1.
Impaired Sensory Mechanisms in Fragile X Syndrome.
| Mechanisms | Auditory | Somatosensory | Visual | Other systems |
|---|---|---|---|---|
| Immature dendritic spine morphology (Comery et al., 1997; Irwin et al., 2001; Nimchinsky et al., 2001; Kogan et al., 2004b; Galvez & Greenough, 2005; Berman et al., 2012; Till et al., 2015) | m | m | ha, m | ha, r |
| Altered critical period plasticity (Dölen et al., 2007; Gonçalves et al., 2013; Kim et al., 2013) | m | m | m | |
| Enhanced mGluR5 signaling (Bear et al., 2004; Dölen et al., 2007; Dölen & Bear, 2008; Hays et al., 2011; Kim et al., 2013) | m | m | m | m |
| Abnormal E/I balance in cortical circuits (Penagarikano et al., 2007; Gibson et al., 2008; Braat & Kooy, 2015; Berzhanskaya et al., 2017) | m | m | r | |
| Abnormal PV cell development (Selby et al., 2007; Nomura et al., 2017; Wen et al., 2017) | m | m | ||
| Enlarged receptive fields and prolong responses (Rotschafer & Razak, 2013; Juczewski et al., 2016) | m | m | ||
| Enhanced long-term depression and deficient cortical long-term potentiation (Li et al., 2002; Zhao et al., 2005; Wilson & Cox, 2007; Ng et al., 2013; Jackson, 2017) | m | m | r, z | |
| Enhanced MMP-9 activity (Bilousova et al., 2009; Gkogkas et al., 2014; Sidhu et al., 2014) | m | ha | ||
| Delayed extracellular matrix and perineuronal net development (Happel & Frischknecht, 2016; Wen et al., 2017) | m | |||
| Abnormally high amplitude of the N1 wave and reduced habituation (Castrén et al., 2003; Schneider et al., 2013; Ethridge et al., 2016) | h, m | |||
| Enhanced resting gamma and reduced phase-locking response (Ethridge et al., 2017; Wang et al., 2017) | h | |||
| Impaired cortical representation of speech sounds (Engineer et al., 2014) | r | |||
| Disruption of thalamocortical synapse during critical period plasticity (Daw et al., 2007b; Harlow et al., 2010) | m | |||
| Delayed depolarizing to hyperpolarizing GABA switch (He et al., 2014) | m | |||
| Altered NMDAR to AMPAR ratio (Harlow et al., 2010) | m | |||
| Abnormal magnocellular pathway (Kogan et al., 2004b) | h | |||
| Disruption of the dorsal stream (Kogan et al., 2004a; Farzin & Rivera, 2010) | h | |||
| Altered retinal function and synaptic structure (Rossignol et al., 2014) | m | |||
| Defects in synaptic transmission in the optic lobe (Zhang et al., 2001) | d | |||
| Impaired long-term olfactory habituation (Sudhakaran et al., 2014) | d | |||
| Reduced specificity in odor coding and alterations (Franco et al., 2017) | d | |||
| Defects at neuromuscular junctions and mushroom bodies (McBride et al., 1999; Zars et al., 2000; Pascual & Préat, 2001; Zhang et al., 2001) | d | |||
| Altered circadian rhythm behaviors (Dockendorff et al., 2002; Inoue et al., 2002; Morales et al., 2002; Zhang et al., 2008) | m, d |
Note. PV = parvalbumin; MMP-9 = matrix metalloproteinase-9; GABA = gamma-aminobutyric acid; NMDAR = N-methyl-D-aspartate receptor; AMPAR = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; h = human; m = mouse; r = rat; d = Drosophila; z = zebrafish.
aPostmortem tissue.
Figure 2.
Auditory, visual, and somatosensory processing phenotypes observed in FXS.
MMP-9 = matrix metalloproteinase-9; LTD = long-term depression; LTP = long-term potentiation; CPP = critical period plasticity; mGluR5 = metabotropic glutamate receptor 5; E/I = excitatory/inhibitory; PV = parvalbumin interneurons; GABA = gamma-aminobutyric acid; NMDAR = N-methyl-D-aspartate receptor; AMPAR = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor.
Indeed, studies of mouse sensory hypersensitivity neurobehavioral phenotypes have led to a better understanding of circuit-level pathophysiology in FXS. The heightened sensory activity seen in humans with FXS may stem from a concurrence of dysfunctional intrinsic excitability or impaired inhibition due to a loss or abnormal development of inhibitory neurons, abnormal dendrite morphology, or reduced GABAergic activity. The molecular and cellular mechanisms of circuit hyperexcitability are beginning to be understood. Fmr1 KO mouse somatosensory and auditory cortex show weakened inhibitory interneuron activity and more excitable pyramidal neurons that may underlie changes in sensory and high-order cognitive behaviors seen in Fmr1 KO mice (Figure 2). Disrupted cytoarchitecture of sensory circuits in Fmr1 KO mice during early development may impair the ability of mice to integrate sensory experiences leading to abnormal sensory circuit development, learning, and high-order cognitive skills that persist into adulthood.
FXS is a neurodevelopmental disorder, but the mechanisms of impaired development of functional neural response selectivity to sensory stimuli are still unclear. The predominant focus of published work in the FXS field has been on characterizing the changes in dendritic spine properties and synaptic or intrinsic properties of neurons. However, the consequences of these synaptic changes to development of behaviorally relevant neural response properties in FXS are not known. Therefore, it is not clear if the observed sensory hypersensitivity in humans with FXS is due to an altered regulation of developmental processes during critical plasticity period that persists into adulthood. The majority of studies using Fmr1 KO mice focus on the neuronal responses and behaviors during a specific developmental window or in adult mice while neglecting to look at any long-term changes in Fmr1 KO mice from early development into adulthood and the long-term impact of early treatment to reverse FXS-associated behavioral deficits. Is the loss of FMRP only detrimental during a critical plasticity period or are the changes attributed to the ongoing absence of FMRP? A recent finding that eliminating FMRP in only the prefrontal cortex of adult mice can lead to abnormal learning suggests FMRP continues to plays a role in neural function even after critical plasticity period ends (Siegel et al., 2017). In addition, some phenotypes are reversed in the adult animal models by acute pharmacological treatments. However, it is not clear whether the acute effects are long-lasting. Moreover, chronic treatments may result in drug tolerance. Further studies are needed to determine developmental versus adult effects of FMRP loss on cortical responses to identify specific time windows, which can be targeted therapeutically.
Questions on whether the animal models are appropriate to study human neurological disorders have arisen due to the inability to translate preclinical therapeutic success to the clinic (Dahlhaus, 2018). Indeed it is important to compare multiple model systems for any neurological disorder. Regardless of the animal model studied (even in nonhuman primates), the manifestation of cognitive and social symptoms will depend on underlying circuits that are quite different across species. The development of these circuits is also difficult to probe. Sensory processing circuits and mechanisms are more likely to show relatively more similarities. This is seen in FXS studies that show very similar baseline and sound-evoked EEG phenotypes in mice and humans (Schneider et al., 2013; Lovelace et al., 2016; Ethridge et al., 2017; Sinclair et al., 2017b; Wang et al., 2017). In addition, few studies of FXS (in humans and mice) have quantified developmental trajectories and roles of FMRP (Table 1). Again, when additional model systems are studied, it is imperative to analyze changes in circuit function over development. Therefore, we argue that development of sensory hypersensitivity may be used as a neurobehavioral probe to more successfully evaluate and translate drug treatments from preclinical models to humans, as well as underlying mechanisms of FXS-associated deficits.
Acknowledgments
The authors thank members of Drs. Ethell, Razak and Binder laboratories for helpful discussions and comments.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research in authors’ laboratories is supported by grants from the National Institutes of Mental Health (1U54 HD082008-01) and the U.S. Army Medical Research (W81XWH-15-1-0436).
References
- Abe M., Furukawa S., Takayama S., Michitaka K., Minami H., Yamamoto K., Horiike N., Onji M. (2003). Drug-induced hepatitis with autoimmune features during minocycline therapy. Intern Med, 42, 48–52. [DOI] [PubMed] [Google Scholar]
- Adusei D. C., Pacey L. K., Chen D., Hampson D. R. (2010). Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology, 59, 167–171. [DOI] [PubMed] [Google Scholar]
- Akin E., Miller L. C., Tucker L. B. (1998). Minocycline-induced lupus in adolescents. Pediatrics, 101, 926. [DOI] [PubMed] [Google Scholar]
- Allen C. B., Celikel T., Feldman D. E. (2003). Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci, 6, 291–299. [DOI] [PubMed] [Google Scholar]
- Ang E. R., Zimmerman J. C., Malkin E. (2002). Pseudotumor cerebri secondary to minocycline intake. J Am Board Fam Pract, 15, 229–233. [PubMed] [Google Scholar]
- Antion M. D., Hou L., Wong H., Hoeffer C. A., Klann E. (2008). mGluR-dependent long-term depression is associated with increased phosphorylation of S6 and synthesis of elongation factor 1A but remains expressed in S6K-deficient mice. Mol Cell Biol, 28, 2996–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett M. T., Herman D. H., McGee A. W. (2014). Deficits in tactile learning in a mouse model of fragile X syndrome. PLoS One, 9, e109116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. T., Sutcliffe J. S., Kunst C. B., Leiner H. A., Eichler E. E., Nelson D. L., Warren S. T. (1993. a). Human and murine FMR-1: Alternative splicing and translational initiation downstream of the CGG-repeat. Nat Genet, 4, 244–251. [DOI] [PubMed] [Google Scholar]
- Ashley C. T., Wilkinson K. D., Reines D., Warren S. T. (1993. b). FMR1 protein: Conserved RNP family domains and selective RNA binding. Science, 262, 563–566. [DOI] [PubMed] [Google Scholar]
- Atkinson J. (2002). The developing visual brain. London: Oxford University Press.
- Bagni C., Greenough W. T. (2005). From mRNP trafficking to spine dysmorphogenesis: The roots of fragile X syndrome. Nat Rev Neurosci, 6, 376–387. [DOI] [PubMed] [Google Scholar]
- Bakker C. E., Verheij C., Willemsen R., Helm, R., Oerlemans, F., Vermey, M., ...Willems, P. J. (1994). Fmr1 knockout mice: A model to study fragile X mental retardation. Cell, 78, 23–33. [PubMed] [Google Scholar]
- Balmer T. S. (2016). Perineuronal nets enhance the excitability of fast-spiking neurons. eNeuro, 3, 1-13. [DOI] [PMC free article] [PubMed]
- Banko J. L., Hou L., Poulin F., Sonenberg N., Klann E. (2006). Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci, 26, 2167–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek G. T., Foster L. G., Berkson G. (1997). Tactile defensiveness and stereotyped behaviors. Am J Occup Ther, 51, 91–95. [DOI] [PubMed] [Google Scholar]
- Baranek G. T., Roberts J. E., David F. J., Sideris J., Mirrett P. L., Hatton D. D., Bailey D. B. (2008). Developmental trajectories and correlates of sensory processing in young boys with fragile X syndrome. Phys Occup Ther Pediatr, 28, 79–98. [DOI] [PubMed] [Google Scholar]
- Barnes E., Roberts J., Long S. H., Martin G. E., Berni M. C., Mandulak K. C., Sideris J. (2009). Phonological accuracy and intelligibility in connected speech of boys with fragile X syndrome or Down syndrome. J Speech Lang Hear Res, 52, 1048–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A. L., Malenka R. C. (2001). NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci, 4, 235–236. [DOI] [PubMed] [Google Scholar]
- Bassell G. J., Warren S. T. (2008). Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron, 60, 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear M. F., Huber K. M., Warren S. T. (2004). The mGluR theory of fragile X mental retardation. Trends Neurosci, 27, 370–377. [DOI] [PubMed] [Google Scholar]
- Bender K. J., Allen C. B., Bender V. A., Feldman D. E. (2006). Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J Neurosci, 26, 4155–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R. F., Murray K. D., Arque G., Hunsaker M. R., Wenzel H. J. (2012). Abnormal dendrite and spine morphology in primary visual cortex in the CGG knock-in mouse model of the fragile X premutation. Epilepsia, 53(Suppl 1), 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzhanskaya J., Phillips M. A., Gorin A., Lai C., Shen J., Colonnese M. T. (2017). Disrupted cortical state regulation in a rat model of fragile X syndrome. Cereb Cortex, 27, 1386–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilousova T. V., Dansie L., Ngo M., Aye J., Charles J. R., Ethell D. W., Ethell I. M. (2009). Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet, 46, 94–102. [DOI] [PubMed] [Google Scholar]
- Bolduc F. V., Bell K., Cox H., Broadie K. S., Tully T. (2008). Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat Neurosci, 11, 1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braat S., Kooy R. F. (2015). The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron, 86, 1119–1130. [DOI] [PubMed] [Google Scholar]
- Brennan F. X., Albeck D. S., Paylor R. (2006). Fmr1 knockout mice are impaired in a leverpress escape/avoidance task. Genes Brain Behav, 5, 467–471. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Kronengold J., Gazula V. R., Chen Y., Strumbos J. G., Sigworth F. J., Navaratnam D., Kaczmarek L. K. (2010). Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci, 13, 819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V., Jin P., Ceman S., Darnell J. C., O'Donnell W. T., Tenenbaum S. A., Jin X., Feng Y., Wilkinson K. D., Keene J. D., Darnell R. B., Warren S. T. (2001). Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell, 107, 477–487. [DOI] [PubMed] [Google Scholar]
- Cascio A., Di Liberto C., D'Angelo M., Iaria C., Scarlata F., Titone L., Campisi G. (2004). No findings of dental defects in children treated with minocycline. Antimicrob Agents Chemother, 48, 2739–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C. J. (2010). Somatosensory processing in neurodevelopmental disorders. J Neurodev Disord, 2, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén M., Pääkkönen A., Tarkka I. M., Ryynänen M., Partanen J. (2003). Augmentation of auditory N1 in children with fragile X syndrome. Brain Topogr, 15, 165–171. [DOI] [PubMed] [Google Scholar]
- Cheever A., Ceman S. (2009). Phosphorylation of FMRP inhibits association with Dicer. RNA, 15, 362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Toth M. (2001). Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience, 103, 1043–1050. [DOI] [PubMed] [Google Scholar]
- Chittajallu R., Isaac J. T. (2010). Emergence of cortical inhibition by coordinated sensory-driven plasticity at distinct synaptic loci. Nat Neurosci, 13, 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill J. D., Grossman A. W., Irwin S. A., Galvez R., Klintsova A. Y., Weiler I. J., Greenough W. T. (2002). A converging-methods approach to fragile X syndrome. Dev Psychobiol, 40, 323–338. [DOI] [PubMed] [Google Scholar]
- Comery T. A., Harris J. B., Willems P. J., Oostra B. A., Irwin S. A., Weiler I. J., Greenough W. T. (1997). Abnormal dendritic spines in fragile X knockout mice: Maturation and pruning deficits. Proc Natl Acad Sci U S A, 94, 5401–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K. M., Munir F., Cross G. (1999). Spatial cognition in males with Fragile-X syndrome: Evidence for a neuropsychological phenotype. Cortex, 35, 263–271. [DOI] [PubMed] [Google Scholar]
- Crair M. C., Malenka R. C. (1995). A critical period for long-term potentiation at thalamocortical synapses. Nature, 375, 325–328. [DOI] [PubMed] [Google Scholar]
- Crawford D. C., Acuña J. M., Sherman S. L. (2001). FMR1 and the fragile X syndrome: Human genome epidemiology review. Genet Med, 3, 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe S. F., Hay D. A. (1990). Neuropsychological dimensions of the fragile X syndrome: Support for a non-dominant hemisphere dysfunction hypothesis. Neuropsychologia, 28, 9–16. [DOI] [PubMed] [Google Scholar]
- Curia G., Papouin T., Séguéla P., Avoli M. (2009). Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb Cortex, 19, 1515–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhaus R. (2018). Of men and mice: Modeling the fragile X syndrome. Front Mol Neurosci, 11, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansie L. E., Phommahaxay K., Okusanya A. G., Uwadia J., Huang M., Rotschafer S. E., Razak K. A., Ethell D. W., Ethell I. M. (2013). Long-lasting effects of minocycline on behavior in young but not adult Fragile X mice. Neuroscience, 246, 186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. C., Fraser C. E., Mostovetsky O., Stefani G., Jones T. A., Eddy S. R., Darnell R. B. (2005). Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev, 19, 903–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. C., Klann E. (2013). The translation of translational control by FMRP: Therapeutic targets for FXS. Nat Neurosci, 16, 1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. C., Van Driesche S. J., Zhang C., Hung K. Y., Mele A., Fraser C. E., Stone E. F., Chen C., Fak J. J., Chi S. W., Licatalosi D. D., Richter J. D., Darnell R. B. (2011). FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell, 146, 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw M. I., Ashby M. C., Isaac J. T. (2007. a). Coordinated developmental recruitment of latent fast spiking interneurons in layer IV barrel cortex. Nat Neurosci, 10, 453–461. [DOI] [PubMed] [Google Scholar]
- Daw M. I., Scott H. L., Isaac J. T. (2007. b). Developmental synaptic plasticity at the thalamocortical input to barrel cortex: Mechanisms and roles. Mol Cell Neurosci, 34, 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Broeder M. J., van der Linde H., Brouwer J. R., Oostra B. A., Willemsen R., Ketting R. F. (2009). Generation and characterization of FMR1 knockout zebrafish. PLoS One, 4, e7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P. Y., Klyachko V. A. (2016). Genetic upregulation of BK channel activity normalizes multiple synaptic and circuit defects in a mouse model of fragile X syndrome. J Physiol, 594, 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P. Y., Rotman Z., Blundon J. A., Cho Y., Cui J., Cavalli V., Zakharenko S. S., Klyachko V. A. (2013). FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron, 77, 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N. S., Casimiro T. M., Gruber S. M., Vanderklish P. W. (2006). Early postnatal plasticity in neocortex of Fmr1 knockout mice. J Neurophysiol, 96, 1734–1745. [DOI] [PubMed] [Google Scholar]
- D'Hulst C., De Geest N., Reeve S. P., Van Dam D., De Deyn P. P., Hassan B. A., Kooy R. F. (2006). Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res, 1121, 238–245. [DOI] [PubMed] [Google Scholar]
- D'Hulst C., Heulens I., Brouwer J. R., Willemsen R., De Geest N., Reeve S. P., De Deyn P. P., Hassan B. A., Kooy R. F. (2009). Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS). Brain Res, 1253, 176–183. [DOI] [PubMed] [Google Scholar]
- Diamond M. E., Arabzadeh E. (2013). Whisker sensory system – From receptor to decision. Prog Neurobiol, 103, 28–40. [DOI] [PubMed] [Google Scholar]
- Diamond M. E., von Heimendahl M., Knutsen P. M., Kleinfeld D., Ahissar E. (2008). ‘ Where’ and ‘what’ in the whisker sensorimotor system. Nat Rev Neurosci, 9, 601–612. [DOI] [PubMed] [Google Scholar]
- Dockendorff T. C., Su H. S., McBride S. M., Yang Z., Choi C. H., Siwicki K. K., Sehgal A., Jongens T. A. (2002). Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron, 34, 973–984. [DOI] [PubMed] [Google Scholar]
- Dölen G., Bear M. F. (2008). Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol, 586, 1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G., Osterweil E., Rao B. S., Smith G. B., Auerbach B. D., Chattarji S., Bear M. F. (2007). Correction of fragile X syndrome in mice. Neuron, 56, 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrn A. L., Yuan K., Barker A. J., Schreiner C. E., Froemke R. C. (2010). Developmental sensory experience balances cortical excitation and inhibition. Nature, 465, 932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek S. M., Bear M. F. (1989). A biochemical correlate of the critical period for synaptic modification in the visual cortex. Science, 246, 673–675. [DOI] [PubMed] [Google Scholar]
- Dziembowska M., Pretto D. I., Janusz A., Kaczmarek L., Leigh M. J., Gabriel N., Durbin-Johnson B., Hagerman R. J., Tassone F. (2013). High MMP-9 activity levels in fragile X syndrome are lowered by minocycline. Am J Med Genet A, 161A, 1897–1903. [DOI] [PubMed] [Google Scholar]
- Dziembowska M., Wlodarczyk J. (2012). MMP9: A novel function in synaptic plasticity. Int J Biochem Cell Biol, 44, 709–713. [DOI] [PubMed] [Google Scholar]
- Eadie B. D., Zhang W. N., Boehme F., Gil-Mohapel J., Kainer L., Simpson J. M., Christie B. R. (2009). Fmr1 knockout mice show reduced anxiety and alterations in neurogenesis that are specific to the ventral dentate gyrus. Neurobiol Dis, 36, 361–373. [DOI] [PubMed] [Google Scholar]
- Edbauer D., Neilson J. R., Foster K. A., Wang C. F., Seeburg D. P., Batterton M. N., Tada T., Dolan B. M., Sharp P. A., Sheng M. (2010). Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron, 65, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edition F. (1994). Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association.
- Eisen D., Hakim M. D. (1998). Minocycline-induced pigmentation. Incidence, prevention and management. Drug Saf, 18, 431–440. [DOI] [PubMed] [Google Scholar]
- El Idrissi A., Ding X. H., Scalia J., Trenkner E., Brown W. T., Dobkin C. (2005). Decreased GABA(A) receptor expression in the seizure-prone fragile X mouse. Neurosci Lett, 377, 141–146. [DOI] [PubMed] [Google Scholar]
- Engineer C. T., Centanni T. M., Im K. W., Rahebi K. C., Buell E. P., Kilgard M. P. (2014). Degraded speech sound processing in a rat model of fragile X syndrome. Brain Res, 1564, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Barreto L., Morales M. (2016). The PI3K signaling pathway as a pharmacological target in autism related disorders and schizophrenia. Mol Cell Ther, 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell I. M., Pasquale E. B. (2005). Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol, 75, 161–205. [DOI] [PubMed] [Google Scholar]
- Ethridge L. E., White S. P., Mosconi M. W., Wang J., Byerly M. J., Sweeney J. A. (2016). Reduced habituation of auditory evoked potentials indicate cortical hyper-excitability in Fragile X Syndrome. Transl Psychiatry, 6, e787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge L. E., White S. P., Mosconi M. W., Wang J., Pedapati E. V., Erickson C. A., Byerly M. J., Sweeney J. A. (2017). Neural synchronization deficits linked to cortical hyper-excitability and auditory hypersensitivity in fragile X syndrome. Mol Autism, 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F., Rivera S. M. (2010). Dynamic object representations in infants with and without fragile X syndrome. Front Hum Neurosci, 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F., Rivera S. M., Whitney D. (2011). Resolution of spatial and temporal visual attention in infants with fragile X syndrome. Brain, 134, 3355–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F., Whitney D., Hagerman R. J., Rivera S. M. (2008). Contrast detection in infants with fragile X syndrome. Vision Res, 48, 1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D. E., Nicoll R. A., Malenka R. C., Isaac J. T. (1998). Long-term depression at thalamocortical synapses in developing rat somatosensory cortex. Neuron, 21, 347–357. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D., Brecht M., Helmchen F., Petersen C. C., Poulet J. F., Staiger J. F., Luhmann H. J., Schwarz C. (2013). Barrel cortex function. Prog Neurobiol, 103, 3–27. [DOI] [PubMed] [Google Scholar]
- Ferraguti F., Baldani-Guerra B., Corsi M., Nakanishi S., Corti C. (1999). Activation of the extracellular signal-regulated kinase 2 by metabotropic glutamate receptors. Eur J Neurosci, 11, 2073–2082. [DOI] [PubMed] [Google Scholar]
- Ferron L., Nieto-Rostro M., Cassidy J. S., Dolphin A. C. (2014). Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat Commun, 5, 3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finestack L. H., Richmond E. K., Abbeduto L. (2009). Language development in individuals with fragile X syndrome. Top Lang Disord, 29, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco L. M., Okray Z., Linneweber G. A., Hassan B. A., Yaksi E. (2017). Reduced lateral inhibition impairs olfactory computations and behaviors in a Drosophila model of fragile X syndrome. Curr Biol, 27, 1111–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland P. W., Wang Y., Rosner B., Shimizu T., Balleine B. W., Dykens E. M., Ornitz E. M., Silva A. J. (2004). Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry, 9, 417–425. [DOI] [PubMed] [Google Scholar]
- Freund L. S., Reiss A. L. (1991). Cognitive profiles associated with the fra(X) syndrome in males and females. Am J Med Genet, 38, 542–547. [DOI] [PubMed] [Google Scholar]
- Gallagher S. M., Daly C. A., Bear M. F., Huber K. M. (2004). Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci, 24, 4859–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R., Gopal A. R., Greenough W. T. (2003). Somatosensory cortical barrel dendritic abnormalities in a mouse model of the fragile X mental retardation syndrome. Brain Res, 971, 83–89. [DOI] [PubMed] [Google Scholar]
- Galvez R., Greenough W. T. (2005). Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am J Med Genet A, 135, 155–160. [DOI] [PubMed] [Google Scholar]
- Gantois I., Vandesompele J., Speleman F., Reyniers E., D'Hooge R., Severijnen L. A., Willemsen R., Tassone F., Kooy R. F. (2006). Expression profiling suggests underexpression of the GABA(A) receptor subunit delta in the fragile X knockout mouse model. Neurobiol Dis, 21, 346–357. [DOI] [PubMed] [Google Scholar]
- Garcia-Pino E., Gessele N., Koch U. (2017). Enhanced excitatory connectivity and disturbed sound processing in the auditory brainstem of fragile X mice. J Neurosci, 37, 7403–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. R., Bartley A. F., Hays S. A., Huber K. M. (2008). Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol, 100, 2615–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkogkas C. G., Khoutorsky A., Cao R., Jafarnejad S. M., Prager-Khoutorsky M., Giannakas N., Kaminari A., Fragkouli A., Nader K., Price T. J., Konicek B. W., Graff J. R., Tzinia A. K., Lacaille J. C., Sonenberg N. (2014). Pharmacogenetic inhibition of eIF4E-dependent Mmp9 mRNA translation reverses fragile X syndrome-like phenotypes. Cell Rep, 9, 1742–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves J. T., Anstey J. E., Golshani P., Portera-Cailliau C. (2013). Circuit level defects in the developing neocortex of Fragile X mice. Nat Neurosci, 16, 903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough W. T., Klintsova A. Y., Irwin S. A., Galvez R., Bates K. E., Weiler I. J. (2001). Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci U S A, 98, 7101–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. S., Walter E., Sherman E., Hoeft F., Reiss A. L. (2009). The neural basis of auditory temporal discrimination in girls with fragile X syndrome. J Neurodev Disord, 1, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S. M., Green J. R., Veeraragavan S., Yuva L., McCoy A., Wu Y., Warren J., Little L., Ji D., Cui X., Weinstein E., Paylor R. (2014). Fmr1 and Nlgn3 knockout rats: Novel tools for investigating autism spectrum disorders. Behav Neurosci, 128, 103–109. [DOI] [PubMed] [Google Scholar]
- Han Y. K., Köver H., Insanally M. N., Semerdjian J. H., Bao S. (2007). Early experience impairs perceptual discrimination. Nat Neurosci, 10, 1191. [DOI] [PubMed] [Google Scholar]
- Happel M. F., Frischknecht R. (2016). Neuronal plasticity in the juvenile and adult brain regulated by the extracellular matrix. In Composition and function of the extracellular matrix in the human body. F Travascio, Ed., INTECH, Rijeka, Croatia.
- Harlow E. G., Till S. M., Russell T. A., Wijetunge L. S., Kind P., Contractor A. (2010). Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron, 65, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. L., Rao B. S., Seo J. S., Choi H. S., Dolan B. M., Choi S. Y., Chattarji S., Tonegawa S. (2007). Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci U S A, 104, 11489–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays S. A., Huber K. M., Gibson J. R. (2011). Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry. J Neurosci, 31, 14223–14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Nomura T., Xu J., Contractor A. (2014). The developmental switch in GABA polarity is delayed in fragile X mice. J Neurosci, 34, 446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert B., Pietropaolo S., Même S., Laudier B., Laugeray A., Doisne N., Quartier A., Lefeuvre S., Got L., Cahard D., Laumonnier F., Crusio W. E., Pichon J., Menuet A., Perche O., Briault S. (2014). Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by a BKCa channel opener molecule. Orphanet J Rare Dis, 9, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch T. K. (2005). Critical period plasticity in local cortical circuits. Nat Rev Neurosci, 6, 877–888. [DOI] [PubMed] [Google Scholar]
- Higashimori H., Morel L., Huth J., Lindemann L., Dulla C., Taylor A., Freeman M., Yang Y. (2013). Astroglial FMRP-dependent translational down-regulation of mGluR5 underlies glutamate transporter GLT1 dysregulation in the fragile X mouse. Hum Mol Genet, 22, 2041–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashimori H., Schin C. S., Chiang M. S., Morel L., Shoneye T. A., Nelson D. L., Yang Y. (2016). Selective deletion of astroglial FMRP dysregulates glutamate transporter GLT1 and contributes to fragile X syndrome phenotypes in vivo. J Neurosci, 36, 7079–7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton V. J., Brown W. T., Wisniewski K., Rudelli R. D. (1991). Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet, 41, 289–294. [DOI] [PubMed] [Google Scholar]
- Hou L., Antion M. D., Hu D., Spencer C. M., Paylor R., Klann E. (2006). Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron, 51, 441–454. [DOI] [PubMed] [Google Scholar]
- Hou L., Klann E. (2004). Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci, 24, 6352–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Qin Y., Bochorishvili G., Zhu Y., van Aelst L., Zhu J. J. (2008). Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J Neurosci, 28, 7847–7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K. M., Gallagher S. M., Warren S. T., Bear M. F. (2002). Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A, 99, 7746–7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K. M., Kayser M. S., Bear M. F. (2000). Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science, 288, 1254–1257. [DOI] [PubMed] [Google Scholar]
- Inoue S., Shimoda M., Nishinokubi I., Siomi M. C., Okamura M., Nakamura A., Kobayashi S., Ishida N., Siomi H. (2002). A role for the Drosophila fragile X-related gene in circadian output. Curr Biol, 12, 1331–1335. [DOI] [PubMed] [Google Scholar]
- Irwin S. A., Patel B., Idupulapati M., Harris J. B., Crisostomo R. A., Larsen B. P., Kooy F., Willems P. J., Cras P., Kozlowski P. B., Swain R. A., Weiler I. J., Greenough W. T. (2001). Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: A quantitative examination. Am J Med Genet, 98, 161–167. [DOI] [PubMed] [Google Scholar]
- Isaac J. T., Crair M. C., Nicoll R. A., Malenka R. C. (1997). Silent synapses during development of thalamocortical inputs. Neuron, 18, 269–280. [DOI] [PubMed] [Google Scholar]
- Jackson A. (2017). Cellular and synaptic pathophysiology in a rat model of Fragile X syndrome (Doctoral dissertation). University of Edinburgh, Scotland.
- Jacobs S., Doering L. C. (2010). Astrocytes prevent abnormal neuronal development in the fragile x mouse. J Neurosci, 30, 4508–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Nathwani M., Doering L. C. (2010). Fragile X astrocytes induce developmental delays in dendrite maturation and synaptic protein expression. BMC Neurosci, 11, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz A., Milek J., Perycz M., Pacini L., Bagni C., Kaczmarek L., Dziembowska M. (2013). The Fragile X mental retardation protein regulates matrix metalloproteinase 9 mRNA at synapses. J Neurosci, 33, 18234–18241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevakumar V., Kroener S. (2016). Ketamine administration during the second postnatal week alters synaptic properties of fast-spiking interneurons in the medial prefrontal cortex of adult mice. Cereb Cortex, 26, 1117–1129. [DOI] [PubMed] [Google Scholar]
- Juczewski K., von Richthofen H., Bagni C., Celikel T., Fisone G., Krieger P. (2016). Somatosensory map expansion and altered processing of tactile inputs in a mouse model of fragile X syndrome. Neurobiol Dis, 96, 201–215. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. E., Moser H. W. (2000). Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex, 10, 981–991. [DOI] [PubMed] [Google Scholar]
- Kidd F. L., Isaac J. T. (1999). Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature, 400, 569–573. [DOI] [PubMed] [Google Scholar]
- Kim H., Gibboni R., Kirkhart C., Bao S. (2013). Impaired critical period plasticity in primary auditory cortex of fragile X model mice. J Neurosci, 33, 15686–15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E., Dever T. E. (2004). Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci, 5, 931–942. [DOI] [PubMed] [Google Scholar]
- Kogan C. S., Bertone A., Cornish K., Boutet I., Der Kaloustian V. M., Andermann E., Faubert J., Chaudhuri A. (2004. a). Integrative cortical dysfunction and pervasive motion perception deficit in fragile X syndrome. Neurology, 63, 1634–1639. [DOI] [PubMed] [Google Scholar]
- Kogan C. S., Boutet I., Cornish K., Zangenehpour S., Mullen K. T., Holden J. J., Der Kaloustian V. M., Andermann E., Chaudhuri A. (2004. b). Differential impact of the FMR1 gene on visual processing in fragile X syndrome. Brain, 127, 591–601. [DOI] [PubMed] [Google Scholar]
- Laggerbauer B., Ostareck D., Keidel E. M., Ostareck-Lederer A., Fischer U. (2001). Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet, 10, 329–338. [DOI] [PubMed] [Google Scholar]
- LaPorta V. N., Nikitakis N. G., Sindler A. J., Reynolds M. A. (2005). Minocycline‐associated intra‐oral soft‐tissue pigmentation: Clinicopathologic correlations and review. J Clin Periodontol, 32, 119–122. [DOI] [PubMed] [Google Scholar]
- Larson J., Jessen R. E., Kim D., Fine A. K., du Hoffmann J. (2005). Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J Neurosci, 25, 9460–9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T. M., Amos N., Bulgen D., Williams B. D. (2001). Minocycline-induced lupus: Clinical features and response to rechallenge. Rheumatology (Oxford), 40, 329–335. [DOI] [PubMed] [Google Scholar]
- Leigh M. J., Nguyen D. V., Mu Y., Winarni T. I., Schneider A., Chechi T., Polussa J., Doucet P., Tassone F., Rivera S. M., Hessl D., Hagerman R. J. (2013). A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile x syndrome. J Dev Behav Pediatr, 34, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensjø K. K., Lepperød M. E., Dick G., Hafting T., Fyhn M. (2017). Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J Neurosci, 37, 1269–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Pelletier M. R., Perez Velazquez J. L., Carlen P. L. (2002). Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci, 19, 138–151. [DOI] [PubMed] [Google Scholar]
- Liu B., Li L., Chen J., Wang Z., Li Z., Wan Q. (2013). Regulation of GABAA receptors by fragile X mental retardation protein. Int J Physiol Pathophysiol Pharmacol, 5, 169–176. [PMC free article] [PubMed] [Google Scholar]
- Lovelace J. W., Ethell I. M., Binder D. K., Razak K. A. (2018). Translation-relevant EEG phenotypes in a mouse model of Fragile X Syndrome. Neurobiol Dis, 115, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace J. W., Wen T. H., Reinhard S., Hsu M. S., Sidhu H., Ethell I. M., Binder D. K., Razak K. A. (2016). Matrix metalloproteinase-9 deletion rescues auditory evoked potential habituation deficit in a mouse model of Fragile X Syndrome. Neurobiol Dis, 89, 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S. J. (2014). An introduction to the event-related potential technique. Cambridge, MA: MIT Press. [Google Scholar]
- McBride S. M., Giuliani G., Choi C., Krause P., Correale D., Watson K., Baker G., Siwicki K. K. (1999). Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron, 24, 967–977. [DOI] [PubMed] [Google Scholar]
- Miller L. J., McIntosh D. N., McGrath J., Shyu V., Lampe M., Taylor A. K., Tassone F., Neitzel K., Stackhouse T., Hagerman R. J. (1999). Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: A preliminary report. Am J Med Genet, 83, 268–279. [PubMed] [Google Scholar]
- Milner D., Goodale M. (2006). The visual brain in action. Oxford, England: Oxford University Press. [Google Scholar]
- Morales J., Hiesinger P. R., Schroeder A. J., Kume K., Verstreken P., Jackson F. R., Nelson D. L., Hassan B. A. (2002). Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron, 34, 961–972. [DOI] [PubMed] [Google Scholar]
- Muddashetty R. S., Nalavadi V. C., Gross C., Yao X., Xing L., Laur O., Warren S. T., Bassell G. J. (2011). Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol Cell, 42, 673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. M., Mazzocco M. M., Gerner G., Henry A. E. (2006). Mathematics learning disability in girls with Turner syndrome or fragile X syndrome. Brain Cogn, 61, 195–210. [DOI] [PubMed] [Google Scholar]
- Musumeci S. A., Bosco P., Calabrese G., Bakker C., De Sarro G. B., Elia M., Ferri R., Oostra B. A. (2000). Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia, 41, 19–23. [DOI] [PubMed] [Google Scholar]
- Musumeci S. A., Calabrese G., Bonaccorso C. M., D'Antoni S., Brouwer J. R., Bakker C. E., Elia M., Ferri R., Nelson D. L., Oostra B. A., Catania M. V. (2007). Audiogenic seizure susceptibility is reduced in fragile X knockout mice after introduction of FMR1 transgenes. Exp Neurol, 203, 233–240. [DOI] [PubMed] [Google Scholar]
- Myrick L. K., Deng P. Y., Hashimoto H., Oh Y. M., Cho Y., Poidevin M. J., Suhl J. A., Visootsak J., Cavalli V., Jin P., Cheng X., Warren S. T., Klyachko V. A. (2015). Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proc Natl Acad Sci U S A, 112, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M. C., Yang Y. L., Lu K. T. (2013). Behavioral and synaptic circuit features in a zebrafish model of fragile X syndrome. PLoS One, 8, e51456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen D. M., Derber W. J., McClellan D. A., Crnic L. S. (2002). Alterations in the auditory startle response in Fmr1 targeted mutant mouse models of fragile X syndrome. Brain Res, 927, 8–17. [DOI] [PubMed] [Google Scholar]
- Nieto Del Rincón P. L. (2008). Autism: Alterations in auditory perception. Rev Neurosci, 19, 61–78. [DOI] [PubMed] [Google Scholar]
- Nimchinsky E. A., Oberlander A. M., Svoboda K. (2001). Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci, 21, 5139–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Musial T. F., Marshall J. J., Zhu Y., Remmers C. L., Xu J., Nicholson D. A., Contractor A. (2017). Delayed maturation of fast-spiking interneurons is rectified by activation of the TrkB receptor in the mouse model of fragile X syndrome. J Neurosci, 37, 11298–11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okray Z., de Esch C. E., Van Esch H., Devriendt K., Claeys A., Yan J., Verbeeck J., Froyen G., Willemsen R., de Vrij F. M., Hassan B. A. (2015). A novel fragile X syndrome mutation reveals a conserved role for the carboxy-terminus in FMRP localization and function. EMBO Mol Med, 7, 423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen E. R., Baumgartner H. A., Rivera S. M. (2013). Using infrared eye-tracking to explore ordinal numerical processing in toddlers with Fragile X Syndrome. J Neurodev Disord, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey L. K., Doering L. C. (2007). Developmental expression of FMRP in the astrocyte lineage: Implications for fragile X syndrome. Glia, 55, 1601–1609. [DOI] [PubMed] [Google Scholar]
- Paixão S., Klein R. (2010). Neuron-astrocyte communication and synaptic plasticity. Curr Opin Neurobiol, 20, 466–473. [DOI] [PubMed] [Google Scholar]
- Paluszkiewicz S. M., Olmos-Serrano J. L., Corbin J. G., Huntsman M. M. (2011). Impaired inhibitory control of cortical synchronization in fragile X syndrome. J Neurophysiol, 106, 2264–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L., Woodruff E., Liang P., Broadie K. (2008). Mechanistic relationships between Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A signaling. Mol Cell Neurosci, 37, 747–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paribello C., Tao L., Folino A., Berry-Kravis E., Tranfaglia M., Ethell I. M., Ethell D. W. (2010). Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol, 10, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A., Préat T. (2001). Localization of long-term memory within the Drosophila mushroom body. Science, 294, 1115–1117. [DOI] [PubMed] [Google Scholar]
- Penagarikano O., Mulle J. G., Warren S. T. (2007). The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet, 8, 109–129. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T., Medini P., Berardi N., Chierzi S., Fawcett J. W., Maffei L. (2002). Reactivation of ocular dominance plasticity in the adult visual cortex. Science, 298, 1248–1251. [DOI] [PubMed] [Google Scholar]
- Porter D., Harrison A. (2003). Minocycline-induced lupus: A case series. N Z Med J, 116, U384. [PubMed] [Google Scholar]
- Reiss A. L., Freund L. (1990). Fragile X syndrome, DSM-III-R, and autism. J Am Acad Child Adolesc Psychiatry, 29, 885–891. [DOI] [PubMed] [Google Scholar]
- Rivera S. M., Menon V., White C. D., Glaser B., Reiss A. L. (2002). Functional brain activation during arithmetic processing in females with fragile X syndrome is related to FMR1 protein expression. Hum Brain Mapp, 16, 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. E., Mirrett P., Burchinal M. (2001). Receptive and expressive communication development of young males with fragile X syndrome. Am J Ment Retard, 106, 216–230. [DOI] [PubMed] [Google Scholar]
- Roberts T. P., Cannon K. M., Tavabi K., Blaskey L., Khan S. Y., Monroe J. F., Qasmieh S., Levy S. E., Edgar J. C. (2011). Auditory magnetic mismatch field latency: A biomarker for language impairment in autism. Biol Psychiatry, 70, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas D. C., Benkers T. L., Rogers S. J., Teale P. D., Reite M. L., Hagerman R. J. (2001). Auditory evoked magnetic fields in adults with fragile X syndrome. Neuroreport, 12, 2573–2576. [DOI] [PubMed] [Google Scholar]
- Ronesi J. A., Huber K. M. (2008). Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci, 28, 543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol R., Ranchon-Cole I., Pâris A., Herzine A., Perche A., Laurenceau D., Bertrand P., Cercy C., Pichon J., Mortaud S., Briault S., Menuet A. (2014). Visual sensorial impairments in neurodevelopmental disorders: Evidence for a retinal phenotype in Fragile X Syndrome. PLoS One, 9, e105996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer S., Razak K. (2013). Altered auditory processing in a mouse model of fragile X syndrome. Brain Res, 1506, 12–24. [DOI] [PubMed] [Google Scholar]
- Rotschafer S. E., &, Cramer K. S. (2017). Developmental emergence of phenotypes in the auditory brainstem nuclei of Fmr1 knockout mice. eNeuro, 4, pii: ENEURO.0264-17.2017. [DOI] [PMC free article] [PubMed]
- Rotschafer S. E., Marshak S., Cramer K. S. (2015). Deletion of Fmr1 alters function and synaptic inputs in the auditory brainstem. PLoS One, 10, e0117266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer S. E., Razak K. A. (2014). Auditory processing in fragile x syndrome. Front Cell Neurosci, 8, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez A. R., Rogers R. S., Sheridan P. J. (2004). Tetracycline and other tetracycline‐derivative staining of the teeth and oral cavity. Int J Dermatol, 43, 709–715. [DOI] [PubMed] [Google Scholar]
- Santoro M. R., Bray S. M., Warren S. T. (2012). Molecular mechanisms of fragile X syndrome: A twenty-year perspective. Annu Rev Pathol, 7, 219–245. [DOI] [PubMed] [Google Scholar]
- Santos A. R., Kanellopoulos A. K., Bagni C. (2014). Learning and behavioral deficits associated with the absence of the fragile X mental retardation protein: What a fly and mouse model can teach us. Learn Mem, 21, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A. (2016). mTOR, a potential target to treat autism spectrum disorder. CNS Neurol Disord Drug Targets, 15, 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerif G., Cornish K., Wilding J., Driver J., Karmiloff-Smith A. (2007). Delineation of early attentional control difficulties in fragile X syndrome: Focus on neurocomputational changes. Neuropsychologia, 45, 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B. L., Fox K., O'Leary D. D. (1993). Postsynaptic control of plasticity in developing somatosensory cortex. Nature, 364, 623–626. [DOI] [PubMed] [Google Scholar]
- Schlienger R. G., Bircher A. J., Meier C. R. (2000). Minocycline-induced lupus. A systematic review. Dermatology, 200, 223–231. [DOI] [PubMed] [Google Scholar]
- Schneider A., Leigh M. J., Adams P., Nanakul R., Chechi T., Olichney J., Hagerman R., Hessl D. (2013). Electrocortical changes associated with minocycline treatment in fragile X syndrome. J Psychopharmacol, 27, 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby L., Zhang C., Sun Q. Q. (2007). Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci Lett, 412, 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Hoeffer C. A., Takayasu Y., Miyawaki T., McBride S. M., Klann E., Zukin R. S. (2010). Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci, 30, 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J. (2002). Severe complication of a commonly prescribed drug: Minocycline-induced lupus. J Am Board Fam Pract, 15, 239–241. [PubMed] [Google Scholar]
- Shetty A. K. (2002). Tetracyclines in pediatrics revisited. Clin Pediatr (Phila), 41, 203–209. [DOI] [PubMed] [Google Scholar]
- Sidhu H., Dansie L. E., Hickmott P. W., Ethell D. W., Ethell I. M. (2014). Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J Neurosci, 34, 9867–9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. J., Chitwood R. A., Ding J. M., Payne C., Taylor W., Gray R., Zemelman B. V., Johnston D. (2017). Prefrontal cortex dysfunction in fragile X mice depends on the continued absence of fragile X mental retardation protein in the adult brain. J Neurosci, 37, 7305–7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons D. J. (1978). Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol, 41, 798–820. [DOI] [PubMed] [Google Scholar]
- Simons D. J., Carvell G. E. (1989). Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol, 61, 311–330. [DOI] [PubMed] [Google Scholar]
- Sinclair D., Featherstone R., Naschek M., Nam J., Du A., Wright S., Pance K., Melnychenko O., Weger R., Akuzawa S., Matsumoto M., Siegel S. J. (2017. a). GABA-B agonist baclofen normalizes auditory-evoked neural oscillations and behavioral deficits in the Fmr1 knockout mouse model of fragile X syndrome. eNeuro, 4, pii: ENEURO.0380-16.2017. [DOI] [PMC free article] [PubMed]
- Sinclair D., Oranje B., Razak K. A., Siegel S. J., Schmid S. (2017. b). Sensory processing in autism spectrum disorders and fragile X syndrome – From the clinic to animal models. Neurosci Biobehav Rev, 76, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K., Leyden J. J. (2005). Safety of doxycycline and minocycline: A systematic review. Clin Ther, 27, 1329–1342. [DOI] [PubMed] [Google Scholar]
- St Clair D. M., Blackwood D. H., Oliver C. J., Dickens P. (1987). P3 abnormality in fragile X syndrome. Biol Psychiatry, 22, 303–312. [DOI] [PubMed] [Google Scholar]
- Strumbos J. G., Brown M. R., Kronengold J., Polley D. B., Kaczmarek L. K. (2010). Fragile X mental retardation protein is required for rapid experience-dependent regulation of the potassium channel Kv3.1b. J Neurosci, 30, 10263–10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakaran I. P., Hillebrand J., Dervan A., Das S., Holohan E. E., Hülsmeier J., Sarov M., Parker R., VijayRaghavan K., Ramaswami M. (2014). FMRP and Ataxin-2 function together in long-term olfactory habituation and neuronal translational control. Proc Natl Acad Sci U S A, 111, E99–E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. J., Wu G. K., Liu B. H., Li P., Zhou M., Xiao Z., Tao H. W., Zhang L. I. (2010). Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature, 465, 927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. S., Nelson D. L., Zhang F., Pieretti M., Caskey C. T., Saxe D., Warren S. T. (1992). DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet, 1, 397–400. [DOI] [PubMed] [Google Scholar]
- Taha S., Stryker M. P. (2002). Rapid ocular dominance plasticity requires cortical but not geniculate protein synthesis. Neuron, 34, 425–436. [DOI] [PubMed] [Google Scholar]
- Teitelbaum J. E., Perez-Atayde A. R., Cohen M., Bousvaros A., Jonas M. M. (1998). Minocycline-related autoimmune hepatitis: Case series and literature review. Arch Pediatr Adolesc Med, 152, 1132–1136. [DOI] [PubMed] [Google Scholar]
- Till S. M., Asiminas A., Jackson A. D., Katsanevaki D., Barnes S. A., Osterweil E. K., Bear M. F., Chattarji S., Wood E. R., Wyllie D. J., Kind P. C. (2015). Conserved hippocampal cellular pathophysiology but distinct behavioural deficits in a new rat model of FXS. Hum Mol Genet, 24, 5977–5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till S. M., Wijetunge L. S., Seidel V. G., Harlow E., Wright A. K., Bagni C., Contractor A., Gillingwater T. H., Kind P. C. (2012). Altered maturation of the primary somatosensory cortex in a mouse model of fragile X syndrome. Hum Mol Genet, 21, 2143–2156. [DOI] [PubMed] [Google Scholar]
- Tournigand C., Généreau T., Prudent M., Diemert M. C., Herson S., Chosidow O. (1999). Minocycline-induced clinical and biological lupus-like disease. Lupus, 8, 773–774. [DOI] [PubMed] [Google Scholar]
- Ullian E. M., Christopherson K. S., Barres B. A. (2004). Role for glia in synaptogenesis. Glia, 47, 209–216. [DOI] [PubMed] [Google Scholar]
- Ungerleider L. G. (1982). Two cortical visual systems In Ingle D. J., Goodale M. A., Mansfield R. J. W. (Eds.), Analysis of visual behavior (pp. 549–586). Cambridge, MA: MIT Press. [Google Scholar]
- van den Ouweland A. M., de Vries B. B., Bakker P. L., Deelen W. H., de Graaff E., van Hemel J. O., Oostra B. A., Niermeijer M. F., Halley D. J. (1994). DNA diagnosis of the fragile X syndrome in a series of 236 mentally retarded subjects and evidence for a reversal of mutation in the FMR-1 gene. Am J Med Genet, 51, 482–485. [DOI] [PubMed] [Google Scholar]
- Vanderklish P. W., Edelman G. M. (2002). Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A, 99, 1639–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Molen M. J., Van der Molen M. W., Ridderinkhof K. R., Hamel B. C., Curfs L. M., Ramakers G. J. (2012. a). Auditory and visual cortical activity during selective attention in fragile X syndrome: A cascade of processing deficiencies. Clin Neurophysiol, 123, 720–729. [DOI] [PubMed] [Google Scholar]
- Van der Molen M. J., Van der Molen M. W., Ridderinkhof K. R., Hamel B. C., Curfs L. M., Ramakers G. J. (2012. b). Auditory change detection in fragile X syndrome males: A brain potential study. Clin Neurophysiol, 123, 1309–1318. [DOI] [PubMed] [Google Scholar]
- van 't Padje S., Engels B., Blonden L., Severijnen L. A., Verheijen F., Oostra B. A., Willemsen R. (2005). Characterisation of Fmrp in zebrafish: Evolutionary dynamics of the fmr1 gene. Dev Genes Evol, 215, 198–206. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J.Pieretti, M., Sutcliffe, J. S., Fu, Y-H., Kuhl, D. P. A., Pizzuti, A., ...Warren, S. T (1991). Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell, 65, 905–914. [DOI] [PubMed] [Google Scholar]
- Volk L. J., Pfeiffer B. E., Gibson J. R., Huber K. M. (2007). Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci, 27, 11624–11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L., Dockendorff T. C., Jongens T. A., Dreyfuss G. (2000). Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol, 20, 8536–8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ethridge L. E., Mosconi M. W., White S. P., Binder D. K., Pedapati E. V., Erickson C. A., Byerly M. J., Sweeney J. A. (2017). A resting EEG study of neocortical hyperexcitability and altered functional connectivity in fragile X syndrome. J Neurodev Disord, 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Sakano H., Beebe K., Brown M. R., de Laat R., Bothwell M., Kulesza R. J., Rubel E. W. (2014). Intense and specialized dendritic localization of the fragile X mental retardation protein in binaural brainstem neurons: A comparative study in the alligator, chicken, gerbil, and human. J Comp Neurol, 522, 2107–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen T. H., Afroz S., Reinhard S. M., Palacios A. R., Tapia K., Binder D. K., Razak K. A., Ethell I. M. (2017). Genetic reduction of matrix metalloproteinase-9 promotes formation of perineuronal nets around parvalbumin-expressing interneurons and normalizes auditory cortex responses in developing Fmr1 knock-out mice. Cereb Cortex. Advance online publication. doi:10.1093/cercor/bhx258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. M., Cox C. L. (2007). Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice. Proc Natl Acad Sci U S A, 104, 2454–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Poidevin M., Han E., Bi J., Jin P. (2012). Circadian rhythm-dependent alterations of gene expression in Drosophila brain lacking fragile X mental retardation protein. PLoS One, 7, e37937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q. J., Rammal M., Tranfaglia M., Bauchwitz R. P. (2005). Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology, 49, 1053–1066. [DOI] [PubMed] [Google Scholar]
- Yau S. Y., Bettio L., Vetrici M., Truesdell A., Chiu C., Chiu J., Truesdell E., Christie B. R. (2018). Chronic minocycline treatment improves hippocampal neuronal structure, NMDA receptor function, and memory processing in Fmr1 knockout mice. Neurobiol Dis, 113, 11–22. [DOI] [PubMed] [Google Scholar]
- Yun S. W., Platholi J., Flaherty M. S., Fu W., Kottmann A. H., Toth M. (2006). Fmrp is required for the establishment of the startle response during the critical period of auditory development. Brain Res, 1110, 159–165. [DOI] [PubMed] [Google Scholar]
- Zalfa F., Giorgi M., Primerano B., Moro A., Di Penta A., Reis S., Oostra B., Bagni C. (2003). The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell, 112, 317–327. [DOI] [PubMed] [Google Scholar]
- Zaman T., De Oliveira C., Smoka M., Narla C., Poulter M. O., Schmid S. (2017). BK channels mediate synaptic plasticity underlying habituation in rats. J Neurosci, 37, 4540–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zars T., Fischer M., Schulz R., Heisenberg M. (2000). Localization of a short-term memory in Drosophila. Science, 288, 672–675. [DOI] [PubMed] [Google Scholar]
- Zhang J., Fang Z., Jud C., Vansteensel M. J., Kaasik K., Lee C. C., Albrecht U., Tamanini F., Meijer J. H., Oostra B. A., Nelson D. L. (2008). Fragile X-related proteins regulate mammalian circadian behavioral rhythms. Am J Hum Genet, 83, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Bonnan A., Bony G., Ferezou I., Pietropaolo S., Ginger M., Sans N., Rossier J., Oostra B., LeMasson G., Frick A. (2014). Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(-/y) mice. Nat Neurosci, 17, 1701–1709. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Brown M. R., Hyland C., Chen Y., Kronengold J., Fleming M. R., Kohn A. B., Moroz L. L., Kaczmarek L. K. (2012). Regulation of neuronal excitability by interaction of fragile X mental retardation protein with slack potassium channels. J Neurosci, 32, 15318–15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Q., Bailey A. M., Matthies H. J., Renden R. B., Smith M. A., Speese S. D., Rubin G. M., Broadie K. (2001). Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell, 107, 591–603. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Q., Matthies H. J., Mancuso J., Andrews H. K., Woodruff E., Friedman D., Broadie K. (2004). The Drosophila fragile X-related gene regulates axoneme differentiation during spermatogenesis. Dev Biol, 270, 290–307. [DOI] [PubMed] [Google Scholar]
- Zhao M. G., Toyoda H., Ko S. W., Ding H. K., Wu L. J., Zhuo M. (2005). Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci, 25, 7385–7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorio D. A., Jackson C. M., Liu Y., Rubel E. W., Wang Y. (2017). Cellular distribution of the fragile X mental retardation protein in the mouse brain. J Comp Neurol, 525, 818–849. [DOI] [PMC free article] [PubMed] [Google Scholar]