Abstract
Introduction:
In recognition of the value of long-term real-world data, a postauthorization safety study of the inhaled corticosteroid (ICS) fluticasone propionate and long-acting β2-agonist (LABA) formoterol fumarate (fluticasone/formoterol; Flutiform®) was conducted.
Methods:
This was a 12-month observational study of outpatients with asthma aged ⩾ 12 years in eight European countries. Patients were prescribed fluticasone/formoterol according to the licensed indication, and independently of their subsequent enrolment in the study. They were then treated according to local standard practice. The study objectives were to evaluate the safety and effectiveness of fluticasone/formoterol under real-world conditions.
Results:
The safety population for this study comprised 2539 patients (mean age 47.7 years; 94.3% aged ⩾ 18 years; 63.4% female). Most patients (1538/2539, 60.6%) had switched to fluticasone/formoterol from another ICS/LABA, primarily due to lack of efficacy (1150/2539, 45.3%). Three quarters (77.4%) of patients were treated for 12 months, and 80.6% continued fluticasone/formoterol treatment after the study. Adverse events (AEs) occurred in 60.0% patients, and 10.2% had AEs considered possibly related to fluticasone/formoterol [most commonly asthma exacerbation (2.0% patients), dysphonia (1.8%) and cough (1.1%)]. Thirty-six severe AEs, but no serious AEs, were considered possibly related to fluticasone/formoterol. The proportion of patients with controlled asthma (based on Asthma Control Test score ⩾ 20) increased from 29.4% at baseline to 67.4% at study end (last observation carried forward). The proportion of patients experiencing at least one severe exacerbation decreased from 35.8% in the year prior to enrolment to 9.8% during the study. Improvements from baseline to study end were also observed in Asthma Quality of Life scores and physician/patient reports of satisfaction with treatment.
Conclusion:
In this real-world postauthorization safety study, fluticasone/formoterol demonstrated a safety profile consistent with that seen in controlled clinical trials, with effectiveness in improving asthma control.
Keywords: asthma, fluticasone propionate, formoterol fumarate, non-interventional study, real world, safety
Introduction
The fixed-dose combination (FDC) of the inhaled corticosteroid (ICS) fluticasone propionate and long-acting β2-agonist (LABA) formoterol fumarate, delivered via pressurized metered-dose inhaler (fluticasone/formoterol; Flutiform®) has been available in Europe for the treatment of asthma in adolescents and adults since 2012.1 The efficacy and safety of fluticasone/formoterol in asthma is supported by a comprehensive clinical dataset.2–9 This includes demonstrated superiority over placebo or the constituent monotherapies in mild–moderate and moderate–severe asthma,6,7 comparable efficacy with other approved ICS/LABA FDC products, though with a more rapid onset of bronchodilation than fluticasone/salmeterol,2–4 and open-label data from up to 12 months of treatment.9
Aside from data specifically relating to fluticasone/formoterol, the use of ICS and LABA in combination is supported by a wealth of preclinical and clinical evidence. At the molecular level, a synergistic mechanism of action of ICS/LABA has been proposed on the basis of in vitro data.10,11 In clinical trials, adding LABA to ICS has been shown to generate better asthma control than increasing the dose of ICS,12–16 and this strategy improves symptoms and lung function with a reduced risk of exacerbations.17 It has also been suggested that the provision of an ICS and a LABA in a single inhaler may also be associated with improved patient adherence relative to the treatments administered by separate inhalers.10,18,19 Together, these lines of evidence are reflected in the 2017 Global Initiative for Asthma (GINA) guidelines, which advise that the preferred approach for the maintenance treatment of asthma in patients over the age of 12 not controlled by low-dose ICS alone [with as-needed short-acting β2-agonist (SABA) as reliever] is to add a LABA.20
Although the value of fluticasone/formoterol and other ICS/LABA combinations in asthma management has been well established through robust clinical trials, the applicability of these data to real-world practice is inevitably limited by restrictions in the eligibility criteria of such trials. Exclusion of patients with comorbidities, for example, prevents trial data from reflecting this critical influence on the effectiveness and safety of a drug in practice. It has been reported that study populations participating in randomized controlled trials (RCTs) in asthma typically represent less than 5% of the population for whom the products become licensed.21–23 Effective treatment of asthma relies upon good inhaler technique, which is carefully controlled and supported by regular retraining in the RCT setting; this is often not reflective of real-world practice, in which poor inhaler technique is a recognized factor limiting the achievement of asthma control.22
In recognition of the discord between the management of asthma in clinical trial and real-world settings and as part of an agreed European Risk Management Plan (EU RMP), a non-interventional postauthorization safety study [PASS; ENCePP EU PAS register number EUPAS4072; AFFIRM (Assessing Fluticasone propionate/Formoterol In Real-life Maintenance treatment)] was conducted to determine the safety and effectiveness of fluticasone/formoterol in clinical practice.
Methods
Study design
This PASS was designed to collect data on the safety and effectiveness of fluticasone/formoterol prescribed for outpatients with moderate to severe asthma aged ⩾ 12 years over a 12-month period. Participants were under the care of general practitioners or respiratory physicians in primary or secondary care in eight European countries: Czech Republic, Denmark, France, Ireland, Norway, Slovak Republic, Sweden and the United Kingdom. Patients were treated with fluticasone/formoterol according to the licensed indication, with data captured for 1 year from first on-study dose of fluticasone/formoterol.
The primary objective was to evaluate the safety of fluticasone/formoterol by collecting data on drug exposure and the frequency of adverse events (AEs) associated with the treatment. Secondary objectives were to assess the effectiveness of fluticasone/formoterol treatment on asthma control under real-world conditions. The study was also intended to provide data on patients’ and physicians’ satisfaction with fluticasone/formoterol therapy.
Assessments
Demographic information, data on asthma history and the reason for initiation of fluticasone/formoterol were recorded at the baseline visit. Details of asthma history included prior asthma treatment (in the last 30 days), asthma control status [according to Asthma Control Test (ACTTM) score], severe asthma exacerbations in the last 12 months and satisfaction with previous treatment. After the baseline visit, physicians’ visits, diagnostic procedures and assessments were performed as clinically indicated in the opinion of the treating physician and according to treatment guidelines. Data available at prespecified timepoints of 1, 3, 6, 9 and 12 months postbaseline were collected, including fluticasone/formoterol exposure (including dose change and reason for discontinuation). Data on AEs were collected via reports from participating patients (either spontaneous report or in response to physicians’ open questioning), as well as those detected by diagnostic procedures during routine clinical practice at the physicians’ discretion.
Asthma control was assessed by means of ACTTM scores (total plus component scores: ability to perform daily activities; shortness of breath; sleep disturbances due to asthma; necessity to use a rescue medication; subject assessment of asthma control). Asthma was defined as ‘controlled’ if the total ACTTM score was ⩾20, ‘somewhat controlled’ if the score was 16–19, and ‘poorly controlled’ if the score was ⩽15.
On-study measurements of asthma-related medication use (including rescue medication use, antibiotics, systemic corticosteroids, etc.), concomitant diseases and medication, consultations and hospitalizations due to asthma, days of absence from work/school/college/university and inability to perform everyday activities due to asthma were also recorded. Lung function parameters [forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and peak expiratory flow (PEF)] were monitored as both absolute and percent predicted values. Severe asthma exacerbations were recorded as AEs; a severe exacerbation defined as worsening of asthma that required either the use of systemic corticosteroids (oral or parenteral) related to the asthma exacerbation, or unscheduled medical consultation (hospitalization, unscheduled physician’s visit or emergency department visit) during which systemic corticosteroids were administered due to asthma symptoms. Asthma exacerbations treated with courses of corticosteroids separated by 1 week or more were treated as separate exacerbations.
Data were also collected on quality of life using the Asthma Quality of Life Questionnaire (AQLQ; total plus component scores: activities limitation; emotional function score; environmental stimuli score; symptoms score), patient- and physician-rated satisfaction with treatment, and physicians’ estimates of patient adherence.
Study data were collated in electronic case report forms by investigator site staff.
Participants
Eligible patients had moderate to severe asthma and had been prescribed fluticasone/formoterol in accordance with the locally approved indication. As such, they were at least 12 years of age (male or female), with asthma that was not controlled on ICS and ‘as required’ inhaled SABAs, or they had been switched to fluticasone/formoterol from another treatment with a fixed or a free ICS/LABA combination. Patients with contraindications as laid out in the summary of product characteristics were excluded.24 Prescription of fluticasone/formoterol was initiated in normal clinical practice, and independent of patients’ subsequent enrolment in the study.
Written informed consent was obtained for all participants according to local requirements. Two sites withdrew from the study; data from these sites are not included.
Statistical analyses
The target study sample size was set at 2500 in order to provide adequate precision in estimating AE rates. Continuous variables were summarized using descriptive statistics; categorical data were summarized as number and percentage of patients with nonmissing data. All analyses were performed on the safety population, which comprised all patients who received at least one dose of study medication during the observational period of the study.
For the safety analysis, treatment-emergent AEs, related AEs, severe AEs, AEs leading to death, serious AEs, AEs leading to discontinuation of the study, AEs requiring additional therapy, AEs leading to dose change, and AEs of special interest were summarized. The annual rate of severe exacerbations was calculated for the period of 12 months prior to study enrolment and during the study.
Treatment effectiveness was analysed based on ACTTM scores, AQLQ scores and lung function parameters. For these parameters, data available at each prespecified time point and at end of study, and corresponding changes from baseline, were summarized descriptively [including 95% confidence intervals (CI) for the mean changes]. Changes from baseline were also analysed using the paired t test; given the non-interventional study design, these statistical tests were exploratory. In general, last observation carried forward (LOCF) imputation for missing data was used for the end-of-study analyses of effectiveness parameters.
Patient- and physician-rated treatment satisfaction and physicians’ estimates of patient adherence at each time point and end of study were summarized by frequency tables.
Results
Patients
A total of 2567 patients consented to participate in the study, and 2539 (98.9%) of these were included in the safety population. A summary of patient disposition is shown in Figure 1. Three quarters (1964, 77.4%) of the safety population completed the 12-month observational period of the study and remained on fluticasone/formoterol during this time. Of those who discontinued from the study or fluticasone/formoterol prematurely (575, 22.6%), the most common primary reasons for discontinuation were loss to follow up (224 patients, 39.0%) and AEs (135, 23.5%). Of the patients who completed the study, nearly all (1893/1964, 96.4%) continued to be prescribed fluticasone/formoterol after the study; 66/1964 (3.4%) were not, and further prescription was unknown for 5/1964 (0.3%) patients. Of the patients who discontinued the study prematurely, a quarter (154/575, 26.8%) subsequently received fluticasone/formoterol after study completion, whilst half (303/575, 52.7%) did not (unknown for the remaining 118, 20.5%). A total of 2047 (80.6%) patients received fluticasone/formoterol further after the study and 369 (14.5%) did not (unknown for 123, 4.8%).
Figure 1.
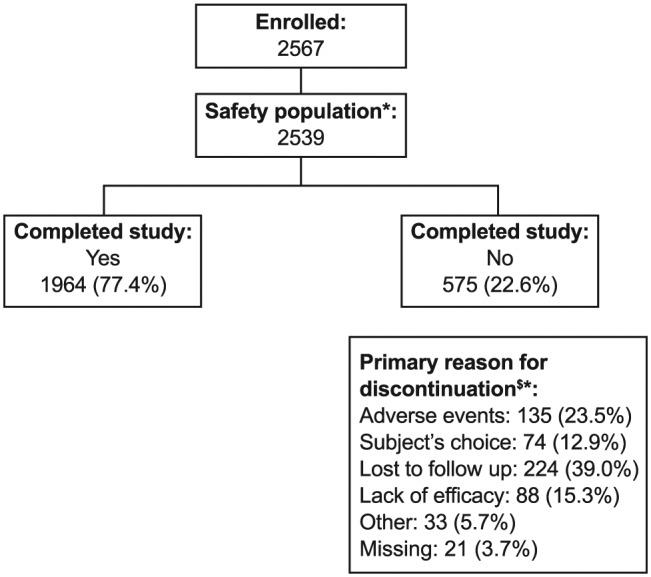
Patient disposition.
*Safety population comprises all patients who received at least one dose of study medication.
$Or why fluticasone/formoterol will not be prescribed any further after study completion.
Percentages are of the safety population.
The baseline characteristics of the safety population are summarized in Table 1. The mean and standard deviation (SD) age was 47.7 (17.5) years, and most patients (2394, 94.3%) were over the age of 18 years. Nearly two thirds (1609, 63.4%) of the safety population were female and 2470 (97.8%) were Caucasian. Although 1350 (60.7%) patients achieved more than 80% predicted FEV1 at baseline, 1076 (43.8%) had poorly controlled asthma (based on ACTTM total score). The median duration of asthma was 8.80 years (range 0–74.9 years). The most common forms of asthma were allergic (1637 patients, 64.5%), intrinsic (897, 35.3%) and exercise-induced (335, 13.2%). Two thirds (1722, 67.8%) of asthma cases were classed as persistently moderate in severity. Two thirds of patients (1856, 73.1%) were nonsmokers, one in ten (274, 10.8%) were current smokers, and 409 (16.1%) were exsmokers. At the baseline visit, 2199 (86.6%) patients had at least one concomitant medical condition; for example, 66 (2.6%) patients had current chronic obstructive pulmonary disease (COPD), 37 (1.5%) had a respiratory tract infection, 324 (12.8%) were obese and 803 (31.6%) had allergic rhinitis.
Table 1.
Baseline demographic characteristics (safety population).
| n | Total (n = 2539) | |
|---|---|---|
| Age, years | 2539 | |
| Mean (SD) | 47.7 (17.5) | |
| Median (range) | 49.0 (11–94) | |
| Age group, n (%) | 2539 | |
| <18 years | 145 (5.7) | |
| ⩾18 years | 2394 (94.3) | |
| Sex, n (%) | 2539 | |
| Male | 930 (36.6) | |
| Female | 1609 (63.4) | |
| Race, n (%)* | 2526 | |
| Caucasian | 2470 (97.8) | |
| Black | 22 (0.9) | |
| Asian | 29 (1.1) | |
| Other | 5 (0.2) | |
| BMI, kg/m2 | 2536 | |
| Mean (SD) | 28.0 (5.9) | |
| Median (range) | 27.4 (12.2–57.3) | |
| ACTTM, n (%) | 2458 | |
| Controlled | 723 (29.4) | |
| Somewhat controlled | 659 (26.8) | |
| Poorly controlled | 1076 (43.8) | |
| Prior treatment, n (%) | 2539 | |
| ICS without LABA | 482 (19.0) | |
| ICS plus LABA (open combination) | 235 (9.3) | |
| LABA without ICS | 14 (0.6) | |
| Beclometasone dipropionate/formoterol | 201 (7.9) | |
| Fluticasone propionate/salmeterol | 400 (15.8) | |
| Budesonide/formoterol | 343 (13.5) | |
| Fluticasone/formoterol | 624 (24.6) | |
| Other | 240 (9.5) | |
| FEV1, % predicted, n (%) | 2225 | |
| 40–60% | 200 (9.0) | |
| >60–80% | 675 (30.3) | |
| >80% | 1350 (60.7) | |
| Smoking situation, n (%) | 2539 | 1856 (73.1) |
| Nonsmoker | 274 (10.8)/409 (16.1) | |
| Smoker/exsmoker | ||
| Smoking duration$, years | 2493 | 4.9 (10.6) |
| Mean (SD) | ||
| Smoking history$, pack-years | 2466 | |
| Mean (SD) | 3.3 (9.1) | |
| Asthma duration, years | 2538 | |
| Mean (SD) | 11.9 (11.7) | |
| Median (range) | 8.8 (0.0–74.9) | |
| Type of asthma, n (%)‡ | 2539 | |
| Allergic | 1637 (64.5) | |
| Intrinsic | 897 (35.3) | |
| Exercise-induced | 335 (13.2) | |
| Analgesic-induced | 13 (0.5) | |
| Other | 62 (2.4) | |
| Severity of asthma, n (%) | 2538 | |
| Intermittent | 163 (6.4) | |
| Persistent mild | 480 (18.9) | |
| Persistent moderate | 1722 (67.8) | |
| Persistent severe | 173 (6.8) |
Race information was not collected in France.
Data from non-smokers were included in calculation of means.
A patient may be counted in more than one type of asthma category.
ACT™, Asthma Control Test; BMI, body mass index; FEV1, forced expiratory volume in 1 s; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; SD, standard deviation.
Details of asthma medications with which study participants were previously treated are presented in Table 1. For the 1538 (60.6%) patients switching to fluticasone/formoterol from another ICS/LABA treatment prior to the study, the most common reason was a lack of efficacy (1150, 45.3%), followed by a lack of compliance/satisfaction (319, 12.6%), side effects (137, 5.4%) or other reasons (138, 5.4%).
Exposure to asthma medication
The mean (SD) time of on-study exposure to fluticasone/formoterol was 344.3 (103.3) days, with 1910 (75.2%) patients having on-study exposure of at least 12 months. Dosing was stable in 2116 (83.3%) patients; the proportions of patients using only low [approximately 50/5 μg, two puffs twice daily (b.i.d.)], medium (approximately 125/5 μg, two puffs b.i.d.) and high (approximately 250/10 μg, two puffs bid) dosage were 4.8%, 48.0% and 30.6%, respectively. Dose increases were recorded in 165 patients (6.5%), dose decreases in 113 (4.5%), multiple dosage adjustments in 145 (5.7%) and interrupted dosage in 41 (1.6%). Nearly half of patients (1152, 45.4%) received a concomitant asthma controller medication at least once during the study; the most commonly used were leukotriene antagonists (675, 26.6%) and systemic corticosteroids (300, 11.8%). Nearly three quarters of patients (1845, 72.7%) received concomitant asthma rescue medication during the study (most commonly SABA: 1559, 61.4%).
Safety
Overall, 1523 patients (60.0%) experienced a total of 4264 AEs, as summarized in Table 2. In 258 patients (10.2%), investigators considered there was a reasonable possibility that AEs (375 in total) were causally related to fluticasone/formoterol treatment. Additional therapy for AEs (related or not related) was required for 1223 patients (48.2%). Most AEs (related or not related to treatment) were mild or moderate in severity; 570 patients (22.4%) experienced at least one mild AE, whilst 634 (25.0%) experienced at least one moderate AE. A total of 508 severe AEs were reported in 319 patients (12.6%), with 29 patients (1.1%) experiencing at least one severe AE considered possibly related to fluticasone/formoterol (total of 36 events). All serious AEs [SAEs; 139, in 107 (4.2%) patients] were considered not to be related to fluticasone/formoterol. Four patients (0.2%) died during the study; these deaths were attributed to bronchopneumonia, liver metastases, cerebrovascular accident and pulmonary embolism, respectively. None of the deaths were considered related to fluticasone/formoterol.
Table 2.
Overall summary of adverse events (safety population).
| Total (n = 2539) | |
|---|---|
| Patients with ⩾1 AE, n (%) | 1523 (60.0) |
| Number of AEs | 4264 |
| Patients with ⩾1 related* AE, n (%) | 258 (10.2) |
| Number of related* AEs | 375 |
| Patients with ⩾1 severe AE, n (%) | 319 (12.6) |
| Number of severe AEs | 508 |
| Patients with ⩾1 related* severe AE, n (%) | 29 (1.1) |
| Number of related* severe AEs | 36 |
| Patients with ⩾1 SAE, n (%) | 107 (4.2) |
| Number of SAEs | 139 |
| Patients who died$ | 4 (0.2) |
| Patients with ⩾1 AE | 152 (6.0) |
| Leading to study/treatment discontinuation‡ | 1223 (48.2) |
| Requiring additional therapy | 39 (1.5) |
| Leading to dose reduction | 28 (1.1) |
| Leading to dose interruption | 134 (5.3) |
| Leading to dose increase | |
| Patients with ⩾1 related* AE leading to study/treatment discontinuation‡ of special interest |
112 (4.4) 211 (8.3) |
Investigator considered reasonable possibility of causal relationship to investigational medicinal product.
Deaths were not considered to be possibly related to fluticasone/formoterol.
An AE was considered as leading to discontinuation from study if other action taken contains ‘discontinued from observation’ or if action taken with fluticasone/formoterol is ‘withdrawn’.
Note that a patient may have findings in more than one category.
AE, adverse event; SAE, serious adverse event.
The most frequently reported AEs by preferred term were asthma exacerbation (647 events in 435 patients, 17.1%), nasopharyngitis (199 events in 165 patients, 6.5%), bronchitis (189 events in 149 patients, 5.9%), and lower respiratory tract infection (143 events in 93 patients, 3.7%). The most frequently observed severe AEs were asthma exacerbation (248 patients, 9.8%), lower respiratory tract infection (10 patients, 0.4%) and bronchitis (8 patients, 0.3%).
The rates of AEs considered related to fluticasone/formoterol were low and are summarized in Table 3. The only AEs that were considered related to fluticasone/formoterol that occurred in more than 1% patients were asthma exacerbation (59 AEs in 50 patients, 2.0%), dysphonia (47 AEs in 46 patients, 1.8%), and cough (28 AEs in 28 patients, 1.1%).
Table 3.
Patients with adverse events considered to be related* to fluticasone/formoterol (observed in more than two patients; safety population).
| System organ class Preferred term |
Total (n = 2539) |
|---|---|
| Cardiac disorders, n (%) | |
| Palpitations | 14 (0.6) |
| Tachycardia | 7 (0.3) |
| Gastrointestinal disorders, n (%) | |
| Dry mouth | 4 (0.2) |
| Dyspepsia | 3 (0.1) |
| Nausea | 6 (0.2) |
| General disorders, n (%) | |
| Chest discomfort | 5 (0.2) |
| Infections and infestations, n (%) | |
| Bronchopneumonia | 3 (0.1) |
| Lower respiratory tract infection | 7 (0.3) |
| Nasopharyngitis | 3 (0.1) |
| Oral candidiasis | 17 (0.7) |
| Oral fungal infection | 4 (0.2) |
| Oropharyngeal candidiasis | 3 (0.1) |
| Respiratory tract infection | 3 (0.1) |
| Upper respiratory tract infection | 4 (0.2) |
| Musculoskeletal and connective tissue disorders, n (%) | |
| Back pain | 3 (0.1) |
| Muscle spasms | 7 (0.3) |
| Nervous system disorders, n (%) | |
| Dizziness | 3 (0.1) |
| Headache | 10 (0.4) |
| Tremor | 16 (0.6) |
| Respiratory, thoracic and mediastinal disorders, n (%) | |
| Asthma | 50 (2.0) |
| Cough | 28 (1.1) |
| Dysphonia | 46 (1.8) |
| Dyspnoea | 5 (0.2) |
| Oropharyngeal pain | 13 (0.5) |
| Upper-airway cough syndrome | 3 (0.1) |
| Vascular disorders, n (%) | |
| Hypertension | 3 (0.1) |
Investigator considered reasonable possibility of causal relationship to investigational medicinal product.
A patient may have more than one adverse event in any category. Highest causal relationship to study medication is counted if an adverse event is reported more than once by the same subject. Adverse events were coded using MedDRA version 16.0 (March 2013, MedDRA MSSO, McLean, VA, USA).
AEs of special interest (Supplementary Table 1) were experienced by 1247 (49.1%) patients, primarily local immunosuppressive effects/infections (899 patients, 35.4%). AEs of special interest that were assessed by the physician as having a reasonable possibility of a causal relationship to fluticasone/formoterol were reported in 211 patients (8.3%). The most common of these were ‘respiratory AEs including cough and paradoxical bronchospasm’ (82 patients, 3.2%, note that no cases of paradoxical bronchospasm were recorded), ‘local immunosuppressive effects, infections’ (57 patients, 2.2%), and ‘asthma worsening/asthma exacerbation’ (50 patients, 2.0%).
Effectiveness
Asthma control
During the course of the study, asthma control improved compared with that achieved with the previous asthma treatment. The mean (SD) ACTTM total score at baseline, related to the prior asthma treatment, was 16.3 (4.8). The mean total score increased to 19.2 (4.2) at 1 month (n = 800), and the improvement was sustained throughout the study period, with a mean (SD) score of 20.4 (4.3) at the end of the study (Table 4). The mean (SD) change from baseline ranged from 3.1 (4.7; at 3 months, n = 1356) to 4.6 (5.1; at 12 months, n = 1771). Correspondingly, the proportion of patients with controlled asthma, that is, with ACTTM total score ⩾ 20, also increased from 29.4% at baseline to 67.4% at end of study (Figure 2).
Table 4.
Asthma Control Test scores (ACTTM).
| Test component | Baseline
(n = 2456) |
Month 12
(n = 1783) |
End of study (LOCF)
(n = 2220) |
Change from baseline to end
of study (LOCF) (n = 2197) |
|
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | 95% CI | |
| Total score | 16.3 (4.8) | 20.8 (4.0) | 20.4 (4.3) | 4.2 (5.2)* | 3.93, 4.37 |
| Ability to perform daily activities | 3.4 (1.1) | 4.2 (0.9) | 4.1 (0.9) | 0.7 (1.2)* | 0.68, 0.77 |
| Shortness of breath | 3.1 (1.3) | 4.0 (1.0) | 3.9 (1.1) | 0.9 (1.4)* | 0.83, 0.95 |
| Sleep disturbances due to asthma | 3.3 (1.4) | 4.3 (1.0) | 4.2 (1.1) | 0.9 (1.5)* | 0.83, 0.95 |
| Necessity to use a rescue medication | 3.2 (1.3) | 4.2 (1.1) | 4.1 (1.1) | 0.8 (1.4)* | 0.79, 0.91 |
| Subject assessment of asthma control | 3.3 (1.1) | 4.2 (0.8) | 4.1 (0.9) | 0.8 (1.2)* | 0.75, 0.85 |
p < 0.001 (paired t test).
Each of the five components assessed on a 5-point scale; total score may range from 5 to 25, with a higher score indicating better control.
CI, confidence interval; LOCF, last observation carried forward, up to 12 months; SD, standard deviation.
Figure 2.
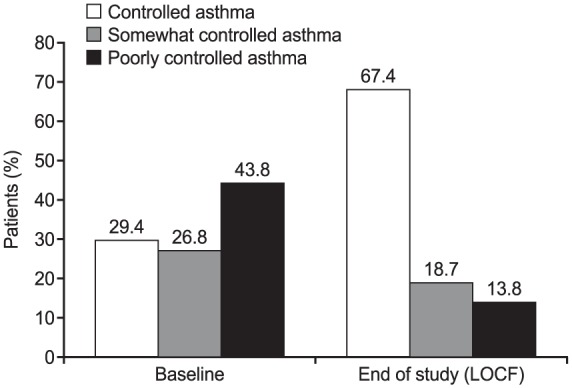
Asthma control status at baseline and end of study (last observation carried forward up to 12 months).
Asthma control based on ACTTM total score (controlled, ACTTM ⩾ 20; somewhat controlled, ACTTM 16–19; poorly controlled, ACTTM ⩽ 15). Percentages are based on number of patients with data available at respective time point: baseline, n = 2456; LOCF, n = 2220.
ACTTM, Asthma Control Test; LOCF, last observation carried forward, up to 12 months.
Severe asthma exacerbations
In the 12 months prior to enrolment in the study, 1629 (64.2%) patients had no severe asthma exacerbations. The remaining 909 (35.8%) patients experienced up to 12 severe asthma exacerbations; 88 (3.5%) reported 6 or more, whilst 461 (18.2%) experienced only 1. During the study period on fluticasone/formoterol treatment, 2291 (90.2%) patients experienced no severe asthma exacerbations. In the 248 (9.8%) patients who experienced a severe asthma exacerbation, the maximum count was seven (in one patient, <0.1%); most (166, 6.5%) experienced only one.
During the study, a total of 370 severe asthma exacerbations were recorded over a cumulative treatment exposure of 2442.1 years; this translates to an overall annualized rate of 0.15 for severe asthma exacerbations whilst receiving fluticasone/formoterol.
Quality of life
The mean (SD) total AQLQ score increased from 4.7 (1.2) at baseline to 5.6 (1.1) at the end of the study (Table 5). Improvements were also seen in all four components of the AQLQ.
Table 5.
Asthma Quality of Life Questionnaire scores.
| Baseline | End of study (LOCF) | Change from baseline | ||
|---|---|---|---|---|
| Total score | ||||
| n | 2381 | 1781 | 1772 | |
| Mean (SD) | 4.69 (1.23) | 5.64 (1.10) | 1.01 (1.12)* | 0.96, 1.06 |
| Activities limitation score | ||||
| n | 2379 | 1779 | 1768 | |
| Mean (SD) | 4.82 (1.25) | 5.66 (1.11) | 0.92 (1.09)* | 0.87, 0.97 |
| Emotional function score | ||||
| n | 2379 | 1781 | 1771 | |
| Mean (SD) | 4.82 (1.44) | 5.78 (1.19) | 1.00 (1.32)* | 0.94, 1.06 |
| Environmental stimuli score | ||||
| n | 2379 | 1780 | 1769 | |
| Mean (SD) | 4.60 (1.44) | 5.46 (1.30) | 0.92 (1.27)* | 0.86, 0.98 |
| Symptoms score | ||||
| n | 2381 | 1782 | 1773 | |
| Mean (SD) | 4.55 (1.30) | 5.61 (1.13) | 1.14 (1.26)* | 1.08, 1.19 |
p < 0.001 (paired t test).
Total AQLQ and components are mean ratings on a 7-point scale; each score may range from 1 to 7, with a higher score indicating less impairment. Note that n is for total score; these differed slightly (by no more than four patients) for most component scores.
AQLQ, Asthma Quality of Life Questionnaire; CI, confidence interval; LOCF, last observation carried forward, up to 12 months; SD, standard deviation.
Lung function
All measured lung function parameters increased between baseline and end of study, as shown in Supplementary Table 2. The mean (SD) baseline and end-of-study values, respectively, for FEV1 were 2.58 l (0.86) and 2.72 l (0.93); for FVC, 3.32 l (1.09) and 3.43 l (1.12); for FEV1/FVC, 0.78 (0.12) and 0.80 (0.12); and for PEF, 356.1 l/min (124.7) and 377.0 l/min (127.9). Changes in percent predicted values for these parameters improved accordingly (Supplementary Table 2).
Satisfaction with asthma treatment
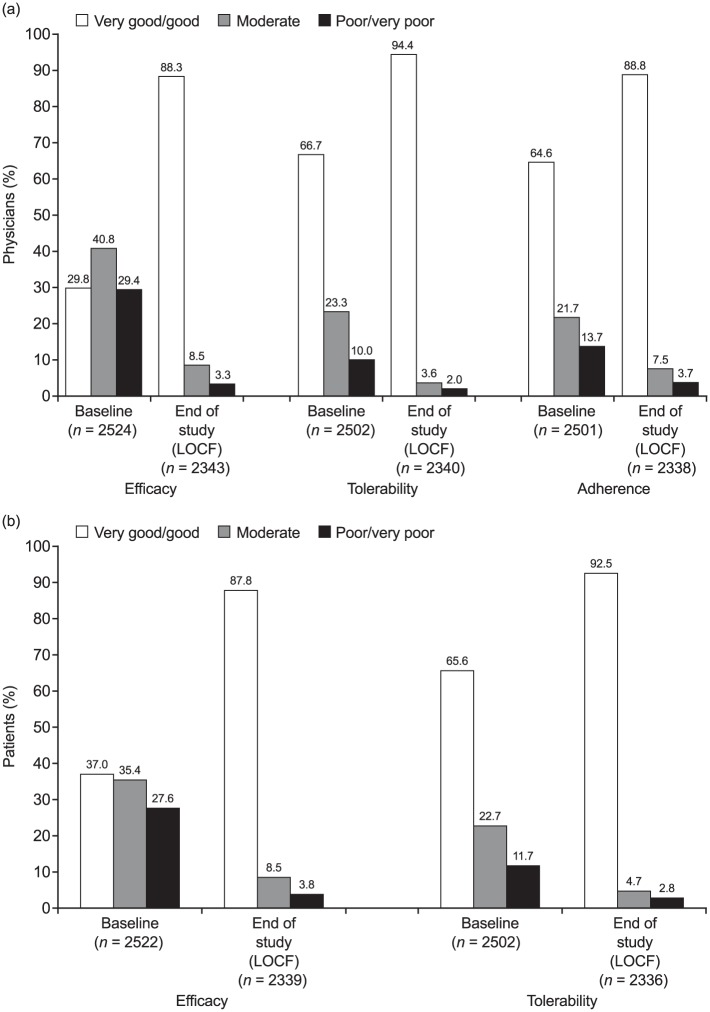
Physicians indicated improvements in their rating of the efficacy and tolerability of fluticasone/formoterol compared with the prior treatment (baseline), as well as in their assessment of their patients’ adherence to the treatment [Figure 3(a)]. The proportion of physicians rating treatment efficacy as ‘good’ or ‘very good’ increased from 29.8% at baseline to 88.3% at end of study, with increases from 66.7% to 94.4% for tolerability and 64.6% to 88.8% for adherence. Patient-rated satisfaction also indicated improvements in opinions of efficacy and safety [Figure 3(b)]. The proportion of patients rating efficacy as ‘good’ or ‘very good’ increased from 37.0% to 87.8%; the proportion for tolerability increased from 65.6% to 92.5%.
Figure 3.
Satisfaction with treatment rated by (a) physicians and (b) patients.
Percentages are based on number of patients with data available at each respective time point.
LOCF, last observation carried forward, up to 12 months.
On-study healthcare resource use
The mean (SD) number of days of absence from work/school/college/university or of days with inability to perform everyday activities due to asthma within a 30-day period decreased from 1.4 (3.9) at baseline to 0.3 (2.0) at the end of study; the median (range) was 0 (0–30) days at both time points.
During the 12-month observation period, in which there was a total on-treatment exposure duration of 874,188 days (2393.4 patient-years), the total number of asthma-related unscheduled visits was 659, with a mean (SD) annualized rate of 0.36 (1.33) and range 0.0–22.8; the total number of asthma-related emergency visits was 72, with mean (SD) annualized rate of 0.04 (0.43) and range 0.0–15.2; the total number of asthma-related hospital admissions was 24, with mean (SD) annualized rate of 0.01 (0.28) and range 0.0–12.2, and the total number of asthma-related days spent at hospital was 138, with mean (SD) annualized rate of 0.1 (2.58) and range 0.0–121.8. The numbers of individual events include participating patients who may have contributed more than one event to each count within the timescale analysed.
During the study, 373 (14.7%) patients had at least one recorded use of systemic corticosteroid due to asthma, and 479 (18.9%) patients had at least one recorded use of antibiotic due to lung/lower respiratory tract infection.
Discussion
The value of supplementing the findings of classical RCTs with observational data from real-world studies is increasingly recognized.22 RCTs maintain a vital role in supporting the approval of new products, owing to their high internal validity and the robustness of evidence they generate.23 More pragmatic trials, however, provide an opportunity to confirm the efficacy and safety of these products in populations that are representative of true clinical practice, by including patients who would typically be excluded from RCTs, such as those who smoke, are overweight, or have comorbidities generally excluded in RCTs.21–23 The need for real-world evidence is especially relevant in respiratory medicine, where treatment efficacy relies upon good inhaler technique and patient engagement in treatment regimens.23 It is important to demonstrate the level of effectiveness that can be achieved in the absence of the rigorous training in inhaler use that typically features in the design of RCTs in patients with asthma. In recognition of these issues, a number of pragmatic RCTs and observational studies have been conducted in heterogeneous asthma populations in recent years.25–27
Together with a 12-month non-interventional study of fluticasone/formoterol conducted in Germany (ffAiRNeSS),28 the PASS reported here was conducted as part of an agreed EU RMP. The current study was designed with the primary objective of collecting safety information during routine clinical use of fluticasone/formoterol, and also monitored the effectiveness of this ICS/LABA in achieving asthma control in real-world practice across eight European countries. The study population was inclusive of patients who would have been excluded from the pivotal RCTs that supported approval of fluticasone/formoterol; for example, smokers (10.8%), and patients with concurrent medical conditions at the baseline visit such as COPD (2.6%) or respiratory tract infection (1.5%).
Notwithstanding the diversity of the enrolled population, incorporating patients with comorbidities and with ‘real-world’ behaviour relating to inhaler technique and adherence to medication, the safety profile of fluticasone/formoterol was consistent with the RCT data. Most AEs recorded in the PASS were considered not to be related to fluticasone/formoterol and few patients experienced SAEs (4.2%; none related to treatment). The rate of SAEs was comparable to that reported in other 12-month studies of fluticasone/formoterol, such as the open-label phase III study (2.1% reported SAEs)9 and the real-world ffAiRNeSS study (4.0%),28 and the 13% of patients who reported SAEs during fluticasone furoate/vilanterol treatment in the asthma Salford Lung Study, for example.29 The most common AEs considered to be related to fluticasone/formoterol were asthma exacerbation (2.0%) and dysphonia (1.8%), which mirrors the finding in the 12-month open-label phase III study.9 Dysphonia was also the most common treatment-related AE in the ffAiRNeSS study.28 The rates of bronchopneumonia and oropharyngeal candidiasis were low (0.1% in both cases).
Analysis of effectiveness of fluticasone/formoterol in this PASS revealed improvements similar to those seen in other observational studies, and indeed RCTs. The change in the mean total ACTTM score from baseline to the end of this study (from 16.3 to 20.4) is similar to the change seen over 12 months in the ffAiRNeSS study (from 16.3 to 19.8).28 Correspondingly, the increase in the proportion of patients with controlled asthma (total ACTTM score ⩾ 20) from 29.4% at baseline to 67.4% at end of study is similar to the increases observed with fluticasone/formoterol in the ffAiRNeSS study (30.9–62.4%).28 In the asthma Salford Lung Study, 28% of patients had controlled asthma (ACTTM total score of ⩾20) at baseline.29 At 24 weeks, 71% of patients with uncontrolled asthma at baseline had achieved asthma control or had an increase of ⩾3 points in ACTTM score from baseline; statistically significant findings were also seen at 12 months. It is important to note the wider definition for treatment success used in the Salford Lung Study compared with the current study.29,30 The mean AQLQ score in the current study increased from 4.7 at baseline to 5.6 at end of study; once more, an improvement consistent with that reported in the ffAiRNeSS study (4.7–5.6).28 The pivotal RCTs of fluticasone/formoterol typically featured predose FEV1 as the primary outcome. In a phase III trial with fluticasone/salmeterol as an active comparator, for example, the least-squares mean (95% CI) change from baseline in predose FEV1 with fluticasone/formoterol at 12 weeks was 0.196 l (0.12, 0.28).4 At 3 months in the current study (data for this time point not presented earlier), there was a mean (95% CI) change from baseline in FEV1 of 0.112 l (0.09, 0.14). It is not appropriate to directly compare these two values, as the former study presented least-squares mean data; nonetheless, the current data support a beneficial effect of fluticasone/formoterol on lung function in real-world practice.
During the observational period of this study, 9.8% patients experienced at least one severe asthma exacerbation, compared with 35.8% in the 12 months prior to the start of the study. This on-study incidence of severe asthma exacerbations was higher than that observed in the ffAiRNeSS study (5.9%)28 or another 12-month phase III open-label study (2%),9 but the observed reduction in exacerbation rate is nonetheless encouraging given the broad population and real-world setting of the current study. Analysis of pooled clinical data from two open-label trials of fluticasone/formoterol has suggested that this treatment is associated with a lower rate of exacerbations requiring oral corticosteroids than other ICS/LABA combinations.31
The current study provided a useful insight into the basis for treatment decisions. Nearly half (45.3%) of patients who were already treated with ICS/LABA switched to fluticasone/formoterol on the basis of a lack of efficacy with their previous asthma medication. The proportion of physicians rating treatment efficacy as ‘good’ or ‘very good’ increased from 29.7% to 88.3% from baseline to the end of the fluticasone/formoterol treatment period, and the patient ratings were similar (37.0% to 87.8%). These improvements are reflected in the high proportion of patients who continued to received fluticasone/formoterol after the study (96% of those who completed the study and 81% overall).
By including patients who may not have met the inclusion criteria for pre-approval RCTs, this PASS has provided data on the long-term safety and effectiveness of fluticasone/formoterol that are more representative of patients observed in normal clinical practice than previously available. For example, the study population contained patients with comorbid conditions such as diabetes mellitus (71 patients, 2.8%). Whilst not contraindicated, conditions such as diabetes present a need for additional caution when prescribing fluticasone/formoterol.24 This was relevant to only a very small proportion of the overall study population; nonetheless, documented data on such patients are valuable for informing practice.
As a PASS of non-interventional design, this study was inevitably associated with certain limitations. Patient visits and assessments were performed according to local clinical practice or standard of care leading to a high variability in the frequency and schedule of patient visits and the assessments conducted at these visits. Consequently, the availability of data at the prespecified time points varied considerably. LOCF was used to manage missing data for the end-of-study assessments of effectiveness, thereby pooling the data of the last known patient status under fluticasone/formoterol treatment within the study, regardless of time of discontinuation from, or completion of, the study.32 Furthermore, since the primary objective of our research was to collect data on the safety of fluticasone/formoterol, the study was not powered for analysis of effectiveness. Thus, results of the exploratory inferential statistical analyses done for the effectiveness parameters (for example, in ACTTM and AQLQ scores, and lung function parameters) must therefore be interpreted in this context.
Conclusion
This 12-month PASS, conducted as part of an agreed EU RMP, supports the use of fluticasone/formoterol in clinical practice as a well tolerated and efficacious therapy for patients with moderate to severe asthma. No specific safety signals were observed. Fluticasone/formoterol was associated with improved asthma control and reduced incidence of severe asthma exacerbations compared with patients’ prior asthma treatment. These improvements were reflected in both physician- and patient-rated assessments of treatment efficacy and tolerability.
Supplemental Material
Supplemental material, Supplementary_data for Non-interventional study of the safety and effectiveness of fluticasone propionate/formoterol fumarate in real-world asthma management by Vibeke Backer, Adam Ellery, Sylvia Borzova, Stephen Lane, Magda Kleiberova, Peter Bengtsson, Tadeusz Tomala, Dominique Basset-Stheme, Carla Bennett, Dirk Lindner, Arthur Meiners and Tim Overend in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors would like to thank all investigators, site personnel, and study participants. We would also like to thank Dr Susanna Cuadripani (Mundipharma Research Limited, Cambridge, UK) for her assistance in reviewing this manuscript. Medical writing support was provided by Dr Becky Fox-Spencer and funded by Mundipharma Research Limited.
Footnotes
Conflict of interest statement: CB is an employee of Mundipharma Research Limited. TO was an employee of Mundipharma Research Limited at the time this study was conducted. DL was a contractor of Mundipharma Research GmbH & Co. KG at the time this study was conducted. AM is currently a consultant for Mundipharma Research GmbH & Co. KG. VB has received a patient fee corresponding to the number of patients included by the study sponsor. PB received grants from Mundipharma during the conduct of the study. AE, SB, SL, MK, TT, and DBS report that they have nothing to disclose.
Funding: This study was funded by Mundipharma Research Limited.
Trademark statement: Flutiform® is a registered trademark of Jagotec AG.
Contributor Information
Vibeke Backer, Department of Respiratory Medicine, Bispebjerg University Hospital, Copenhagen, Denmark.
Adam Ellery, Cape Cornwall Surgery, Penzance, Cornwall, UK St. Just.
Sylvia Borzova, FELICEM, s.r.o., Michalovce, Slovakia.
Stephen Lane, Professorial Respiratory Centre, Tallaght Hospital, Dublin, Ireland.
Magda Kleiberova, ALERGO Kleiberova, s.r.o., Rychnov nad Kneznou, Czech Republic.
Peter Bengtsson, Kvartersakuten Morby Centrum, Danderyd, Sweden.
Tadeusz Tomala, Svelvik legesenter, Svelvik, Norway.
Dominique Basset-Stheme, Allergology Practice, Valence, France.
Carla Bennett, Mundipharma Research Limited, Cambridge Science Park, Milton Rd, Cambridge, CB4 0GW UK.
Dirk Lindner, Mundipharma Research GmbH and Co. KG, Limburg, Germany.
Arthur Meiners, Mundipharma Research GmbH and Co. KG, Limburg, Germany.
Tim Overend, Mundipharma Research Limited, Cambridge, UK.
References
- 1. European Medicines Agency Decision. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Flutiform_29/WC500125700.pdf (2012, accessed 10 April 2018).
- 2. Bodzenta-Lukaszyk A, Buhl R, Balint B, et al. Fluticasone/formoterol combination therapy versus budesonide/formoterol for the treatment of asthma: a randomized, controlled, non-inferiority trial of efficacy and safety. J Asthma 2012; 49(10): 1060–1070. [DOI] [PubMed] [Google Scholar]
- 3. Aalbers R, Brusselle G, McIver T, et al. Onset of bronchodilation with fluticasone/formoterol combination versus fluticasone/salmeterol in an open-label, randomized study. Adv Ther 2012; 29(11): 958–969. [DOI] [PubMed] [Google Scholar]
- 4. Bodzenta-Lukaszyk A, Dymek A, McAulay K, et al. Fluticasone/formoterol combination therapy is as effective as fluticasone/salmeterol in the treatment of asthma, but has a more rapid onset of action: an open-label, randomized study. BMC Pulm Med 2011; 11: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bodzenta-Lukaszyk A, Pulka G, Dymek A, et al. Efficacy and safety of fluticasone and formoterol in a single pressurized metered dose inhaler. Respir Med 2011; 105(5): 674–682. [DOI] [PubMed] [Google Scholar]
- 6. Corren J, Mansfield LE, Persteva T, et al. Efficacy and safety of fluticasone/formoterol combination therapy in patients with moderate-to-severe asthma. Respir Med 2013; 107(2): 180–195. [DOI] [PubMed] [Google Scholar]
- 7. Nathan RA, D’Urzo A, Blazhko V, et al. Safety and efficacy of fluticasone/formoterol combination therapy in adolescent and adult patients with mild-to-moderate asthma: a randomised controlled trial. BMC Pulm Med 2012; 12: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bodzenta-Lukaszyk A, Van Noord J, Schröder-Babo W, et al. Efficacy and safety profile of fluticasone/formoterol combination therapy compared to its individual components administered concurrently in asthma: a randomised controlled trial. Curr Med Res Opin 2013; 29(5): 579–588. [DOI] [PubMed] [Google Scholar]
- 9. Mansur AH, Kaiser K. Long-term safety and efficacy of fluticasone/formoterol combination therapy in asthma. J Aerosol Med Pulm Drug Deliv 2013; 26(4): 190–199. [DOI] [PubMed] [Google Scholar]
- 10. Barnes PJ. Scientific rationale for using a single inhaler for asthma control. Eur Respir J 2007; 29(3): 587–595. [DOI] [PubMed] [Google Scholar]
- 11. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J 2002; 19(1): 182–191. [DOI] [PubMed] [Google Scholar]
- 12. Woolcock A, Lundback B, Ringdal N, et al. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med 1996; 153(5): 1481–1488. [DOI] [PubMed] [Google Scholar]
- 13. Greening AP, Ind PW, Northfield M, et al. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Allen & Hanburys Limited UK Study Group. Lancet 1994; 344(8917): 219–224. [DOI] [PubMed] [Google Scholar]
- 14. Szefler SJ, Martin RJ, King TS, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol 2002; 109(3): 410–418. [DOI] [PubMed] [Google Scholar]
- 15. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev 2010(4): CD005533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Powell H, Gibson PG. Inhaled corticosteroid doses in asthma: an evidence-based approach. Med J Aust 2003; 178(5): 223–225. [DOI] [PubMed] [Google Scholar]
- 17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev 2010(5): CD005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naedele-Risha R, Dorinsky P, Craig TJ. Dual components of optimal asthma therapy: scientific and clinical rationale for the use of long-acting beta-agonists with inhaled corticosteroids. J Am Osteopath Assoc 2001; 101(9): 526–533. [PubMed] [Google Scholar]
- 19. Marceau C, Lemiere C, Berbiche D, et al. Persistence, adherence, and effectiveness of combination therapy among adult patients with asthma. J Allergy Clin Immunol 2006; 118(3): 574–581. [DOI] [PubMed] [Google Scholar]
- 20.Global initiative for asthma. Global strategy for asthma management and prevention, 2017, http://www.ginasthma.org (accessed 17 May 2017). [Google Scholar]
- 21. Wong GW, Miravitlles M, Chisholm A, et al. Respiratory guidelines: which real world? Ann Am Thorac Soc 2014; 11(Suppl. 2): S85–S91. [DOI] [PubMed] [Google Scholar]
- 22. Price D, Brusselle G, Roche N, et al. Real-world research and its importance in respiratory medicine. Breathe (Sheff) 2015; 11(1): 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roche N, Reddel H, Martin R, et al. Quality standards for real-world research: focus on observational database studies of comparative effectiveness. Ann Am Thorac Soc 2014; 11(Suppl. 2): S99–S104. [DOI] [PubMed] [Google Scholar]
- 24. Napp Pharmaceuticals Ltd. Flutiform summary of product characteristics. https://www.medicines.org.uk/emc/product/4277/smpc (accessed 10 April 2018).
- 25. Collier S, Harvey C, Brewster J, et al. Monitoring safety in a phase III real-world effectiveness trial: use of novel methodology in the Salford Lung Study. Pharmacoepidemiol Drug Saf 2017; 26(3): 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. New JP, Diar Bakerly N, Leather D, et al. Obtaining real-world evidence: the Salford Lung Study. Thorax 2014; 69(12): 1152–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Usmani OS, Kemppinen A, Gardener E, et al. A randomized pragmatic trial of changing to and stepping down fluticasone/formoterol in asthma. J Allergy Clin Immunol Pract 2017; 5(5): 1378–1387. [DOI] [PubMed] [Google Scholar]
- 28. Schmidt O, Petro W, Hoheisel G, et al. Real-life effectiveness of asthma treatment with a fixed-dose fluticasone/formoterol pressurised metered-dose inhaler: results from a non-interventional study. Respir Med 2017; 131: 166–174. [DOI] [PubMed] [Google Scholar]
- 29. GlaxoSmithKline. Relvar Ellipta significantly improved asthma control in Salford Lung Study patients compared with their usual care. http://www.gsk.com/en-gb/media/press-releases/relvar-ellipta-significantly-improved-asthma-control-in-salford-lung-study-patients-compared-with-their-usual-care/ (2017, accessed 17 May 2017).
- 30. Leather D, Vestbo J, Diar Bakerly N, et al. Effectiveness of fluticasone furoate/vilanterol (FF/VI) compared to usual care (UC) in patients with asthma: the Salford Lung Study (SLS). Eur Respir J 2017; 50: OA3193. [Google Scholar]
- 31. Papi A, Mansur AH, Pertseva T, et al. Long-term fluticasone propionate/formoterol fumarate combination therapy is associated with a low incidence of severe asthma exacerbations. J Aerosol Med Pulm Drug Deliv 2016; 29(4): 346–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med 2012; 367(14): 1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_data for Non-interventional study of the safety and effectiveness of fluticasone propionate/formoterol fumarate in real-world asthma management by Vibeke Backer, Adam Ellery, Sylvia Borzova, Stephen Lane, Magda Kleiberova, Peter Bengtsson, Tadeusz Tomala, Dominique Basset-Stheme, Carla Bennett, Dirk Lindner, Arthur Meiners and Tim Overend in Therapeutic Advances in Respiratory Disease