Abstract
Objective: To describe vision-related quality of life (VRQoL) in a Norwegian population of patients with newly diagnosed neovascular age-related macular degeneration (nAMD), and to evaluate the association with demographic data of age, gender, and civil status (married, cohabitants/unmarried, not cohabitants). Method: The Norwegian version of the National Eye Institute Visual Functioning Questionnaire–25 (NEI VFQ-25) questionnaire was administered to 145 patients recently diagnosed with nAMD. We used descriptive statistics and bivariate analysis to determine the distribution of demographic parameters and a possible association between demographic parameters and NEI VFQ-25 scores. Spearman correlation was employed to analyze the NEI VFQ-25 items and subscales scores. Results: Mean (SD) VFQ-25 total score was 78.5 (14.7). The scores per subscales varied from 64.7 to 89.7. No significant difference was revealed between demographic parameters and the NEI VFQ-25 items, except for one item (being with others) when comparing paired and nonpaired participants. Conclusion: In a Norwegian population with newly diagnosed nAMD, VRQoL is reported at a high baseline level. This is an important information for the health care personnel when discussing expectations during treatment with the patient. Age, gender, and civil status did not affect VRQoL.
Keywords: age-related macular degeneration, NEI VFQ-25, demographics
Introduction
Age-related macular degeneration (AMD) is a progressive disease, affecting the central retina, and causing destructive and irreversible changes in the sharp-sightedness. There are two basic subtypes of AMD: nonneovascular or dry AMD and neovascular AMD (nAMD), also called exudative or wet AMD. Wet AMD is characterized by choroidal neovascularization, with formation of abnormal blood vessels, leading to subretinal and intraretinal macular edema, hemorrhage, fibrosis, or a combination, causing rapid central vision loss. Dry AMD is much more common and causes a slow vision declining over many years (Lindsley, Li, Ssemanda, Virgili, & Dickersin, 2016). Worldwide, AMD is increasing and becoming a prevalent condition (Ferris et al., 2013). The expected almost 200 million in 2020 will increase to almost 290 million in 2040 (Wong et al., 2014). Based on the findings in the European population 65 years and older, the authors estimate that 3.3% have AMD in at least one eye (Augood et al., 2006). Studies from the Nordic countries show a prevalence of late AMD between 3.5% and 9.5% with the highest prevalence from Greenland (Andersen et al., 2008; Erke, 2013; Erke et al., 2012; Gunnlaugsdottir, Arnarsson, & Jonasson, 2013). AMD increases dramatically with age (Andersen et al., 2008; Björnsson, Syrdalen, Bird, Peto, & Kinge, 2006; Korb et al., 2014). Gender is rarely a factor that influences its prevalence (Erke, 2013; Korb et al., 2014; Wong et al., 2014). However, one study indicates an association of nAMD with female gender (Rudnicka et al., 2012).
Studies have highlighted treatment effects, epidemiology, genetics, risk factors, and quality of life (QoL) to better understand how people live with AMD (Lim, Mitchell, Seddon, Holz, & Wong, 2012; Slakter & Stur, 2005; Yuzawa, Fujita, Tanaka, & Wang, 2013). Reduction in QoL is associated with wet AMD and has been measured with standardized instruments leaving additional knowledge regarding aspects of the disease (Matamoros et al., 2015; Swamy, Chia, Wang, Rochtchina, & Mitchell, 2009). Aspects such as psychological well-being and daily functioning cannot be captured by one single clinical measurement. However, the impact of the disease on patient’s QoL is also an important factor (Yuzawa et al., 2013), and vision-related quality of life (VRQoL) is one of the main outcomes measured in existing registries and major clinical trials. Using specific patient reported outcome measures (PROM) instruments among AMD patients is recommended by the International Consortium for Health Outcomes Measurement (ICHOM; Rodrigues et al., 2016).
To clarify the impact of AMD on QoL requires large clinical trials where researchers use the same QoL instrument (Slakter & Stur, 2005). The National Eye Institute Visual Functioning Questionnaire–25 (NEI VFQ-25) is a disease-specific instrument that measures VRQoL. Studies show that this instrument is suitable to measure vision-related function in patients with AMD (Suner et al., 2009). Two studies from Scandinavia, a Swedish and a Danish study, have used NEI VFQ-25 in samples comprising persons with eye diseases (Granström, 2016; Sørensen, Andersen, Henningsen, Larsen, & Sørensen, 2011). To date, no Norwegian studies within the same area have been performed. NEI VFQ-25 has been used in many studies examining different eye diseases, however, mostly within AMD. The instrument has been tested in several studies concluding that it has good psychometric properties as a measure of visual function-related outcomes on patients with AMD (Owen et al., 2006).
The aim of this study is to describe VRQoL measured by NEI VFQ-25 in a Norwegian patient population recently diagnosed with exudative AMD, and to evaluate the association with demographic data of age, gender, and civil status.
Method
Participants and Study Design
This cross-sectional study comprises patients with clinical diagnoses of nAMD in at least one eye. Participants were recruited at Department of Ophthalmology, Oslo University Hospital in Norway, between February 2015 and September 2016. Adult Scandinavian-speaking patients of both genders, without any documented cognitive impairment, were eligible for participation in this study. All attendees left a written consent form before taking part in this study. Patients who had received treatment with intravitreal injections earlier were excluded. The study was approved by the Regional Committees for Medical and Health Research (REK South East).
Data Collection Procedures and Measures
This study evaluated baseline data from 145 consecutive patients, who were newly diagnosed with wet AMD at the outpatient clinic. The data collection was by interviewer-administered questionnaire because of the difficulties for the patients to read the questionnaire themselves. The patients who were unable to respond to the questionnaire at the hospital completed the form by telephone interview with the first or second author. As two different investigators performed the interviews, a previous training was given to administer the form in the same way according to the protocol.
The NEI VFQ-25 Questionnaire
The NEI VFQ-25 was developed at the National Eye Institute, in Maryland, USA. It is a shorter version of the 51-item NEI VFQ, containing 25 items, which generate 12 subscales for the following dimensions of vision-target QoL: general vision, difficulty with near-vision activities, difficulty with distance-vision activities, limitations in social functioning, role limitations, dependency on others, mental health symptoms, driving difficulties, limitations with peripheral, color vision, ocular pain, and general health. The score produced for the NEI VFQ-25 converts the pre-coded numeric values of items to a score from 0 to 100. Higher scores reflect better QoL (Mangione et al., 1998; Mangione et al., 2001). Using the Rasch model to determine the validity of NEI VFQ-25 for use in people with low vision, Marella et al. (2010) suggests that the NEI VFQ-25 has better validity when split into visual functioning and socioemotional scales. Other studies suggest that the instrument has demonstrated good reliability and construct validity to measure vision-related functioning outcomes in patients with AMD (Mangione et al., 2001; Revicki, Rentz, Harnam, Thomas, & Lanzetta, 2010). Correlations between the NEI VFQ-25 versions of each subscale and their respective long-form version greater than .90 (Mangione et al., 2001) support good validity of the instrument. The construct validity of the NEI VFQ-25 is supported by moderate to strong correlations observed in another study (Orr et al., 2011). Correlations between NEI VFQ-25 scores and visual acuity in the better (r = .68, p < .0001) and worse (r = .46, p < .0001) seeing eyes support the convergent validity of the NEI VFQ-25 (Revicki et al., 2010). The Norwegian version of this instrument, translated into the Norwegian language by Mapi Research Trust (Lyon, France), was used in the present study after permission.
Statistical Methods
Data were analyzed by IBM using SPSS for PC, version 23. Descriptive statistics were used to determine the distribution of demographic and clinical characteristics. The primary outcome VRQoL was measured with the NEI VFQ-25 corresponding to the concept’s content identified by Mangione et al. (1998) including work-related duties, seeing in brightness, seeing movies and sports, general trouble seeing, low illumination, driving at night, mood, mental health, seeing clearly, driving in daytime, and reading.
The description of the population included the following parameters: age, gender, and civil status. To determine whether there was an association between age (divided by the median age into groups of those younger and those older than 82 years, respectively), gender, and civil status (dichotomized to paired vs. nonpaired according to whether they were married/cohabitants or not), and NEI VFQ-25 composite and subscales score, bivariate analyses were performed. The t test was used to compare mean values between groups. We employed the Spearman approach to analyze the correlation between the NEI VFQ-25 subscales scores.
Results
Descriptive Analyses—Patient Demographics and Characteristics
A total of 145 patients completed the questionnaire. Their mean age and the standard deviation (SD) were 81.7 (7.5) years (range: 61-99 years). The sample consisted of 73% females, 58% were in nonpaired relationship, and 89% were retired. As the majority of the respondents were retired, we decided not to include the variable “employment” in the statistical analyses. Patient’s characteristics are summarized in Table 1.
Table 1.
Demographic Data of the Study Participants.
| Total patient sample (n = 145) | % | |
|---|---|---|
| Female/male | 106/39 | 73/27 |
| Age, years | 100 | |
| M (SD) | 81.7 (7.6) | |
| Range | 61-99 | |
| Age 60-70 | 12 | 8.3 |
| Age 71-80 | 48 | 33.1 |
| Age 81-90 | 65 | 44.8 |
| Age >90 | 20 | 13.7 |
| Paired/nonpaired | 60/83 | 42/58 |
| Retired/non-retired | 129/16 | 89/11 |
NEI VFQ-25 Scores
Mean (SD) NEI VFQ-25 total score was 78.5 (14.7), range (36.4-99.4). The scores per subscales varied from 47.2 (22.3) for the subscale “General health” to 93.6 (14.5) for the subscale “Color vision.” “General health” and “General vision” revealed the lowest scores. Mean scores for the NEI VFQ-25 are summarized in Table 2.
Table 2.
Scores for Each Subscale and the Total Score of NEI VFQ-25.
| Subscale NEI VFQ-25 questionnaire |
M | SD | Range | n |
|---|---|---|---|---|
| General health | 47.28 | 22.33 | 0-100 | 138 |
| General vision | 56.95 | 18.22 | 20-100 | 138 |
| Ocular pain | 86.14 | 20.5 | 12.5-100 | 138 |
| Near activities | 71.55 | 22.4 | 8.3-100 | 138 |
| Distance activities | 73.75 | 24.2 | 0-100 | 137 |
| Social functioning | 83.33 | 22.3 | 0-100 | 138 |
| Mental health | 76.79 | 20.18 | 12.5-100 | 138 |
| Role difficulties | 68.29 | 29.01 | 0-100 | 138 |
| Dependency | 89.73 | 17.03 | 8.3-100 | 138 |
| Driving | 64.67 | 37.51 | 0-100 | 71 |
| Color vision | 93.65 | 14.50 | 25-100 | 138 |
| Peripheral vision | 92.93 | 15.98 | 25-100 | 138 |
| Total score | 78.48 | 14.69 | 36.4-99.4 | 138 |
Note. NEI VFQ = National Eye Institute Visual Functioning Questionnaire.
NEI VFQ-25 Scores by Age
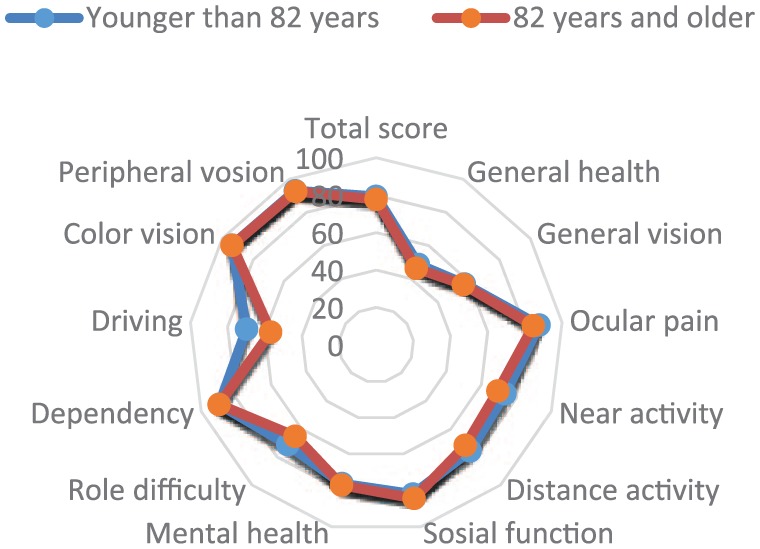
The NEI VFQ-25 mean (SD) composite scores by age were 79.4 (13.8) for participants younger than 82 years, and 77.73 (15.5) for participants 82 years and older. The scores per subscales varied from 48.5 (24.8) (General health) to 93.7 (15.2) (Color vision) among participants younger than 82 years, and from 46.0 (19.8) (General health) to 93.6 (13.9) (Color vision) for participants 82 years and older. The results did not reveal any significant difference between the two groups either by the composite score or by subscale scores. Figure 1 presents the comparison between age groups and the NEI VFQ-25 items, in terms of mean scores and connected SD.
Figure 1.
NEI VFQ-25 subscales scores by age.
Note. NEI VFQ = National Eye Institute Visual Functioning Questionnaire.
NEI VFQ-25 Scores by Gender
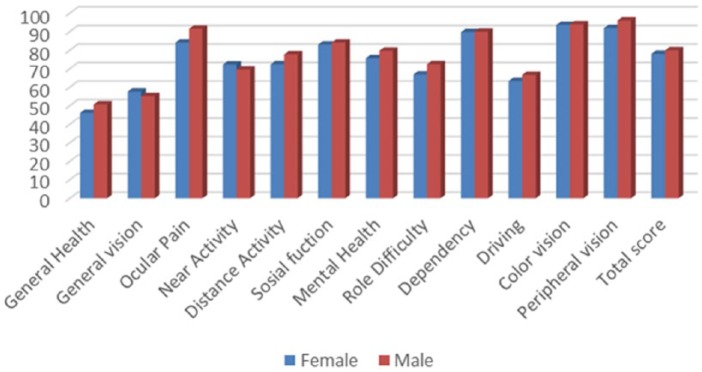
Mean (SD) composite scores by gender were 78.0 (15.0) for women and 79.9 (13.7) for men. The scores per subscales varied from 46.0 (22.0) (General health) to 93.6 (15.2) (Color vision) among the female participants, and from 50.7 (23.2) (General health) to 95.6 (13.8) (Peripheral vision) for men.
The highest difference between groups, while still nonsignificant, was for the “Ocular pain” subscale for which men had a higher score. Mean scores by gender are represented in Figure 2.
Figure 2.
NEI VFQ-25 subscales scores by gender.
Note. NEI VFQ = National Eye Institute Visual Functioning Questionnaire.
NEI VFQ-25 Scores by Civil Status
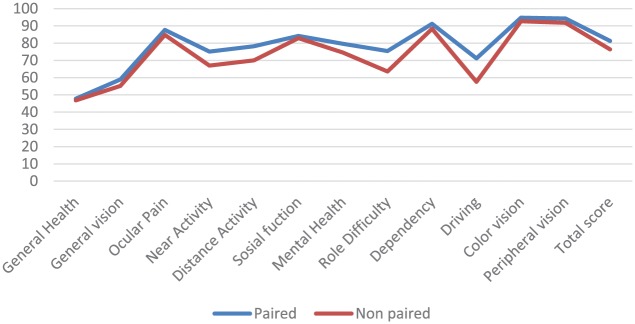
The total, composite scores (mean and SD) for the NEI VFQ-25 were 81.4 (12.4) for paired and 76.4 (16.0) for nonpaired participants. The subscales varied from 47.8 (22.3) (General health) to 94.7 (12.2) (Ocular pain) for paired and from 46.8 (22.8) (General health) to 92.7 (16.0) (Color vision) for nonpaired. Mean NEI VFQ-25 total scores by civil status showed no significant differences (see Figure 3).
Figure 3.
NEI VFQ-25 subscales scores by civil status.
Note. NEI VFQ = National Eye Institute Visual Functioning Questionnaire.
Analyzing the 25 single items scores of the NEI VFQ-25 questionnaire by civil status showed significant difference between groups for Item 13, “Being with others.” The nonpaired group had a score of 2.2, and the paired group a score of 1.6 (p = .038).
Correlation Analyses
Table 3 shows Spearman’s correlation coefficients between the 12 subscales of the NEI VFQ-25 questionnaire. For all subscales except “General health,” “Ocular pain,” “Color vision,” and “Peripheral vision,” the p value was lower than .05. The four subscales described above comprehend different aspects, that is, health, pain, and vision. There were correlations between subscales affected by AMD such as “Distance activities” and “Near activities.”
Table 3.
Correlation Matrix.
| General health | General vision | Ocular pain | Near activities | Distance activities | Social functioning | Mental health | Role difficulties | Dependency | Driving | Color vision | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| General vision | .09 p = .31 |
||||||||||
| Ocular pain | .18 p = .04 |
.20 p = .01 |
|||||||||
| Near activities | .93 p = .27 |
.57 p < .001 |
.18 p = .03 |
||||||||
| Distance activities | .20 p = .02 |
.52 p < .001 |
.31 p < .001 |
.63 p < .01 |
|||||||
| Social functioning | .20 p = .02 |
.48 p < .001 |
.16 p = .06 |
.60 p < .001 |
.63 p < .001 |
||||||
| Mental health | .19 p = .03 |
.56 p < .001 |
.28 p < .001 |
.53 p < .001 |
.57 p < .001 |
.60 p < .001 |
|||||
| Role difficulties | .23 p < .001 |
.55 p < .001 |
.35 p < .001 |
.59 p < .001 |
.58 p < .001 |
.54 p < .01 |
.58 p < .001 |
||||
| Dependency | .23 p < .01 |
.47 p < .01 |
.21 p = .01 |
.63 p < .001 |
.61 p < .001 |
.67 p < .001 |
.65 p < .001 |
.59 p < .001 |
|||
| Driving | −.01 p = .92 |
.55 p < .01 |
−.01 p = .90 |
.58 p < .001 |
.73 p < .01 |
.68 p < .001 |
.50 p < .001 |
.54 p < .001 |
.62 p < .001 |
||
| Color vision | .17 p = .84 |
.14 p = .08 |
.02 p = .78 |
.21 p = .01 |
.11 p = .19 |
.14 p = .11 |
.05 p = .52 |
.15 p = .07 |
.16 p = .05 |
.25 p = .03 |
|
| Peripheral vision | .03 p = .68 |
.20 p = .01 |
.21 p = .01 |
.22 p = .01 |
.24 p = .00 |
.39 p < .001 |
.34 p < .001 |
.21 p = .01 |
.26 p < .001 |
.32 p < .001 |
.14 p = .10 |
Comparison Internationally
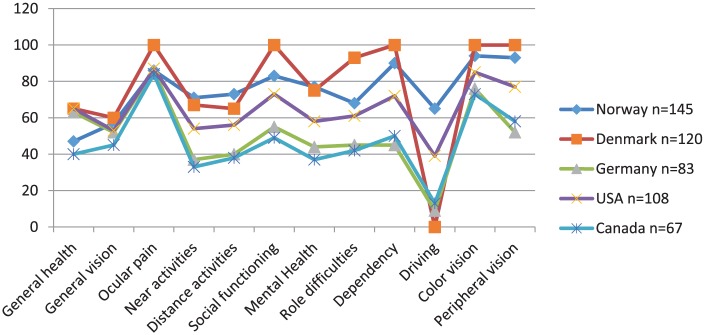
We compared the results in terms of NEI VFQ-25 scores from this Norwegian study with data from Denmark, Germany, United States, and Canada using the same instrument (Cruess, Zlateva, Xu, & Rochon, 2007; Mangione et al., 2001; Pauleikhoff et al., 2009; Sørensen et al., 2011). As Figure 4 shows, scorings from the studies performed in Denmark and Norway reveal the highest values. This finding could be expected due to these countries’ close connection to each other regarding citizenship, geographic distance, and similar health care system.
Figure 4.
Overview of NEI VFQ-25 subscales scores in different studies with AMD patients.
Note. NEI VFQ = National Eye Institute Visual Functioning Questionnaire; AMD = age-related macular degeneration.
Discussion
Results from the present study show that in a Norwegian population with newly diagnosed nAMD, VRQoL is reported at a high baseline level. The highest scores were for the subscales “Ocular pain,” “Color vision,” “Peripheral vision,” and “Distance activities,” which are subscales typically insensitive to AMD (Gyawali, Paudel, & Adhikari, 2012; Marella et al., 2010; Miskala et al., 2003; Sengupta et al., 2014); and low scores for the subscales “General vision,” “Near activities,” and “Driving,” which are the most sensitive subscales to AMD (Broman et al., 2002; Frennesson, Nilsson, Peebo, & Nilsson, 2010). This indicates that most of the study’s participants do not feel pain or discomfort in or around their eyes, and have no difficulties seeing colors, picking out, and matching own clothes. Furthermore, they have no problems noticing objects off to the side while they are walking along, they can read street signs or the names of stores, and they still go out to see movies, plays, or enjoy sports events. On the contrary, they report difficulties with their general vision, reading ordinary print in newspapers, doing work or hobbies that require seeing well up close, and driving during day and night time.
The lowest mean value from the NEI VFQ-25 scores was shown for “General health.” This finding might be explained by the high mean age when comparing with another Scandinavian study, which included younger participants (Sørensen et al., 2011). Furthermore, as we have no data on comorbidity, other diseases in addition to the AMD may affect these patients leaving a low rating for the participant’s self-reported general health. However, other studies present similar findings for this subscale (Bertelmann et al., 2016; Broman et al., 2002). The lowest score second to “General health” is shown by the “General vision” subscale. Our results can coincide with the fact that “General vision” is impaired by AMD and is one of the greatest decreases in scores of those with monocular impairment (Broman et al., 2002). Compared with the other Scandinavian study (Sørensen et al., 2011), our results showed a high score (86.14) on the subscale “Ocular pain.” This is one of the most insensitive subscales to nAMD. Different studies show mean scores corresponding to the general population and reference samples, suggesting that nonvascular AMD is a disease, which does not cause ocular pain (Marella et al., 2010).
The “Near activities” subscale score shows a value of 71.0, and we found a frequency of 8.3% of the respondents who reported that they had given up reading because of impaired vision. A total of 29.7% reported limited difficulties reading. As this subscale is one of the most sensitive to AMD disease, our results suggest moderate impairment.
Low scores in the “Social function” subscale might indicate considerably poor vision and/or high mean age. Our study revealed a score of 83.0 in this subscale, although the samples’ high mean age. This could indicate that our participants may have relatively good subjective vision.
The high score of 76.7 by the subscale “Mental health” leaves a pattern typically the same as the “Social functioning” scale, which also show similar scores when comparing scoring-values from other studies. This can indicate a close relationship between “Social functioning” and “Mental health.” A study reports that people with decreased vision show a higher percentage of individuals feeling irritable, fatigued, and disinterested in everyday experiences. This can be accompanied by sadness, tearfulness, and depression (Mojon-Azzi, Sousa-Poza, & Mojon, 2008). Our participants’ high score can indicate a modest influence of AMD on “Mental health.”
The subscale “Role difficulties” implies that patients accomplish less because of impaired vision that limits the length of activities. A low score in this subscale can be related to considerably poor vision (Gyawali et al., 2012). If such assumed, we can expect that our participants’ score of 68 show a moderate visual impairment.
The “Dependency” subscale score of 89.7 follows the same trend as “Social functioning” and “Mental health.” This finding can indicate that these subscales correlate with each other. From the correlation matrix, we read relatively high values which is in accordance to other studies (Simão, Lana-Peixoto, Araújo, Moreira, & Teixeira, 2008; Wang, Chan, & Jin, 2008). Our study’s high score can indicate that Norwegian participants reported modest “Social difficulties.”
Results from “Driving subscale” are difficult to interpret, as almost 50% of our participants did not drive. This result with high levels of missing data for the driving items corresponds to other studies (Marella et al., 2010). Driving has a particularly strong association with QoL, especially in some countries where public transportation is limited or not available. Being able to drive offers independency to older individuals (Sengupta et al., 2014). The scores left for the “Dependency” subscale are high (90.0), while the scores given for the subscale “Driving” are lower. This could be interpreted as satisfaction, that is, that the participants in our study have good transportation alternatives.
Subjects with AMD have well-preserved peripheral vision (Sengupta et al., 2014). Low scores for this subscale can indicate that participants suffer from other eye diseases, which cause changes in the peripheral vision. Our study’s high mean score (92.9) may indicate that most of our participants do not suffer from other eye diseases than AMD.
To compare our results with other studies performed in countries both close and distant from Norway, we used data from Denmark investigating QoL in patients with AMD by using the NEI VFQ-25 questionnaire (Sørensen et al., 2011). In addition, one study from Canada (Cruess, Zlateva, Xu, & Rochon, 2007), one from Germany, (Pauleikhoff et al., 2009), and the original study for the development of the instrument (Mangione et al., 2001) were selected. These studies present results for NEI VFQ-25 scores in participants without visual impairment, and total scores showed a high self-reported vision-related QoL in control groups (Cruess et al., 2007; Lotery, Xu, Zlatava, & Loftus, 2007; Mangione et al., 2001; Pauleikhoff et al., 2009; Sørensen et al., 2011). Sørensen found that the control group scored higher on the subscales where AMD is known to affect “Visual performance,” while there was no difference between the control group and patients on the subscales “Ocular pain,” “Color vision,” and “Peripheral vision.” Those subscales are less affected by AMD. Cruess et al.’s (2007) study found that subjects with nAMD scored significantly lower than controls for all but one scale of the NEI VFQ-25 (Ocular pain). Results from the comparison of these studies showed NEI VFQ-25 total scores varying from 48.0 to 78.0. Our study presents a total score of 78.0, which is equal to the Danish score, and higher than the other studies’ results. VRQoL from our study are negatively affected by AMD; however, patients still scored high on QoL measured with NEI VFQ-25. The overview of NEI VFQ-25 subscales scores for the different studies leaves reflection on cultural suitability of the instrument. The original NEI VFQ-25 is American and as Norway and Denmark, the neighbors US and Canada have corresponding values presented. However, the values from Germany and Canada are directly overlapping. We have no explanation for this finding except that these countries represent the Western world and we assume alike health care systems. When we look at the scorings “Near activities,” higher values were found in the Norwegian sample, which may be attributed to a higher level of visual status. Another explanation is clinically important declines shown for visual acuity, which do not result in declines in the NEI VFQ-25 scales. This may be due to adaption to vision loss. Patients may have low expectations for their vision-targeted QoL because of their high age. The presence of other diseases may also cause impairment in daily life. The high score from our participants could possibly be explained by the health system efficiency in identifying the disease before serious consequences occurred. The interviewer-administered format could have influenced patient ability to answer honestly for fear of appearing to complain too much about their health issues.
Strengths and Limitations
The use of a standardized instrument is always an advantage. In addition, availability of other studies presenting comparative data on the same instrument is crucial. However, further research is needed to examine which factors affect VRQoL in patients with wet AMD, especially their “General health.”
Some limitations should be considered when interpreting the results. First, we were unable to compare the scores of the NEI VFQ-25 with healthy participants as a reference group. Second, non-Norwegian speaking patients were excluded thus the sample represents only Norwegian speaking people, thus the participants may not be completely generalized to the greater AMD population. However, we think that the study sample is representative for patients with classic or occult wet forms of AMD who are seeking treatment. Another limitation is that the NEI VFQ-25 was designed and validated for the American language and culture (Mangione et al., 2001), and it has not yet been validated after its translation to Norwegian. A main limitation in our analyses of VRQoL was the lack of data connected to the AMD multiple dimensions of mono-/bi-lateral, visual acuity and comorbidity. Inclusion of those aspects in further research will leave important knowledge.
This study being the first using the NEI VFQ-25 questionnaire in Norway reveals that the participants had a very high overall NEI VFQ-25 score compared with similar studies in other countries. We found no significant differences when comparing gender, age groups, and civil status. Overall, “General health,” “General vision,” and “Driving” are the most affected aspects in this study. “Ocular pain,” “Peripheral vision,” “Dependency,” and “Color vision” are least affected, followed by “Mental health,” “Distance activity,” “Near activity,” and “Role difficulty.” Being aware that age, gender, and civil status, which are nonmodifiable factors, do not affect VRQoL on patients with wet AMD gives us an indication that other modifiable aspects may affect it. These findings can be of interest to clinicians, researchers, and health service managers for the development of appropriate help for these patients.
Acknowledgments
The authors thank Mapi Research Trust for providing the National Eye Institute Visual Functioning Questionnaire–25 (NEI VFQ-25) translation into Norwegian.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ana Carla Schippert  https://orcid.org/0000-0002-3993-2337
https://orcid.org/0000-0002-3993-2337
Ellen Grov  https://orcid.org/0000-0002-5347-8812
https://orcid.org/0000-0002-5347-8812
References
- Andersen M. V. N., Rosenberg T., la Cour M., Kiilgaard J. F., Prause J. U., Alsbirk P. H., . . . Bird A. C. (2008). Prevalence of age-related maculopathy and age-related macular degeneration among the Inuit in Greenland: The Greenland Inuit Eye Study. Ophthalmology, 115, 700-707. [DOI] [PubMed] [Google Scholar]
- Augood C. A., Vingerling J. R., de Jong P. T., Chakravarthy U., Seland J., Soubrane G., . . . Rahu M. (2006). Prevalence of age-related maculopathy in older Europeans: The European Eye Study (EUREYE). Archives of Ophthalmology, 124, 529-535. [DOI] [PubMed] [Google Scholar]
- Bertelmann T., Feltgen N., Scheffler M., Hufenbach U., Wiedon A., Wilhelm H., Ziemssen F. (2016). Vision-related quality of life in patients receiving intravitreal ranibizumab injections in routine clinical practice: Baseline data from the German OCEAN study. Health and Quality of Life Outcomes, 14(1), Article 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsson Ó. M., Syrdalen P., Bird A. C., Peto T., Kinge B. (2006). The prevalence of age-related maculopathy (ARM) in an urban Norwegian population: The Oslo Macular Study. Acta Ophthalmologica Scandinavica, 84, 636-641. [DOI] [PubMed] [Google Scholar]
- Broman A. T., Munoz B., Rodriguez J., Sanchez R., Quigley H. A., Klein R., . . . West S. K. (2002). The impact of visual impairment and eye disease on vision-related quality of life in a Mexican-American population: Proyecto VER. Investigative Ophthalmology & Visual Science, 43, 3393-3398. [PubMed] [Google Scholar]
- Cruess A., Zlateva G., Xu X., Rochon S. (2007). Burden of illness of neovascular age-related macular degeneration in Canada. Canadian Journal of Ophthalmology, 42, 836-843. [DOI] [PubMed] [Google Scholar]
- Erke M. G. (2013). Age-related macular degeneration: Prevalence and risk factors—a cross-sectional study: The Tromsø Study 2007/2008 (Doctoral thesis). University of Tromsø, Norway. [Google Scholar]
- Erke M. G., Bertelsen G., Peto T., Sjølie A. K., Lindekleiv H., Njølstad I. (2012). Prevalence of age-related macular degeneration in elderly Caucasians: The Tromsø Eye Study. Ophthalmology, 119, 1737-1743. [DOI] [PubMed] [Google Scholar]
- Ferris F. L., Wilkinson C., Bird A., Chakravarthy U., Chew E., Csaky K, . . . Beckman Initiative for Macular Research Classification Committee. (2013). Clinical classification of age-related macular degeneration. Ophthalmology, 120, 844-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frennesson C., Nilsson U. L., Peebo B. B., Nilsson S. E. (2010). Significant improvements in near vision, reading speed, central visual field and related quality of life after ranibizumab treatment of wet age-related macular degeneration. Acta Ophthalmologica, 88, 420-425. [DOI] [PubMed] [Google Scholar]
- Granström T. (2016). Anti-VEGF treatment of patients with diabetic macular edema: Studies of visual acuity, macular edema and patient-reported outcomes. Uppsala, Sweden: Uppsala Universitet. [DOI] [PubMed] [Google Scholar]
- Gunnlaugsdottir E., Arnarsson Á., Jonasson F. (2013). Visual impairment and blindness in Icelanders aged 50 years and older—The Reykjavik Eye Study. Laeknabladid, 99(3), 123-127. [DOI] [PubMed] [Google Scholar]
- Gyawali R., Paudel N., Adhikari P. (2012). Quality of life in Nepalese patients with low vision and the impact of low vision services. Journal of Optometry, 5, 188-195. [Google Scholar]
- Korb C. A., Kottler U. B., Wolfram C., Hoehn R., Schulz A., Zwiener I., . . . Mirshahi A. (2014). Prevalence of age-related macular degeneration in a large European cohort: Results from the population-based Gutenberg Health Study. Graefe’s Archive for Clinical and Experimental Ophthalmology, 252, 1403-1411. [DOI] [PubMed] [Google Scholar]
- Lim L. S., Mitchell P., Seddon J. M., Holz F. G., Wong T. Y. (2012). Age-related macular degeneration. The Lancet, 379, 1728-1738. [DOI] [PubMed] [Google Scholar]
- Lindsley K., Li T., Ssemanda E., Virgili G., Dickersin K. (2016). Interventions for age-related macular degeneration: Are practice guidelines based on systematic reviews? Ophthalmology, 123, 884-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotery A., Xu X., Zlatava G., Loftus J. (2007). Burden of illness, visual impairment and health resource utilisation of patients with neovascular age-related macular degeneration: Results from the UK cohort of a five-country cross-sectional study. British Journal of Ophthalmology, 91, 1303-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangione C. M., Berry S., Spritzer K., Janz N. K., Klein R., Owsley C., Lee P. P. (1998). Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire. Archives of Ophthalmology, 116, 227-233. [DOI] [PubMed] [Google Scholar]
- Mangione C. M., Lee P. P., Gutierrez P. R., Spritzer K., Berry S., Hays R. D. (2001). Development of the 25-list-item National Eye Institute Visual Function Questionnaire. Archives of Ophthalmology, 119, 1050-1058. [DOI] [PubMed] [Google Scholar]
- Marella M., Pesudovs K., Keeffe J. E., O’Connor P. M., Rees G., Lamoureux E. L. (2010). The psychometric validity of the NEI VFQ-25 for use in a low-vision population. Investigative Ophthalmology & Visual Science, 51, 2878-2884. [DOI] [PubMed] [Google Scholar]
- Matamoros E., Maurel F., Léon N., Solomiac A., Bardoulat I., Joubert M., . . . Souied E. H. (2015). Quality of life in patients suffering from active exudative age-related macular degeneration: The EQUADE Study. Ophthalmologica, 234, 151-159. [DOI] [PubMed] [Google Scholar]
- Miskala P. H., Hawkins B. S., Mangione C. M., Bass E. B., Bressler N. M., Dong L. M., . . . Submacular Surgery Trials Research Group. (2003). Responsiveness of the National Eye Institute Visual Function Questionnaire to changes in visual acuity: Findings in patients with subfoveal choroidal neovascularization—SST Report No. 1. Archives of Ophthalmology, 121, 531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojon-Azzi S., Sousa-Poza A., Mojon D. S. (2008). Impact of low vision on well-being in 10 European countries. Ophthalmologica, 222, 205-212. [DOI] [PubMed] [Google Scholar]
- Orr P., Rentz A. M., Margolis M. K., Revicki D. A., Dolan C. M., Colman S., . . . Bressler N. M. (2011). Validation of the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25) in age-related macular degeneration. Investigative Ophthalmology & Visual Science, 52, 3354-3359. [DOI] [PubMed] [Google Scholar]
- Owen C. G., Rudnicka A. R., Smeeth L., Evans J. R., Wormald R. P., Fletcher A. E. (2006). Is the NEI-VFQ-25 a useful tool in identifying visual impairment in an elderly population? BMC Ophthalmology, 6(1), Article 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauleikhoff D., Scheider A., Wiedmann P., Gelisken F., Scholl H., Roider I., . . . Xu X. (2009). [Neovascular age-related macular degeneration in Germany. Encroachment on the quality of life and the financial implications]. Der Ophthalmologe: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft, 106, 242-251. [DOI] [PubMed] [Google Scholar]
- Revicki D. A., Rentz A. M., Harnam N., Thomas V. S., Lanzetta P. (2010). Reliability and validity of the National Eye Institute Visual Function Questionnaire-25 in patients with age-related macular degeneration. Investigative Ophthalmology & Visual Science, 51, 712-717. [DOI] [PubMed] [Google Scholar]
- Rodrigues I. A., Sprinkhuizen S. M., Barthelmes D., Blumenkranz M., Cheung G., Haller J., . . . McKibbin M. (2016). Defining a minimum set of standardized patient-centered outcome measures for macular degeneration. American Journal of Ophthalmology, 168, 1-12. [DOI] [PubMed] [Google Scholar]
- Rudnicka A. R., Jarrar Z., Wormald R., Cook D. G., Fletcher A., Owen C. G. (2012). Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: A meta-analysis. Ophthalmology, 119, 571-580. [DOI] [PubMed] [Google Scholar]
- Sengupta S., Van Landingham S. W., Solomon S. D., Do D. V., Friedman D. S., Ramulu P. Y. (2014). Driving habits in older patients with central vision loss. Ophthalmology, 121, 727-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão L. M., Lana-Peixoto M. A., Araújo C. R., Moreira M. A., Teixeira A. L. (2008). The Brazilian version of the 25-item National Eye Institute Visual Function Questionnaire: Translation, reliability and validity. Arquivosbrasileiros de oftalmologia, 71, 540-546. [DOI] [PubMed] [Google Scholar]
- Slakter J. S., Stur M. (2005). Quality of life in patients with age-related macular degeneration: Impact of the condition and benefits of treatment. Survey of Ophthalmology, 50, 263-273. [DOI] [PubMed] [Google Scholar]
- Sørensen M. S., Andersen S., Henningsen G., Larsen C. T., Sørensen T. L. (2011). Danish version of Visual Function Questionnaire-25 and its use in age-related macular degeneration. Danish Medical Bulletin, 58(6), A4290. [PubMed] [Google Scholar]
- Suner I. J., Kokame G. T., Yu E., Ward J., Dolan C., Bressler N. M. (2009). Responsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: Validation studies from two phase 3 clinical trials. Investigative Ophthalmology & Visual Science, 50, 3629-3635. [DOI] [PubMed] [Google Scholar]
- Swamy B. N., Chia E. M., Wang J. J., Rochtchina E., Mitchell P. (2009). Correlation between vision- and health-related quality of life scores. Acta Ophthalmologica, 87, 335-339. [DOI] [PubMed] [Google Scholar]
- Wang C.-W., Chan L.-W., Jin H.-Y. (2008). Psychometric properties of the Chinese version of the 25-item National Eye Institute Visual Function Questionnaire. Optometry and Vision Science, 85, 1091-1099. [DOI] [PubMed] [Google Scholar]
- Wong W. L., Su X., Li X., Cheung C. M. G., Klein R., Cheng C.-Y., Wong T. Y. (2014). Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. The Lancet Global Health, 2(2), e106-e116. [DOI] [PubMed] [Google Scholar]
- Yuzawa M., Fujita K., Tanaka E., Wang E. C. (2013). Assessing quality of life in the treatment of patients with age-related macular degeneration: Clinical research findings and recommendations for clinical practice. Clinical Ophthalmology, 7, 1325-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]