Abstract
Background:
Kidney biopsy is considered the gold standard for diagnosis of renal disease. It is increasingly performed in cases of diagnostic uncertainty, including in patients with coexistent diabetes and hypertension, for which a presumptive clinical diagnosis can be made. Little is known about the incidence and distribution of biopsy-proven kidney diseases. Changes in the distribution of biopsy diagnoses over time may have significant implications for resource allocation and future research.
Objective:
We studied the relative frequency of kidney diseases in Southern Alberta over the past 30 years, to determine whether the population-standardized annual biopsy rate and incidence of selected diagnostic categories have changed. We hypothesized an increasing incidence of renal biopsies and a growing proportion of nonglomerular diseases (eg, tubulointerstitial disorders) likely due to evolving indications for biopsy. Given the rise in obesity, diabetes, and aging population with chronic kidney disease (CKD), we anticipated a rise in nephroangiosclerosis and diabetic nephropathy over time.
Design:
Retrospective population-based cohort study using the Biobank for the Molecular Classification of Kidney Disease (BMCKD).
Setting:
Southern Alberta, Canada.
Patients:
All patients who underwent renal biopsy between 1985 and 2015 in our database.
Measurements:
We used descriptive and quantitative analysis to characterize demographics and biopsy-based diagnoses.
Methods:
We conducted a retrospective population-based cohort study to analyze all consecutive patients who underwent at least one kidney biopsy over a 30-year period in Southern Alberta (1985-2015). We considered the first adequate biopsy. We described the annual standardized incidence of biopsy-proven kidney diseases over time and summarized associated patient characteristics. We assumed a Poisson distribution for biopsy counts and used provincial demographic information to standardize rates.
Results:
During the study period, 6434 people (58% male; mean age: 47.9 years) underwent a kidney biopsy. The population-standardized annual biopsy rate increased from 10.8 biopsies per 100 000 person-years in the first 5 years of the study (1985-1989) to 18.2 biopsies per 100 000 person-years in the last 5 years (2010-2014). The mean age at the time of biopsy increased from 42.5 years (1985-1989) to 51.4 years (2010-2014). Glomerular diseases remained the most prevalent histopathological group, with a growing representation of diabetic kidney disease from 3.69% to 16.18%, and a relative decrease in the proportion of other glomerular diseases from 72.32% to 62.92% of glomerular diagnoses. Tubulointerstitial diseases increased from 5.87% to 7.36% of total diagnoses.
Limitations:
Classification schemes have changed over time, so recently recognized conditions may have been misclassified in earlier data. There was a changing group of pathologists and nephrologists over this period. Variations in interpretation and application of biopsy indications by physician may influence recorded prevalence of certain diagnoses. We do not yet have complete information on indications or patient outcomes linked to the database.
Conclusions:
In Southern Alberta, kidney biopsy is being utilized more frequently and in older people. Diabetic nephropathy is increasingly diagnosed, which may reflect either or both changes in the prevalence of causative factors and local biopsy practices.
Keywords: kidney biopsy, renal pathology, demographics, biopsy registry, database, epidemiology, incidence, glomerulonephritis, diabetic nephropathy, tubulointerstitial disease
Abrégé
Contexte:
La biopsie rénale est considérée comme la méthode par excellence pour diagnostiquer des néphropathies. Elle est de plus en plus pratiquée dans les cas de diagnostics incertains, notamment chez les patients souffrant également de diabète et d’hypertension, et pour lesquels un diagnostic clinique présomptif peut être posé. On en sait encore peu sur l’incidence et la distribution statistique des néphropathies avérées par biopsie. Des variations dans la distribution statistique des diagnostics par biopsie au fil du temps pourraient avoir des répercussions significatives sur l’affectation des ressources et sur les recherches futures.
Objectifs:
Nous voulions savoir si le taux de biopsies annuel normalisé selon la population et l’incidence des catégories de diagnostic sélectionnées avaient varié. Nous avions émis l’hypothèse d’une incidence croissante des biopsies rénales et d’une proportion croissante d’affections non glomérulaires (notamment les troubles tubulo-interstitiels) susceptibles d’être attribuables à une demande croissante pour des biopsies. Compte tenu de l’augmentation des cas d’obésité et de diabète, et du vieillissement de la population atteinte de néphropathies chroniques, nous avions anticipé une augmentation des cas de néphroangiosclérose et de néphropathie diabétique au fil du temps.
Type d’étude:
Une étude de cohorte rétrospective sur des données provenant de la biobanque BMCKD (Biobank for the Molecular Classification of Kidney Disease).
Cadre de l’étude:
Le sud de la province de l’Alberta, au Canada.
Sujets:
Tous les patients répertoriés dans notre base de données en raison d’une biopsie rénale subie entre 1985 et 2015.
Mesures:
Nous avons employé l’analyse descriptive et quantitative pour caractériser les données démographiques des patients et le diagnostic posé à la suite d’une biopsie.
Méthodologie:
Nous avons mené une étude de cohorte rétrospective pour examiner tous les patients consécutifs ayant subi au moins une biopsie rénale sur une période de 30 ans (1985 à 2015) dans le sud de l’Alberta. Nous avons tenu compte de la première biopsie satisfaisante. Nous avons mesuré l’incidence annuelle normalisée des néphropathies avérées par biopsie au fil du temps et nous avons résumé les caractéristiques des patients qui y étaient associés. Une distribution de Poisson a permis d’établir le nombre de biopsies, alors que les données démographiques provinciales ont servi à la normalisation des taux.
Résultats:
Au cours de la période étudiée, 6 434 personnes (âge moyen : 47,9 ans), dont 58 % étaient des hommes, ont subi une biopsie rénale. Entre les cinq premières années (1985-1989) et les cinq dernières années de l’étude (2010-2014), le taux de biopsies annuel normalisé selon la population est passé de 10,8 par 100 000 années-personnes à 12,8 par 100 000 années-personnes; l’âge moyen au moment de subir la biopsie est passé de 42,5 ans à 51,4 ans. Le groupe histopathologique des atteintes glomérulaires est demeuré le plus prévalent des diagnostics glomérulaires avec une représentation croissante des cas de néphropathies diabétiques (de 3,69 % à 16,18 %) et une diminution relative de la proportion des autres atteintes glomérulaires (de 72,32 % à 62,92 %). Quant aux affections tubulo-insterstitielles, elles sont passées de 5,87 % à 7,36 % de tous les diagnostics posés au cours de la période étudiée.
Limites:
Plusieurs variables ont évolué au cours de la période étudiée : les équipes de pathologistes et de néphrologues ont changé, et les systèmes de classification ont été modifiés; de sorte que certaines affections diagnostiquées récemment pourraient avoir été mal classées dans les données antérieures. De plus, des différences dans l’interprétation et l’application des biopsies de la part des médecins pourraient avoir influencé la prévalence consignée pour certains diagnostics. Nous ne disposons toujours pas d’informations complètes sur les indications et les résultats des patients liés à la base de données.
Conclusion:
Dans le sud de l’Alberta, la biopsie rénale est de plus pratiquée, et ce, chez des patients plus âgés. La néphropathie diabétique est de plus en plus diagnostiquée; ce qui pourrait indiquer des variations dans la prévalence des facteurs en cause et/ou des changements dans les pratiques locales quant à l’usage de la biopsie.
What was known before
Little is known about the incidence and distribution of biopsy-proven kidney diseases. Changes in the distribution of biopsy diagnoses over time may have significant implications for resource allocation and future research.
What this adds
This population-based study characterizes patient demographics and the evolution of biopsy-based kidney disease diagnosis over time in Southern Alberta.
Introduction
Since its establishment as a key diagnostic tool, kidney biopsy has become widely regarded as the gold standard for classifying renal disease.1-5 It is now a common procedure that informs both diagnosis and management of many kidney diseases. When biopsy occurs promptly, it can prevent or delay the course of progression to kidney failure with its associated costs, morbidity, and mortality.
Despite a recent trend toward the development of kidney biopsy registries, population-based data on clinical and histologic parameters at the time of kidney biopsy are scant. Until recently, these efforts have been limited to national or international referral center registries, which are subject to referral bias due to overrepresentation of rarer conditions.6-11 As distribution and incidence of these etiologies vary globally over time and may be highly affected by local standard practice patterns, population-based efforts are required to inform trends in disease spectrum and biopsy practices. These data can be used to inform health policies and resource allocation, refine pretest probabilities in clinical practice, and facilitate research collaborations.6-11
The recently developed Biobank for the Molecular Classification of Kidney Disease (BMCKD) provides a comprehensive registry to gain insight into biopsy patterns in a large Southern Alberta population. The aim of our retrospective study was to gather descriptive data to evaluate demographic characteristics and histopathologic diagnoses in a population-based cohort.
We sought to determine whether the population-standardized annual biopsy rate and incidence of selected diagnostic categories among first native kidney biopsies have changed over the last 3 decades in Southern Alberta. Given the rise in obesity, diabetes, and aging population with chronic kidney disease (CKD), we anticipated an increasing annual biopsy rate and changing distribution of disease to reflect our more elderly and comorbid patient population.
Methods
Study Design and Eligibility Criteria
We used the BMCKD to conduct a retrospective population-based cohort study of renal biopsies performed in Southern Alberta between 1985 and 2015. Kidney biopsies were performed as clinically indicated by the nephrologist, in both inpatient and outpatient settings as a day procedure. If patients had undergone more than one biopsy, we included data from the first adequate sample. We also separately grouped normal samples and those considered insufficient to make a diagnosis.
Population and Sources
The province of Alberta has the fastest growing population in Canada, totaling over 4.2 million people.12 In Southern Alberta, which includes Calgary and the surrounding area, the processing of renal pathology is centralized and conducted at Calgary Laboratory Services (CLS), located at the Foothills Medical Centre, in collaboration with Alberta Health Services (AHS) and the University of Calgary’s Department of Pathology and Laboratory Medicine (DPLM). The centralized organization of renal pathology services has enabled the assembly of a population-based cohort of adult and pediatric kidney biopsies performed in Southern Alberta. The BMCKD was established at the University of Calgary in 2015, in partnership with CLS, AHS, researchers, clinicians, and patients.13 The banked materials include paraffin and frozen tissue blocks, plastic-embedded tissue blocks for light and electron microscopy, histopathology slides, detailed pathology reports, and electron microscopy images. We extracted data on demographic characteristics, including age and sex at biopsy, biopsy date, and diagnoses from the Biobank database. Currently, we do not have information on indications for biopsy or patient outcomes linked to the biopsy data.
Classification Approach
We considered native and transplant biopsies separately and categorized native histopathologic diagnoses into 7 groups: glomerular diseases, diabetic nephropathy, deposition diseases, tubulointerstitial diseases, vascular diseases, hereditary kidney diseases, and any conditions not accounted for in the above groupings. Inadequate samples were excluded, and normal results grouped separately. In the event that categories were unclear based on the diagnostic information provided, a nephrologist and pathologist adjudicated the discrepancy. For instance, primary glomerulonephritis (GN) took precedence if found in a patient with coexisting diabetes- or hypertension-related changes.
Glomerular diseases were subclassified into proliferative and nonproliferative types. The proliferative diseases were divided into 5 main categories: immune (including IgA nephropathy, post-infectious GN, lupus nephritis, membranoproliferative GN), pauci-immune, anti-GBM (glomerular basement membrane), GN with monoclonal Ig deposits, and C3 glomerulopathy, as well as proliferative GN that could not be further subclassified. We classified glomerular diseases according to the 2016 consensus report on pathologic classification, diagnosis, and reporting of GN.14 Nonproliferative glomerular disease was separated into focal segmental glomerulosclerosis, minimal change disease, and membranous nephropathy. All other glomerular disease was included in a “not otherwise specified” group.
We reported diabetic nephropathy as a separate category. Deposition diseases were mainly amyloidosis. Tubulointerstitial diseases included acute tubular necrosis, interstitial nephritis, and other processes resulting in significant tubulointerstitial inflammation or damage. Vascular diseases were subgrouped into hypertensive pathologies as well as thrombotic microangiopathies. Alport, thin basement membrane, and polycystic kidney disease were classified as hereditary. Any conditions not accounted for in the above groupings were labeled as such.
Data Analysis
We used descriptive statistics to summarize age, sex, and histological diagnosis. Categorical values were expressed as absolute frequencies and percentages; age was summarized using mean and standard deviation. We summarized biopsy information as counts over person-time (rates). We obtained census information to standardize data according to the annual population in Southern Alberta. We represented rates as biopsies per 100 000 person-years over time in 5-year increments from 1985 to 2014 (periods A to F). We assumed a Poisson distribution to estimate measures of central tendency and variance of rates. Data were analyzed using the STATA statistical software package, version 13.1 (StataCorp LP, College Station, Texas).
Results
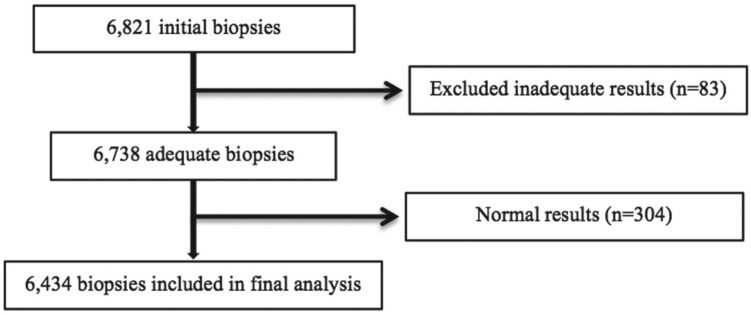
During the study period, 7327 patients underwent a renal biopsy, and 6434 of their biopsies (58% male; mean age: 47.9 ± 19.8 years) were ultimately included in the analysis (Figure 1 shows the enrollment process).
Figure 1.
Study population.
Note. From 1985 onward, the Biobank includes 6821 total renal biopsy specimens. This represents the first sample from each patient if multiple biopsies were done during this time frame. In all, 387 biopsies were inadequate or had no identifiable pathology. Thus, we analyzed 6434 samples in total.
The population-standardized annual biopsy rate has increased from 10.8 biopsies per 100 000 persons per year (1985-1989) to 18.2 biopsies per 100 000 persons per year (2010-2014) (Figure 2).
Figure 2.
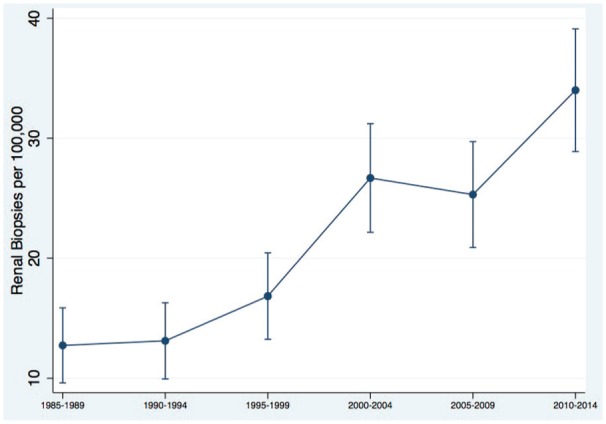
Population-standardized annual biopsy rate.
Biopsy samples tended to include older people in more recent years: mean age at the time of renal biopsy has risen from 42.5 ± 19 to 51.4 ± 16 years over that interval, with a larger proportion of people older than age 70 (Figure 3). There was a wide variation in study population age and sex by disease category. Hereditary diseases tended to be found among the youngest patients whereas deposition diseases were most often found in older patients (Figure 4).
Figure 3.
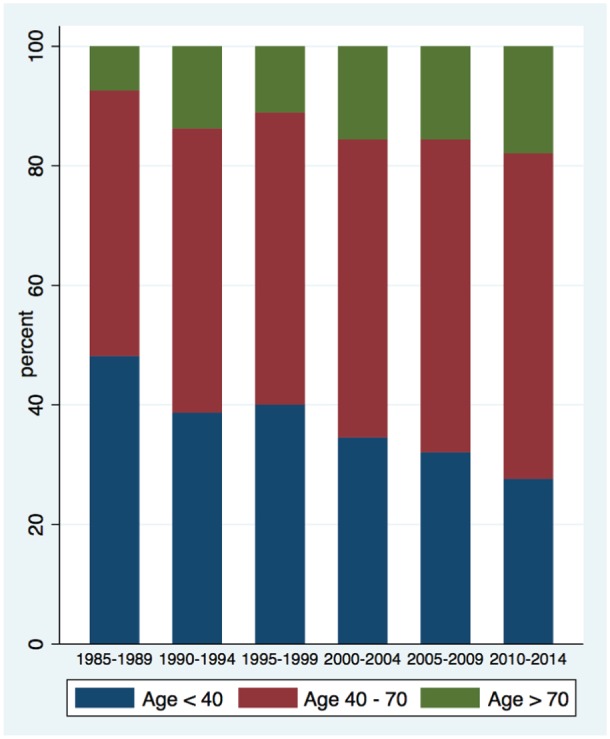
Proportion of renal biopsies performed on elderly patients over study period, by time period.
Figure 4.
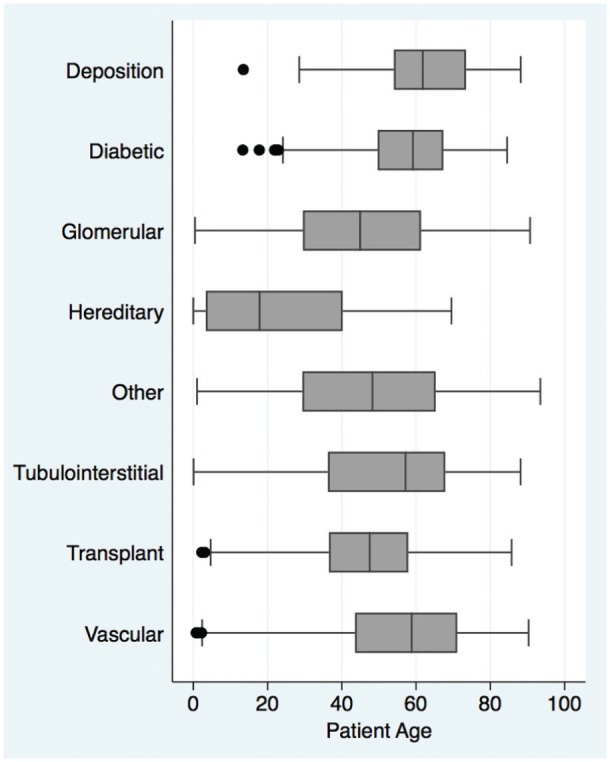
Age at time of biopsy according to diagnostic class.
The population-adjusted rate of normal biopsies varied between 0.24 and 1.26 biopsies per 100 000 persons per year but this did not consistently increase over time. Overall, the most prevalent etiology was glomerular disease (n = 3754), followed by transplant (n = 836), vascular (n = 566), diabetic (n = 523), tubulointerstitial (n = 411), deposition (n = 149), other (n = 124), and hereditary (n = 71) (Table 1).
Table 1.
Study Population Demographics and Distribution by Disease Category.
| Diagnostic categories | n (%) | Age at biopsy | Female (%) |
|---|---|---|---|
| Glomerular diseases | 3754 (58.35) | 45.04 ± 20.27 | 43.77 |
| Diabetic nephropathy | 523 (8.13) | 57.59 ± 13.50 | 41.30 |
| Deposition diseases | 149 (2.32) | 62.06 ± 13.76 | 43.62 |
| Tubulointerstitial diseases | 411 (6.39) | 51.89 ± 20.70 | 43.07 |
| Vascular diseases | 566 (8.80) | 56.34 ± 18.15 | 36.75 |
| Hereditary diseases | 71 (1.10) | 21.77 ± 20.37 | 33.80 |
| Transplant biopsies | 836 (12.99) | 46.59 ± 15.90 | 37.80 |
| Other | 124 (1.93) | 47.24 ± 21.60 | 38.71 |
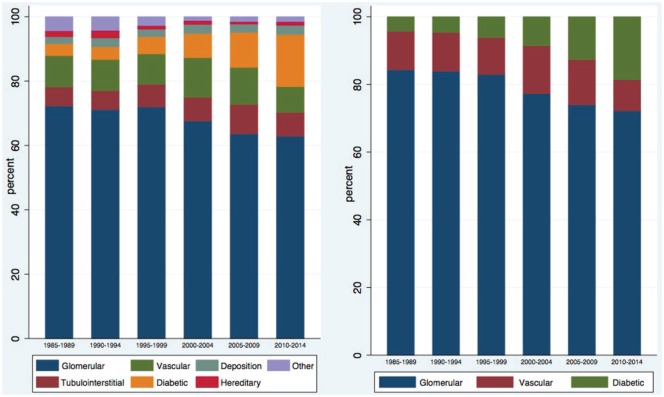
During this period, glomerular diseases remained the most prevalent pathologic grouping, though their relative proportion of the total has decreased from 72.32% to 62.92% (Table 2). There was an increasing representation of diabetic kidney disease from 3.69% to 16.18%, and tubulointerstitial diseases from 5.87% to 7.36%, as expected (Table 2 and Figure 5).
Table 2.
The Relative Frequencies of Histopathologic Diagnoses in Native Renal Biopsies by Time Period.
| Category | 1985-1989 | 1990-1994 | 1995-1999 | 2000-2004 | 2005-2009 | 2010-2014 |
|---|---|---|---|---|---|---|
| Glomerular | 431 (72.32%) | 434 (71.15%) | 525 (72.02%) | 739 (67.61%) | 719 (63.63%) | 906 (62.92%) |
| Diabetic | 22 (3.69%) | 24 (3.93%) | 39 (5.35%) | 82 (7.50%) | 123 (10.88%) | 233 (16.18%) |
| Deposition | 13 (2.18%) | 17 (2.79%) | 17 (2.33%) | 31 (2.84%) | 29 (2.57%) | 42 (2.92%) |
| Tubulointerstitial | 35 (5.87%) | 36 (5.90%) | 51 (7.00%) | 80 (7.32%) | 103 (9.12%) | 106 (7.36%) |
| Vascular | 58 (9.73%) | 59 (9.67%) | 69 (9.47%) | 135 (12.35%) | 130 (11.50%) | 115 (7.99%) |
| Hereditary | 11 (1.85%) | 14 (2.30%) | 8 (1.10%) | 13 (1.19%) | 9 (0.80%) | 16 (1.11%) |
| Other | 26 (4.36%) | 26 (4.26%) | 20 (2.74%) | 13 (1.19%) | 17 (1.50%) | 22 (1.53%) |
| Total | 596 | 610 | 729 | 1093 | 1130 | 1440 |
Figure 5.
The relative frequencies of histopathologic diagnoses by time period.
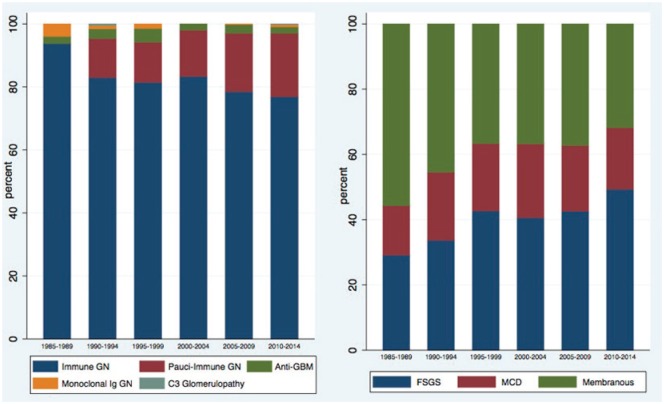
Further analysis of the proliferative and nonproliferative glomerular diseases revealed a growing incidence of pauci-immune GN and focal segmental glomerulosclerosis (FSGS) (Table 3 and Figure 6).
Table 3.
The Relative Frequencies of Glomerular Lesions by Time Period.
| Category | 1985-1989 | 1990-1994 | 1995-1999 | 2000-2004 | 2005-2009 | 2010-2014 |
|---|---|---|---|---|---|---|
| Proliferative | ||||||
| Immune | 166 (49.55%) |
161 (43.28%) |
224 (47.26%) |
335 (50.53%) |
340 (50.22%) |
416 (49.00%) |
| Pauci-immune | 0 | 24 (6.45%) |
35 (7.38%) |
59 (8.90%) |
80 (11.82%) |
109 (12.84%) |
| Anti-GBM | 4 (1.19%) |
6 (1.61%) |
12 (2.53%) |
8 (1.21%) |
12 (1.77%) |
11 (1.30%) |
| Monoclonal Ig | 7 (2.09%) |
2 (0.54%) |
4 (0.84%) |
0 | 1 (0.15%) |
4 (0.47%) |
| C3 Glomerulopathy | 0 | 1 (0.27%) |
0 | 0 | 0 | 1 (0.12%) |
| Nonproliferative | ||||||
| FSGS | 46 (13.73%) |
60 (16.13%) |
85 (17.93%) |
106 (15.99%) |
104 (15.36%) |
152 (17.90%) |
| MCD | 24 (7.16%) |
37 (9.95%) |
41 (8.65%) |
59 (8.90% |
49 (7.24%) |
58 (6.83%) |
| Membranous | 88 (26.27%) |
81 (21.77%) |
73 (15.40%) |
96 (14.48%) |
91 (13.44%) |
98 (11.54%) |
| Total | 335 | 372 | 474 | 663 | 677 | 849 |
Note. GBM = glomerular basement membrane; FSGS = focal segmental glomerulosclerosis; MCD = minimal change disease.
Figure 6.
The relative frequencies of proliferative and nonproliferative glomerular lesions by time period.
Note. GN = glomerulonephritis; GBM = glomerular basement membrane; FSGS = focal segmental glomerulosclerosis; MCD = minimal change disease.
Discussion
This study is the first to characterize the evolution of renal biopsy trends over time in Canada. We are performing biopsies more frequently, which may reflect improved accessibility of the procedure15,16 as well as changes in the clinical approach to CKDs, especially with the advent of new therapies. In our center, nephrologists performed renal biopsies until the mid-1990s, when interventional radiology began to provide this service. This change may have influenced the observed increased rates of kidney biopsy. We perform more renal biopsies annually compared with 30 years ago, even when this is adjusted for the rapid population growth of our province. Other similar studies from the United States have used referral center databases, which may influence the patient population included.10 In contrast, the BMCKD reflects the nature of renal biopsy in Canada, which is universally accessible and without cost to the patient. Many studies throughout North America, Europe, and Asia focus on GN alone.6-10 In one of the more comparable analyses from Denmark, the authors state their number of annual biopsies has remained relatively stable at 570, though this was not population-adjusted.11
The average age at time of biopsy is also increasing, which likely reflects population aging as well as a growing proportion of complex elderly patients referred to nephrology care. In keeping with national trends, the proportion of elderly individuals in Alberta is rising, and we expect this will continue to impact the distribution of biopsy-based diagnoses. About 12% of our population was older than 65 years in 2016, but this is projected to rise to at least 19% over the next 25 years.17
The incidence of diabetic nephropathy as diagnosed by biopsy has increased substantially over the observation period. It is unclear if this relates primarily to a changing incidence of this condition, a baseline population that has more preexisting diabetes, or a better appreciation for the potential of other kidney diseases to occur in a diabetic patient. Provincial data suggest the incident diabetes rate nearly doubled from 3 to 6 per 1000 between 1996 and 2008.18 In years past, longstanding diabetics with proteinuric kidney disease were likely less often confirmed by biopsy. However, with easier access to the procedure, we have observed an increasing interest in pathologic confirmation, especially to rule out other coexisting conditions that may significantly alter clinical management.19,20
Within the subgroups of proliferative and nonproliferative glomerular diseases, there appears to be a growing incidence of biopsy-proven pauci-immune GN and FSGS with a relatively decreased proportion accounted for by other conditions. In the case of FSGS, this may reflect a higher prevalence of risk factors including obesity in our patient population, as well as change in biopsy practice overall. Recent data show that 67% of Albertans are at least overweight, with 28% of those being obese, which is higher than the national average.21
The demographics of our population have changed over time, which we have documented in our biopsy registry. From a resource utilization perspective, this information informs individual and regional nephrologist practice in a unique way. Aggressive diseases that require immediate treatment, such as lupus nephritis and ANCA (antineutrophil cytoplasmic antibody)-associated vasculitis, benefit from rapid biopsy by interventional radiology and processing by pathology. We advocate for these services. Future studies are needed to correlate clinical indications for biopsy, biopsy practices and diagnosis, and patient outcomes. As the use of similar databases becomes more widespread, there will be opportunities for multicenter collaboration and data linkages.
This study is limited by its retrospective design and the fact that renal biopsy may not provide complete information as to the etiology of a patient’s kidney disease. Furthermore, as in the case of membranoproliferative GN, classification schemes and nomenclature have changed over time, so recently recognized conditions such as C3 glomerulopathy may have been misclassified in earlier data. Although it is considered the diagnostic gold standard, disease can be localized, and the biopsy may be subject to sampling error, which can affect all studies of this nature.22 In addition, there was a changing group of pathologists and nephrologists over this period. Variations in interpretation and application of biopsy indications by physician may influence recorded prevalence of certain diagnoses. We did not audit biopsy results and independently confirm recorded diagnoses for this study. We do not yet have complete information on indications or patient outcomes linked to the database. Despite these limitations, this study provides important and novel information on the epidemiology of biopsy-proven kidney disease diagnosis.
Conclusion
In this study, the incidence of performing renal biopsies has increased significantly over the past 30 years. The trend is toward performing more biopsies in older patients, who tend to be more comorbid and have patterns of renal disease consistent with this. We found an increasing proportion of diabetic nephropathy and tubulointerstitial diseases over time, which likely reflects changes in the prevalence of causative factors as well as local biopsy practices. Transplant patients account for a larger percentage of total annual biopsies in recent years.
These results have implications for nephrologists, as well as resource stewardship and associated costs. Future studies are needed to clarify biopsy indications, safety, and patient outcomes. While with the advent of renal biomarkers, this landscape may change significantly in coming years, renal biopsy still remains the most valuable means of accurately diagnosing kidney diseases.
Acknowledgments
We thank Michelle Nelson and Kevin Chapman for their assistance with the Biobank. Dr Susanna McRae assisted with categorizing histopathologic diagnoses.
Footnotes
Ethics Approval and Consent to Participate: The University of Calgary Research Ethics Board approved this study.
Consent for Publication: All authors give consent to the publication of this study.
Availability of Data and Materials: Supplementary data for this study is available upon request.
Author Contributions: AC performed the databank review, analyzed the data, and drafted the article. PR conceived the study, obtained the ethics approved, and assisted with the data analysis. All authors contributed to the completion of the article and approved the final version.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Biobank for the Molecular Classification of Kidney Disease (BMCKD) was supported by an infrastructure grant from the Canadian Foundation for Innovation. The authors received no other financial support for the research and authorship of this article.
ORCID iD: Pietro Ravani  https://orcid.org/0000-0001-6973-8570
https://orcid.org/0000-0001-6973-8570
References
- 1. Iversen P, Brun C. Aspiration biopsy of the kidney. Am J Med. 1951;11(3):324-330. [DOI] [PubMed] [Google Scholar]
- 2. Madaio MP. Renal biopsy. Kidney Int. 1990;38(3):529-543. [DOI] [PubMed] [Google Scholar]
- 3. Fuiano G, Mazza G, Comi N, et al. Current indications for renal biopsy: a questionnaire-based survey. Am J Kidney Dis. 2000;35(3):448-457. [DOI] [PubMed] [Google Scholar]
- 4. Paone DB, Meyer LE. The effect of biopsy on therapy in renal disease. Arch Intern Med. 1981;141(8):1039-1041. [PubMed] [Google Scholar]
- 5. Dhaun N, Bellamy CO, Cattran DC, Kluth DC. Utility of renal biopsy in the clinical management of renal disease. Kidney Int. 2014;85(5):1039-1048. [DOI] [PubMed] [Google Scholar]
- 6. McGrogan A, Franssen C, De Vries C. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):414-430. [DOI] [PubMed] [Google Scholar]
- 7. Briganti EM, Dowling J, Finlay M, et al. The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant. 2001;16(7):1364-1367. [DOI] [PubMed] [Google Scholar]
- 8. Simon P, Ramee MP, Boulahrouz R, et al. Epidemiologic data of primary glomerular diseases in Western France. Kidney Int. 2004;66(3):905-908. [DOI] [PubMed] [Google Scholar]
- 9. Rivera F, López Gómez JM, Pérez García R. Spanish registry of glomerulonephritis: clinicopathologic correlations of renal pathology in Spain. Kidney Int. 2004;66:898-904. [DOI] [PubMed] [Google Scholar]
- 10. Sim JJ, Batech M, Hever A, et al. Distribution of biopsy-proven presumed primary glomerulonephropathies in 2000-2011 among a racially and ethnically diverse US population. Am J Kidney Dis. 2016;68(4):533-544. [DOI] [PubMed] [Google Scholar]
- 11. Heaf J. The Danish renal biopsy register. Kidney Int. 2004;66:895-897. [DOI] [PubMed] [Google Scholar]
- 12. Statistics Canada. Alberta census profile 2016. Statistics Canada. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo02a-eng.htm. Published 2017. Accessed January 22, 2018.
- 13. Muruve DA, Mann MC, Chapman K, et al. The biobank for the molecular classification of kidney disease: research translation and precision medicine in nephrology. BMC Nephrol. 2017;18:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sethi S, Haas M, Markowitz GS, et al. Mayo clinic/renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol. 2016;27(5):1278-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torres Muñoz A, Valdez-Ortiz R, González-Parra C, Espinoza-Dávila E, Morales-Buenrostro LE, Correa-Rotter R. Percutaneous renal biopsy of native kidneys: efficiency, safety and risk factors associated with major complications. Arch Med Sci. 2011;7(5):823-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stiles KP, Yuan CM, Chung EM, Lyon RD, Lane JD, Abbott KC. Renal biopsy in high-risk patients with medical diseases of the kidney. Am J Kidney Dis. 2000;36(2):419-433. [DOI] [PubMed] [Google Scholar]
- 17. Hansen J. Population projection: Alberta and census divisions, 2016-2041. Treasury Board and Finance: Office of Statistics and Information—Demography. https://open.alberta.ca/dataset/90a09f08-c52c-43bd-b48a-fda5187273b9/resource/8aa3a7b0-fd9f-4b63-b985-ad06643991c9/download/2017-2041-Alberta-Population-Projections.pdf. Published 2017. Accessed July 9, 2018.
- 18. Johnson JA, Vermeulen SU. Epidemiological trends of diabetes in Alberta. Institute of Health Economics: Alberta Diabetes Atlas. https://www.ihe.ca/publications/alberta-diabetes-atlas-2007. Published 2007. Accessed January 22, 2018.
- 19. Richards NT, Greaves I, Lee SJ, Howie AJ, Adu D, Michael J. Increased prevalence of renal biopsy findings other than diabetic glomerulopathy in type II diabetes mellitus. Nephrol Dial Transplant. 1992;7(5):397-399. [PubMed] [Google Scholar]
- 20. Pham TT, Sim JJ, Kujubu DA, Liu IL, Kumar VA. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol. 2007;27:322-328. [DOI] [PubMed] [Google Scholar]
- 21. Diabetes, Obesity and Nutrition Strategic Clinical Network. A look at obesity in Alberta. Alberta Health Services. https://www.albertahealthservices.ca/assets/about/scn/ahs-scn-don-obesity-facts.pdf. Published 2014. Accessed July 9, 2018.
- 22. Corwin HL, Schwartz MM, Lewis EJ. The importance of sample size in the interpretation of the renal biopsy. Am J Nephrol. 1988;8:85-89. [DOI] [PubMed] [Google Scholar]