Abstract
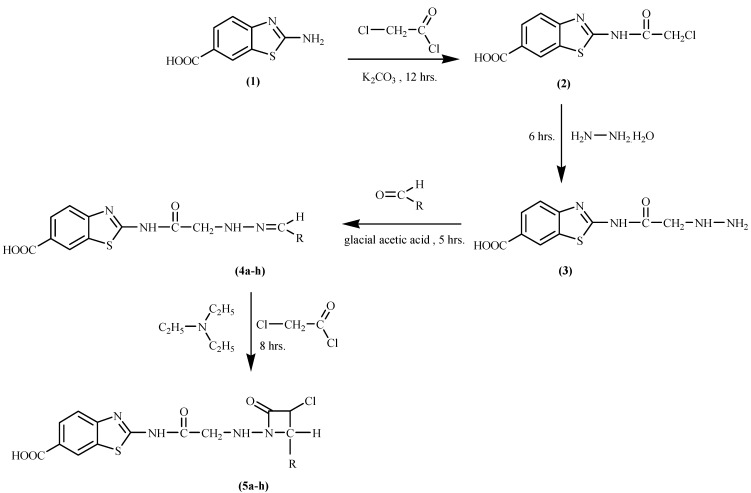
2-Aminobenzothiazole-6-carboxylic acid (1), on condensation with chloroacetyl chloride yielded 2-(2-chloroacetylamino)benzothiazole-6-carboxylic acid (2), which on amination with hydrazine hydrate yielded in turn 2-(2-hydrazinoacetylamino)benzo-thiazole-6-carboxylic acid (3). Compound 3, on condensation with various aromatic aldehydes afforded a series of 2-{2-[N’-(arylidene)hydrazino]acetylamino}benzothiazole-6-carboxylic acids 4a-h, which upon dehydrative annulation in the presence of chloroacetyl chloride and triethylamine yielded 2-{2-[3-chloro-2-(aryl)-4-oxoazetidin-1-ylamino]-acetylamino}benzothiazole-6-carboxylic acids 5a-h. The synthesized compounds 4a-h and 5a-h were screened for their antibacterial activity against four microorganisms: Staphylococcus aureus (Gram positive), Bacillus subtilis (Gram positive), Psuedomonas aeruginosa (Gram negative) and Escherichia coli (Gram negative). They were found to exhibit good to moderate antibacterial activity. The antifungal activity of these compounds were also tested against three different fungal species. None of them were active against the species tested.
Keywords: 2-Aminobenzothiazole, azetidinones, antibacterial activity.
Introduction
The β-lactam heterocycles are still the most prescribed antibiotics used in medicine. They are considered as an important contribution of science to humanity [1]. The most widely used antibiotics such as the penicillins, cephalosporins, carumonam, aztreonam, thienamycine and the nocardicins all contain β-lactam rings [2]. The long-term use of β-lactam antibiotics exerts selective pressure on bacteria and permits the proliferation of resistant organisms [3]. A comparative study of current antibiotics with those from previous decades shows an alarming increase in bacterial resistance to β-lactam antibiotics [4]. The development of several synthetic and semi-synthetic β-lactam antibiotics by the pharmaceutical industry was due to the growing resistance of bacteria towards the β-lactam antibiotics and the need for medicines with a more specific antibacterial activity [5]. An interesting group of β-lactams are the monocyclic β-lactams, which are molecules that do not contain another ring fused to the β-lactam one.
Azetidinones, which are part of the antibiotic structure, are known to exhibit interesting biological activities [6]. A large number of 3-chloro monocyclic β-lactams possess powerful antibacterial, antimicrobial, anti-inflammatory, anticonvulsant and antitubercular activity [7,8,9,10,11]. They also function as enzyme inhibitors and are effective on the central nervous system [12]. 2-Aminobenzothiazoles constitute another class of heterocycles that possess antimicrobial and various other pharmacological activities like diuretic, antiulcer, antihistamine and anticancer properties [13,14,15,16].
Rey et al. have described methods for the preparation of N-substituted-2-azetidinones, which are useful in the synthesis of taxol and taxol derivatives [17]. Patel. et al. have carried out the synthesis of azetidinone and thiazolidinone derivatives from 2-amino-6-(2-naphthalenyl)thiazolo[3,2-d]thiadiazole [18]. Singh and co-workers have prepared some new 2-azetidinones from N-(salicylidene)amines and 2-diazo-1,2-diarylethanones [19]. Singh has also reviewed β-lactams in the new millennium, i.e. monobactams and carbapenems [20].
Hence, with a view to further assess the pharmacological profile of this class of compounds, it was thought worthwhile to synthesize some new congeners of β-lactam heterocycles by incorporating the 2-aminobenzothiazole and azetidinone moieties in a single molecular framework. The present work deals with the synthesis of the title compounds starting from 2-aminobenzothiazole-6-carboxylic acid, followed by their antimicrobial screening.
Results and Discussion
Synthesis
2-Aminobenzothiazole-6-carboxylic acid (1) was prepared in quantitative yield according to a known method [21]. This compound, on condensation with chloroacetyl chloride yielded 2-(2-chloro-acetylamino)benzothiazole-6-carboxylic acid (2). Compound 2 on amination with hydrazine hydrate afforded 2-(2-hydrazinoacetylamino)benzothiazole-6-carboxylic acid (3). The condensation reaction of compound 3 with various aromatic aldehydes yielded 2-{2-[N’-(arylidene)hydrazino]acetylamino}-benzothiazole-6-carboxylic acids 4a-h. Compounds 4a-h, on reaction with chloroacetyl chloride in the presence of triethylamine underwent dehydrative annulation to afford 2-{2-[3-chloro-2-(aryl)-4-oxoazetidin-1-ylamino]acetylamino}benzothiazole-6-carboxylic acids 5a-h. These reactions are summarized in Scheme 1. Yields were moderate to fair (50-77%). The purity of the compounds was monitored by TLC and the structure of the compounds was deduced on the basis of their elemental analysis and spectral data. The substituents of the compounds are given in Table 1.
Scheme 1.
Table 1.
Substituents of compounds 4a-h and 5a-h.
| Compd. | Substituent R |
|---|---|
| 4a, 5a | 3-BrC6H4 |
| 4b, 5b | 4-OHC6H4 |
| 4c, 5c | C6H5 |
| 4d, 5d | 4-OCH3C6H4 |
| 4e, 5e | 2-ClC6H4 |
| 4f, 5f | 4-ClC6H4 |
| 4g, 5g | 3-NO2C6H4 |
| 4h, 5h | 4- NO2C6H4 |
Antibacterial activity
To determine the antibacterial activity of these agents, the cup plate method was used, with Ampicillin and Streptomycin as the reference antibiotics [22]. The prepared compounds were examined against two strains each of gram positive and gram negative bacteria. The test results, presented in Table 2, suggest that compounds 5e, 5g, 5h and 4e, 4f, 4g, 5f, 5h are highly active against S. aureus and E. coli respectively. Compounds 4g and 4h are also highly active against P. aeruginosa. The rest of the compounds were found to be either moderately active, slightly active or inactive against the tested microorganisms.
Table 2.
Antibacterial activity of the compounds 4a-h and 5a-h.
| Compound | Gram positive bacteria | Gram negative bacteria | ||
|---|---|---|---|---|
| S. aureus | B. subtilis | P. aeruginosa | E.coli | |
| Ampicillin | + + + | + + | + + | + + + |
| Streptomycin | + + + | + + + | + + + | + + + |
| 4a | + + | - | + | + + |
| 4b | + | - | - | + |
| 4c | - | + | - | + |
| 4d | + + | + | + | + + |
| 4e | + + | + | + + | + + + |
| 4f | + + | + + | - | + + + |
| 4g | + | - | + + + | + + + |
| 4h | + | + + | + + + | + + |
| 5a | + | + + | + + | + |
| 5b | - | + | - | + |
| 5c | + | + | + | + + |
| 5d | + + | + + | - | + |
| 5e | + + + | - | + | + + |
| 5f | - | + | + | + + + |
| 5g | + + + | - | + + | + + |
| 5h | + + + | + + | + + | + + + |
Key to symbols: Inactive = - (inhibition zone < 6 mm); Slightly active = + (inhibition zone 6-9 mm); Moderately active = + + (inhibition zone 9-12 mm); Highly active = + + + (inhibition zone > 12 mm).
Antifungal activity
The antifungal activities of the prepared compounds were tested against three different fungi such as C. tropicans, A. niger and F. heterosporium by filter paper disc technique [23]. None of the compounds were found to be active against the fungi species tested.
Experimental
General
Melting points were determined in open capillaries on Thomas Hoover apparatus and are uncorrected. 1H-NMR spectra were recorded on a Bruker AM400 (400 MHz) instrument using tetramethylsilane (TMS) as an internal standard and DMSO-d6 as a solvent. Chemical shifts are given in parts per million (ppm). Mass spectra (MS) were recorded on Schimadzu GC-MS. Infrared spectra were recorded on Schimadzu-IR Prestige 21. Elemental analysis (C, H, N) was performed on Perkin Elmer 240 analyzer and all compounds are within ±0.4% of theory unless otherwise specified. All products were purified by recrystallisation. The reactions were followed up and the purity of products was carried out on pre-coated TLC plates (Silica gel 60 F254 , Merck), visualizing the spots under ultraviolet light. Column chromatography was performed on Merck silicagel (60-120 mesh). The antimicrobial screening was carried out at Chemo Test Laboratory. 2-Aminobenzothiazole-6-carboxylic acid (1): was prepared by a reported method [21].
Synthesis of 2-(2-chloroacetylamino)benzothiazole-6-carboxylic acid (2)
Equimolar amounts of 2-aminobenzothiazole-6-carboxylic acid (1, 0.1 mole) and chloroacetyl chloride (0.1 mole) in chloroform (30 mL) was refluxed in the presence of K2CO3 (0.1 mole) for about 12 h. Excess of solvent was removed in vacuo and the residue was stirred with water (50 mL). The residue was washed with 5% NaHCO3 (30 mL) and subsequently with water (30 mL). The crude product was dried and crystallized from methanol to furnish a pale yellow solid. Yield 81%; m.p. 162 °C; IR (KBr, cm-1) 3430 (NH), 1710 (C=O), 1635 (CONH); 1H-NMR δ 4.29 (s, 2H, CH2), 8.0 (s, 1H, NH), 8.42-8.99 (m, 3H, benzothiazole), 11.1 (s, 1H, COOH); Anal. calcd. for C10H7ClN2O3S: C 44.36, H 2.59, N 10.35, S 11.83 %. Found: C 44.38, H 2.63, N 10.33, S 11.85 %.
Synthesis of 2-(2-hydrazinoacetylamino)benzothiazole-6-carboxylic acid (3)
A mixture of 2-(2-chloroacetylamino)benzothiazole-6-carboxylic acid (2, 0.1 mole) and hydrazine hydrate (0.1 mole) in ethanol (30 mL) was refluxed for about 6 h. After cooling the resulting solid was filtered, dried and crystallized from CHCl3-MeOH mixture to give light brown solid. Yield 75%; m.p. 173 °C; IR (KBr, cm-1) 3455 (NH), 3350 (NHNH2), 2886 (CH2NH), 1705 (C=O), 1650 (CONH); 1H-NMR δ 2.0 (s, 2H, NH2), 2.2 (s, 1H, NH), 3.58 (s, 2H, CH2), 8.16 (s, 1H, CONH), 8.40-8.96 (m, 3H, benzothiazole), 11.2 (s, 1H, COOH); Anal. calcd. for C10H10N4O3S: C 45.11, H 3.76, N 21.05, S 12.03 %. Found: C 45.08, H 3.74, N 21.09, S 12.06 %.
General procedure for the synthesis of 2-{2-[N’-(arylidene)hydrazino]acetylamino}benzothiazole-6-carboxylic acids 4a-h
A mixture of 2-(2-hydrazinoacetylamino)benzothiazole-6-carboxylic acid (3, 0.01 mole), aromatic aldehyde (0.01 mole) and 2-3 drops of glacial acetic acid in ethanol (30 mL) was refluxed for 5 h. The solvent was removed under reduced pressure. The residue was stirred with ice cold water (50 mL), filtered and dried. The crude product obtained was purified by column chromatography over silica gel (eluent: n-hexane/EtOAc 9:1).
2-{2-[N’-(3-Bromobenzylidene)hydrazino]acetylamino}benzothiazole-6-carboxylic acid (4a). Yield 77%; m.p. 188-190 °C; IR (KBr, cm-1) 3472 (NH), 1719 (C=O), 1638 (CONH), 1548 (N=CH); 1H-NMR δ 2.1 (s, 1H, NH), 3.59 (s, 2H, CH2), 7.9 (s, 1H, CONH), 8.1 (s, 1H, N=CH), 7.22-7.84 (m, 4H, ArH), 8.40-8.97 (m, 3H, benzothiazole), 11.12 (s, 1H, COOH); Anal. calcd. for C17H13BrN4O3S: C 47.11, H 3.00, N 12.93, S 7.39 %. Found: C 47.14, H 3.04, N 12.89, S 7.37 %.
2-{2-[N’-(4-Hydroxybenzylidene)hydrazino]acetylamino}benzothiazole-6-carboxylic acid (4b). Yield 72%; m.p. 172 °C; I.R (KBr, cm-1) 3464 (NH), 3590 (ArOH), 1704 (C=O), 1652 (CONH), 1554 (N=CH); 1H-NMR δ 2.14 (s, 1H, NH), 3.50 (s, 2H, CH2), 5.1 (s, 1H, ArOH), 6.82-7.46 (m, 4H, ArH), 7.94 (s, 1H, CONH), 8.16 (s, 1H, N=CH), 8.38-8.99 (m, 3H, benzothiazole), 11.10 (s, 1H, COOH); Anal. calcd. for C17H14N4O4S: C 55.13, H 3.78, N 15.14, S 8.65 %. Found: C 55.11, H 3.81, N 15.12, S 8.68 %.
2-[2-(N’-Benzylidenehydrazino)acetylamino]benzothiazole-6-carboxylic acid (4c). Yield 68%; m.p. 166-168 °C; IR (KBr, cm-1) 3477 (NH), 1715 (C=O), 1648 (CONH), 1550 (N=CH); 1H-NMR δ 2.08 (s, 1H, NH), 3.58 (s, 2H, CH2), 7.28-7.64 (m, 5H, ArH), 7.90 (s, 1H, CONH), 8.04 (s, 1H, N=CH), 8.35-8.92 (m, 3H, benzothiazole), 11.02 (s, 1H, COOH); Anal. calcd. for C17H14N4O3S: C 57.63, H 3.95, N 15.82, S 9.04 %. Found: C 57.59, H 3.92, N 15.87, S 9.08 %.
2-{2-[N’-(4-Methoxybenzylidene)hydrazino]acetylamino}benzothiazole-6-carboxylic acid (4d). Yield 64%; m.p. 181-183 °C; IR (KBr, cm-1) 3469 (NH), 1700 (C=O), 1641 (CONH), 1545 (N=CH); 1H-NMR δ 2.05 (s, 1H, NH), 3.54 (s, 2H, CH2), 3.75 (s, 3H, Ar-OCH3), 6.82-7.54 (m, 4H, ArH), 7.96 (s, 1H, CONH), 8.2 (s, 1H, N=CH), 8.38-9.03 (m, 3H, benzothiazole), 11.08 (s, 1H, COOH); Anal. calcd. for C18H16N4O4S: C 56.25, H 4.17, N 14.58, S 8.33 %. Found: C 56.31, H 4.13, N 14.55, S 8.32 %.
2-{2-[N’-(2-Chlorobenzylidene)hydrazino]acetylamino}benzothiazole-6-carboxylic acid (4e). Yield 61%; m.p. 155-157 °C; IR (KBr, cm-1) 3460 (NH), 1707 (C=O), 1645 (CONH), 1551 (N=CH); 1H-NMR δ 2.0 (s, 1H, NH), 3.48 (s, 2H, CH2), 7.18-7.58 (m, 4H, ArH), 7.92 (s, 1H, CONH), 8.14 (s, 1H, N=CH), 8.39-8.96 (m, 3H, benzothiazole), 11.0 (s, 1H, COOH); Anal. calcd. for C17H13ClN4O3S: C 52.51, H 3.35, N 14.41, S 8.24 %. Found: C 52.49, H 3.39, N 14.43, S 8.27 %.
2-{2-[N’-(4-Chlorobenzylidene)hydrazino]acetylamino}benzothiazole-6-carboxylic acid (4f). Yield 69%; m.p. 165 °C; IR (KBr, cm-1) 3462 (NH), 1713 (C=O), 1647 (CONH), 1549 (N=CH); 1H-NMR δ 2.04 (s, 1H, NH), 3.52 (s, 2H, CH2), 7.28-7.64 (m, 4H, ArH), 7.88 (s, 1H, CONH), 8.1 (s, 1H, N=CH), 8.42-8.99 (m, 3H, benzothiazole), 11.04 (s, 1H, COOH); Anal. calcd. for C17H13ClN4O3S: C 52.51, H 3.35, N 14.41, S 8.24 %. Found: C 52.48, H 3.33, N 14.44, S 8.26 %.
2-{2-[N’-(3-Nitrobenzylidene)hydrazino]acetylamino}benzothiazole-6-carboxylic acid (4g). Yield 76%; m.p. 218-220 °C; IR (KBr, cm-1) 3473 (NH), 1720 (C=O), 1666 (CONH), 1559 (N=CH), 1345,1520 (NO2); 1H-NMR δ 2.12 (s, 1H, NH), 3.58 (s, 2H, CH2), 7.62-8.64 (m, 4H, ArH), 7.98 (s, 1H, CONH), 8.18 (s, 1H, N=CH), 8.40-8.97 (m, 3H, benzothiazole), 11.06 (s, 1H, COOH); Anal. calcd. for C17H13N5O5S: C 51.13, H 3.26, N 17.54, S 8.02 %. Found: C 51.16, H 3.29, N 17.52, S 8.01 %.
2-{2-[N’-(4-Nitrobenzylidene)hydrazino]acetylamino}benzothiazole-6-carboxylic acid (4h). Yield 73%; m.p. 208 °C; IR (KBr, cm-1) 3468 (NH), 1708 (C=O), 1669 (CONH), 1556 (N=CH), 1338,1515 (NO2); 1H-NMR δ 2.1 (s, 1H, NH), 3.54 (s, 2H, CH2), 7.86-8.16 (m, 4H, ArH), 7.94 (s, 1H, CONH), 8.16 (s, 1H, N=CH), 8.36-8.93 (m, 3H, benzothiazole), 11.1 (s, 1H, COOH); Anal. calcd. for C17H13N5O5S: C 51.13, H 3.26, N 17.54, S 8.02 %. Found: C 51.15, H 3.29, N 17.55, S 7.98 %.
General procedure for the synthesis of 2-{2-[3-chloro-2-(aryl)-4-oxoazetidin-1-yl-amino]acetyl-amino}benzothiazole-6-carboxylic acids 5a-h
To a stirred solution of Schiff base 4a-h (0.05 mole) and Et3N (0.01 mole) in dioxane (50 mL), ClCH2COCl (0.01 mole) was added dropwise at 0-5 °C. The reaction mixture was stirred for about 5 h and the precipitated amine hydrochloride was filtered off. The filtrate was refluxed for about 3 h and excess of solvent was evaporated under reduced pressure. The solid obtained was washed with water (30 mL), filtered and dried. The crude product obtained was purified by column chromatography technique (eluent: n-hexane/EtOAc 8:2).
2-{2-[2-(3-Bromophenyl)-3-chloro-4-oxoazetidin-1-ylamino]acetylamino}benzothiazole-6-carboxylic acid (5a). Yield 58%; m.p. 261-264 °C; I.R (KBr, cm-1) 3456 (NH), 1646 (CONH), 1755 (CO, β-lactam); 1H-NMR δ 3.1 (s, 1H, NH), 3.57 (s, 2H, CH2), 5.2 (d, 1H, CH-Ar), 5.52 (d, 1H, CH-Cl), 7.08-7.31 (m, 4H, ArH), 8.0 (s, 1H, CONH), 8.32-8.90 (m, 3H, benzothiazole), 11.06 (s, 1H, COOH); 13C-NMR δ 52.5 (CH2), 61.6 (CH), 64.2 (CH-Cl), 122.7-145.9 (6 aromatic carbons), 121.4-174.7 (7 carbons, benzothiazole), 163.7 (CO, β-lactam), 168.3 (CONH), 169.5 (COOH); MS (m/e) 509.91 (M+); Anal. calcd. for C19H14BrClN4O4S: C 44.75, H 2.74, N 10.99, S 6.28 %. Found: C 44.71, H 2.76, N 10.97, S 6.32 %.
2-{2-[3-Chloro-2-(4-hydroxyphenyl)-4-oxoazetidin-1-ylamino]acetylamino}benzothiazole-6-carboxylic acid (5b). Yield 50%; m.p. 220-222 °C; IR (KBr, cm-1) 3462 (NH), 1648 (CONH), 1750 (CO, β-lactam), 3585 (Ar-OH); 1H-NMR δ 3.05 (s, 1H, NH), 3.56 (s, 2H, CH2), 5.0 (s, 1H, Ar-OH), 5.24 (d, 1H, CH-Ar), 5.48 (d, 1H, CH-Cl), 6.64-6.99 (m, 4H, ArH), 8.06 (s, 1H, CONH), 8.38-8.95 (m, 3H, benzothiazole), 11.1 (s, 1H, COOH); 13C-NMR δ 52.2 (CH2), 62.4 (CH), 64.7 (CH-Cl), 115.4-156.6 (6 aromatic carbons), 121.9-174.2 (7 C, benzothiazole), 163.2 (CO, β-lactam), 168.7 (CONH), 169.1 (COOH); MS (m/e) 446.07 (M+); Anal. calcd. for C19H15ClN4O5S: C 51.06, H 3.36, N 12.54, S 7.17 %. Found: C 51.08, H 3.34, N 12.50, S 7.14 %.
2-[2-(3-Chloro-2-oxo-4-phenylazetidin-1-ylamino)acetylamino]benzothiazole-6-carboxylic acid (5c). Yield 53%; m.p. 215 °C; IR (KBr, cm-1) 3452 (NH), 1656 (CONH), 1742 (CO, β-lactam); 1H-NMR δ 3.08 (s, 1H, NH), 3.59 (s, 2H, CH2), 5.1 (d, 1H, CH-Ar), 5.50 (d, 1H, CH-Cl), 7.06-7.23 (m, 5H, ArH), 8.12 (s, 1H, CONH), 8.44-8.97 (m, 3H, benzothiazole), 11.0 (s, 1H, COOH); 13C-NMR δ 52.9 (CH2), 62.3 (CH), 64.5 (CH-Cl), 126.4-143.7 (6 aromatic carbons), 121.7-174.3 (7 C, benzothiazole), 163.9 (CO, β-lactam), 168.1 (CONH), 169.7 (COOH); MS (m/e) 430.09 (M+); Anal. calcd. for C19H15ClN4O4S: C 52.96, H 3.48, N 13.01, S 7.43 %. Found: C 52.93, H 3.52, N 13.04, S 7.44 %.
2-{2-[3-Chloro-2-(4-methoxyphenyl)-4-oxoazetidin-1-ylamino]acetylamino}benzothiazole-6-carboxylic acid (5d). Yield 59%; m.p. 231-233 °C; IR (KBr, cm-1) 3459 (NH), 1650 (CONH), 1745 (CO, β-lactam); 1H-NMR δ 3.15 (s, 1H, NH), 3.54 (s, 2H, CH2), 3.77 (s, 3H, Ar-OCH3), 5.16 (d, 1H, CH-Ar), 5.46 (d, 1H, CH-Cl), 6.74-7.03 (m, 4H, ArH), 8.04 (s, 1H, CONH), 8.42-8.96 (m, 3H, benzothiazole), 11.2 (s, 1H, COOH); 13C-NMR δ 52.6 (CH2), 55.7 (OCH3), 62.6 (CH), 64.8 (CH-Cl), 114.4-158.5 (6 aromatic carbons), 122.1-173.9 (7 C, benzothiazole), 163.1 (CO, β-lactam), 168.5 (CONH), 169.8 (COOH); MS (m/e) 460.06 (M+); Anal. calcd. for C20H17ClN4O5S: C 52.12, H 3.69, N 12.16, S 6.95 %. Found: C 52.10, H 3.73, N 12.11, S 6.97 %.
2-{2-[3-Chloro-2-(2-chlorophenyl)-4-oxoazetidin-1-ylamino]acetylamino}benzothiazole-6-carboxylic acid (5e). Yield 52%; m.p. 238 °C; IR (KBr, cm-1) 3445 (NH), 1659 (CONH), 1749 (CO, β-lactam); 1H-NMR δ 3.0 (s, 1H, NH), 3.50 (s, 2H, CH2), 5.12 (d, 1H, CH-Ar), 5.48 (d, 1H, CH-Cl), 7.02-7.24 (m, 4H, ArH), 8.08 (s, 1H, CONH), 8.36-8.93 (m, 3H, benzothiazole), 11.16 (s, 1H, COOH); 13C-NMR δ 52.1 (CH2), 61.3 (CH), 63.5 (CH-Cl), 126.1-143.8 (6 aromatic carbons), 121.2-174.4 (7 C, benzothiazole), 163.5 (CO, β-lactam), 168.9 (CONH), 169.1 (COOH); MS (m/e) 464.10 (M+); Anal. calcd. for C19H14Cl2N4O4S: C 49.03, H 3.01, N 12.04, S 6.88 %. Found: C 49.07, H 3.04, N 12.07, S 6.83 %.
2-{2-[3-Chloro-2-(4-chlorophenyl)-4-oxoazetidin-1-ylamino]acetylamino}benzothiazole-6-carboxylic acid (5f). Yield 55%; m.p. 244-246 °C; IR (KBr, cm-1) 3449 (NH), 1657 (CONH), 1752 (CO, β-lactam); 1H-NMR δ 3.12 (s, 1H, NH), 3.53 (s, 2H, CH2), 5.08 (d, 1H, CH-Ar), 5.40 (d, 1H, CH-Cl), 7.08-7.26 (m, 4H, ArH), 8.02 (s, 1H, CONH), 8.38-8.95 (m, 3H, benzothiazole), 11.12 (s, 1H, COOH); 13C-NMR δ 52.4 (CH2), 62.5 (CH), 64.9 (CH-Cl), 128.6-141.8 (6 aromatic carbons), 121.4-174.1 (7 C, benzothiazole), 163.2 (CO, β-lactam), 168.3 (CONH), 169.4 (COOH); MS (m/e) 464.05 (M+); Anal. calcd. for C19H14Cl2N4O4S: C 49.03, H 3.01, N 12.04, S 6.88 %. Found: C 49.05, H 3.02, N 12.06, S 6.90 %.
2-{2-[3-Chloro-2-(3-nitrophenyl)-4-oxoazetidin-1-ylamino]acetylamino}benzothiazole-6-carboxylic acid (5g). Yield (54%); m.p. 273-277 °C; IR (KBr, cm-1) 3460 (NH), 1654 (CONH), 1760 (CO, β-lactam), 1342,1525 (NO2); 1H-NMR δ 3.09 (s, 1H, NH), 3.60 (s, 2H, CH2), 5.04 (d, 1H, CH-Ar), 5.42 (d, 1H, CH-Cl), 7.49-8.07 (m, 4H, ArH), 8.06 (s, 1H, CONH), 8.41-8.98 (m, 3H, benzothiazole), 11.1 (s, 1H, COOH); 13C-NMR δ 52.7 (CH2), 61.5 (CH), 64.8 (CH-Cl), 119.5-148.4 (6 aromatic carbons), 122.3-173.9 (7 C, benzothiazole), 163.9 (CO, β-lactam), 168.9 (CONH), 170.1 (COOH); MS (m/e) 475.03 (M+); Anal. calcd. for C19H14ClN5O6S: C 47.95, H 2.94, N 14.72, S 6.73 %. Found: C 47.97, H 2.92, N 14.75, S 6.77 %.
2-{2-[3-Chloro-2-(4-nitrophenyl)-4-oxoazetidin-1-ylamino]acetylamino}benzothiazole-6-carboxylic acid (5h). Yield 60%; m.p. 276-278 °C; IR (KBr, cm-1) 3457 (NH), 1651 (CONH), 1754 (CO, β-lactam), 1333,1523 (NO2); 1H-NMR δ 3.04 (s, 1H, NH), 3.56 (s, 2H, CH2), 5.12 (d, 1H, CH-Ar), 5.46 (d, 1H, CH-Cl), 7.36-8.12 (m, 4H, ArH), 8.12 (s, 1H, CONH), 8.38-8.95 (m, 3H, benzothiazole), 11.08 (s, 1H, COOH); 13C-NMR δ 52.2 (CH2), 62.5 (CH), 64.7 (CH-Cl), 120.6-149.2 (6 aromatic carbons), 121.3-174.1 (7 C, benzothiazole), 163.6 (CO, β-lactam), 168.2 (CONH), 169.7 (COOH); MS (m/e) 475.11 (M+); Anal. calcd. for C19H14ClN5O6S: C 47.95, H 2.94, N 14.72, S 6.73 %. Found: C 47.98, H 2.91, N 14.67, S 6.76 %.
Antibacterial activity
The cup plate method using Hi-Media agar medium was employed to study the antibacterial activity of 4a-h and 5a-h against S. aureus (ATCC6538P), B. subtilis (ATCC6633), P. aeruginosa (ATCC9027) and E. coli (ATCC10536) [22]. Preparation of nutrient broth, subculture, base layer medium, agar medium and peptone water was done as per the standard procedure. Each test compound (50 mg) was dissolved in dimethylformamide (50 mL, 1000 μg/mL), which was used as sample solution. Sample size for all the compounds was fixed at 0.1 mL. Using a sterilized cork borer cups were scooped out of agar medium contained in a petri dish which was previously inoculated with the microorganisms. The test compound solution (0.1 mL) was added in the cups and the petri dishes were subsequently incubated at 37 °C for 48 h. Ampicillin and Streptomycin were used as reference drugs and dimethylformamide as a negative control. Zones of inhibition produced by each compound was measured in mm, and the results are listed in Table 2.
Antifungal activity
The antifungal activities of compounds 4a-h and 5a-h were tested against three different fungi such as C. tropicans (ATCC9763), A. niger (ATCC16404) and F. heterosporium by the filter paper disc technique [23].
Acknowledgments
The authors are thankful to Dr. Atul Pusalkar and Dr. Borna Basu, Chemo Test Laboratory, Sewri (W), Mumbai-400 015, India; for antimicrobial screening of the compounds. The authors are also grateful to the U.G.C. for the financial assistance.
Footnotes
Sample Availability: Not available.
References
- 1.a) Southgate R. The synthesis of natural β-lactam antibiotics. Contemp. Org. Synth. 1994;1:417–431. [Google Scholar]; b) Morin R. B., Gorman M. Chemistry and Biology of β-Lactam Antibiotics. Academic Press; New York: 1982. [Google Scholar]
- 2.Mata E. G., Fraga M. A., Delpiccolo C. M. L. An Efficient, Stereoselective Solid-Phase Synthesis of β-Lactams Using Mukaiyama’s Salt for the Staudinger Reaction. J. Comb. Chem. 2003;5:208–210. doi: 10.1021/cc020107d. [DOI] [PubMed] [Google Scholar]
- 3.Page E. I. The Chemistry of β-Lactams. Blackie Academic and Professional; New York: 1992. [Google Scholar]
- 4.a) Niccolai D., Trasi L., Thomas R. J. The renewed challenge of antibacterial chemotherapy. Chem. Commun. 1997:2333–2342. [Google Scholar]; b) Chu D. T. W., Plattner J.I., Katz L. New Directions in Antibacterial Research. J. Med. Chem. 1996;39:3853–3874. doi: 10.1021/jm960294s. [DOI] [PubMed] [Google Scholar]
- 5.Van der Steen F. H., Van Koten G. Synthesis of 3-amino-2-azetidinones: A literature survey. Tetrahedron. 1991;47:7503–7524. doi: 10.1016/S0040-4020(01)88276-4. [DOI] [Google Scholar]
- 6.Durckheimer W., Blumbach J., Lattrell R., Scheunemann K. H. Recent Developments in the Field of β-Lactam Antibiotics. Angew. Chem. Int. Ed. Engl. 1985;24:180–202. doi: 10.1002/anie.198501801. [DOI] [Google Scholar]
- 7.Abdulla R. F., Fuhr K. H. Monocyclic antibiotic beta-lactams. J. Med. Chem. 1975;18:625–627. doi: 10.1021/jm00240a022. [DOI] [PubMed] [Google Scholar]
- 8.Feigelson G. B., Curran W. V., Ziegler C. B. 4-Substituted azetidinones as precursors to 2-substituted-3-carboxy carbapenem antibiotics and a method of producing them. US 5,371,215. 1994
- 9.Doherty J. B., Dorn C. P., Durette P. L., Finke P. E., MacCoss M., Mills S. G., Shah S. K., Sahoo S. P., Polo S. A., Hagmann W. K. Substituted azetidinones as anti-inflammatory and antidegenerative agents. WO 94,10,143. 1994
- 10.Khalafallah A. K., Selim M. A., El-Hamd R. M. A., Elmaghraby M. A., Soleiman H. A., Raslan M. A. Novel synthesis of some new fused/spiro heterocyclic compounds and their biological activity. Indian J. Chem. 1995;34B:1066–1070. [Google Scholar]
- 11.Parikh K. A., Oza P. S., Parikh A. R. Synthesis of some new 2-azetidinones as potential antitubercular agents. Indian J. Chem. 2000;39B:716. [Google Scholar]
- 12.Vashi B. S., Mehta D. S., Shah V. H. Synthesis and biological activity of 4-thiazolidinones, 2-azetidinones, 4-imidazolinone derivatives having thymol moiety. Indian J. Chem. 1995;34B:802–808. [Google Scholar]
- 13.Russo F., Romeo G., Santagati N. A., Caruso A., Cutuli V., Amore D. Synthesis of new thienopyrimidobenzothiazoles and thienopyrimidobenzoxazoles with analgesic and anti-inflammatory properties. Eur. J. Med. Chem. 1994;29:569–578. doi: 10.1016/0223-5234(94)90149-X. [DOI] [Google Scholar]
- 14.Katsura Y., Inoue Y., Nishino S., Tomoi M., Takasugi H. Studies on antiulcer drugs. IV. Synthesis and antiulcer activities of imidazo[1,2-a]pyridinylethylbenzothiazoles and benzimidazoles. Chem. Pharm. Bull (Tokyo) 1992;40:1818–1822. doi: 10.1248/cpb.40.1818. [DOI] [PubMed] [Google Scholar]
- 15.Kuhler T. C., Swanson M., Shcherbuchin V., Larsson H., Mellgard B., Sjostrom J. E. Structure-Activity Relationship of 2-[[(2-Pyridyl)methyl]thio]-1H-benzimidazoles as Anti-Helicobacter pylori Agents in Vitro and Evaluation of their in Vivo Efficacy. J. Med. Chem. 1998;41:1777–1788. doi: 10.1021/jm970165r. [DOI] [PubMed] [Google Scholar]
- 16.Baltork I. M., Khosropour A. R., Hojati S. F. Mild and Efficient Synthesis of Benzoxazoles, Benzothiazoles, Benzimidazoles and Oxazolo[4,5-b]pyridines Catalyzed by Bi(III) Salts Under Solvent-Free Conditions. Monatshe Chem. 2007;138:663–667. doi: 10.1007/s00706-007-0655-9. [DOI] [Google Scholar]
- 17.Rey A. W., Vemishetti P., Droghini R. 5412092. U.S. Pat. 1995
- 18.Patel K. H., Mehta A. G. Synthesis and Antifungal Activity of Azetidinone and Thiazolidinones Derivatives of 2-Amino-6-(2-naphthalenyl)thiazolo[3,2-d]thiadiazole. Eur. J. Chem. 2006;3:267–273. doi: 10.1155/2006/186294. [DOI] [Google Scholar]
- 19.Singh G. S., Mbukwa E., Pheko T. Synthesis and antimicrobial activity of new 2-azetidinones from N-(salicylidene)amines and 2-diazo-1,2-diarylethanones. Arkivoc. 2007:80–90. [Google Scholar]
- 20.Singh G. S. β-Lactams in the New Millennium. Part-I: Monobactams and Carbapenems. Mini-Rev. Med. Chem. 2004;4:69–92. doi: 10.2174/1389557043487501. [DOI] [PubMed] [Google Scholar]
- 21.Nikolyukin Y. A., Gibboni D. J. 5710012. U.S. Pat. 1998
- 22.British Pharmacopoeia. 2005;Vol. IV:A300. Appendix XIV. [Google Scholar]
- 23.Vincent J. G., Vincent H. W. Filter paper disc modification of the Oxford cup penicillin determination. Proc. Soc. Exptl. Biol. Med. 1944;55:162–164. doi: 10.3181/00379727-55-14502. [DOI] [Google Scholar]