Abstract
The reaction products in the presence of Lewis acid of isoeugenol (1) with ethanethiol, thiophenol, 2-mercaptothiazoline or 2-mercapto-1-methylimidazole (ISO-S1 – ISO-S-4) were obtained. The radical-scavenging activity of these compounds was investigated using the induction period method for polymerization of methyl methacrylate (MMA) initiated by thermal decomposition of 2,2'-azobisisobutyronitrile (AIBN) and benzoyl peroxide (BPO) and monitored by differential scanning calorimetry (DSC). For BPO, the stoichiometric factor (number of free radicals trapped by one mole of antioxidant moiety, n) declined in the order isoeugenol (1.8) > ISO-S-1 (1.6) > ISO-S-2 (1.2 ) > ISO-S-3 (0.9) > ISO-S-4 (0.3), whereas for AIBN, their n values were about 1, except for ISO-S-3 (0.6). The ratio of the rate constant of inhibition to that of propagation (kinh/kp) for BPO declined in the order ISO-S-4 (56) > ISO-S-3 (15) > ISO-S-2 (11) >ISO-S-1 (9) > isoeugenol (8). Similarly, for AIBN the kinh/kp of the reaction products (33-57) was greater than that of isoeugenol (31). The reaction products of isoeugenol with a SH group showed greater inhibition rate constants (kinh) than the parent compound isoeugenol.
Keywords: radical-scavenging activity; thioisoeugenols, BPO, AIBN; induction period method
Introduction
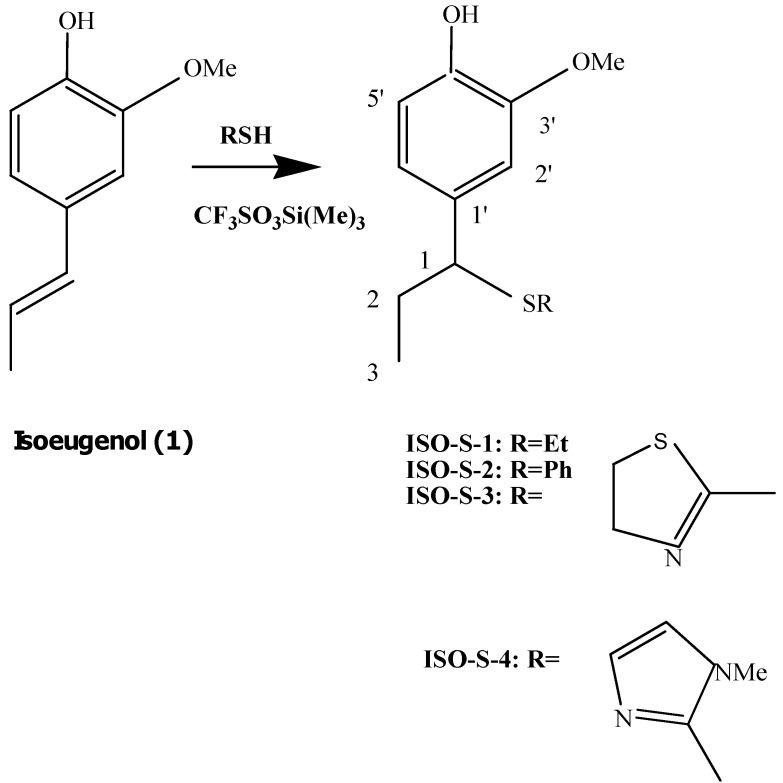
Isoeugenol (4-propenyl-2-methoxyphenol, 1) is used in perfumes, soaps, detergents, air fresheners and cosmetics. However, isoeugenol is also an important allergen [1]. Furthermore, isoeugenol has a moderate acute toxicity by dermal and oral routes. Although isoeugenol exhibited cytotoxic and the mentioned allergenic activity, it is a potent antioxidant [1]. It has been generally assumed that the antioxidant effects of phenolic antioxidants are responsible for their anti-inflammatory and chemopreventive effects. We previously synthesized a dimer of isoeugenol, dehydrodiisoeugenol, demonstrating that this compound, but not isoeugenol, displays potent cyclooxygenase-2 (COX-2) inhibition, an anti-inflammatory activity [2]. Also, we previously reported that the cytotoxicity of isoeugenol may be attributed to its induction of reactive oxygen species (ROS) and the reduction of gluthathione (GSH) levels, possibly due to its prooxidative activity [3]. Curcumin, an isoeugenol- related compound, was previously reported to form conjugates with GSH by separating mono- and diglutathionyl adducts of curcumin. This suggested that formation of curcumin-GSH adducts lead to inactivation of the parent curcumin’s prooxidative activity [4]. These results led us a hypothesis: thioisoeugenols may reduce the cytotoxic and allergenic activity without decreasing their antioxidant activity. Thus, we synthesized ISO-S-1, a reaction product of isoeugenol (1) with ethanethiol; ISO-S-2, a reaction product of 1 with thiophenol; ISO-S-3, its reaction product with 2-mercaptothiazoline and ISO-S-4, a reaction product of 1 with 2-mercapto-1-methylimidazole (see Scheme 1).
Scheme 1.
Preparation of products ISO-S-1 – ISO-S-4.
We have previously used DSC and the induction period method to investigate the radical scavenging activity of phenolic antioxidants under nearly anaerobic conditions, and this method has been proven to be reliable for evaluating the activity of these compounds [5,6].
In an extension of this work, we investigated here the radical-scavenging activity of ISO-S-1, 2, 3 and 4, and their parent compound, isoeugenol, by determining the induction period for polymerization of methyl methacrylate (MMA) initiated by thermal decomposition of 2,2'-azobisisobutyronitrile (AIBN, a R. radical) and benzoyl peroxide (BPO, a PhCOO. radical).
Results and Discussion
Radical scavenging activities determined by the induction period method
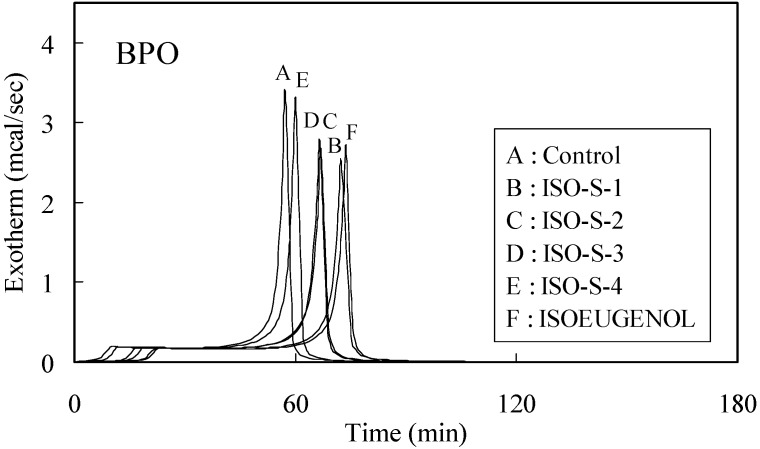
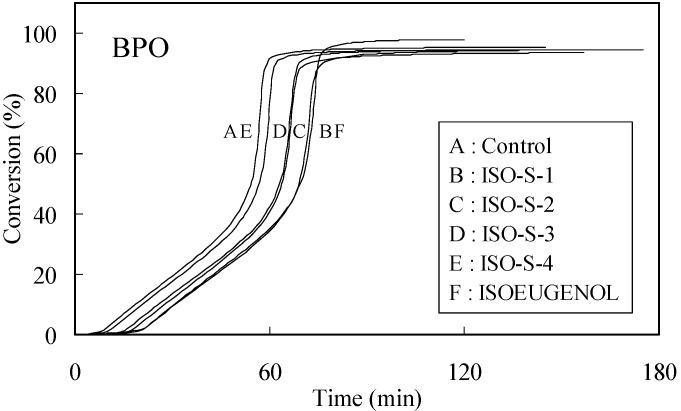
The time-exotherm and time-conversion curves for ISO-S-1, 2, 3, 4 and isoeugenol are shown in Figure 1 and Figure 2, respectively.
Figure 1.
Exothermic curves for the polymerization of MMA initiated by thermal decomposition of BPO in the presence of 0.01 mol% additives.
Figure 2.
Time-conversion curves for the polymerization of MMA initiated by thermal decomposition of BPO in the presence of 0.01 mol% additives. Curves were obtained from findings shown in Figure 1.
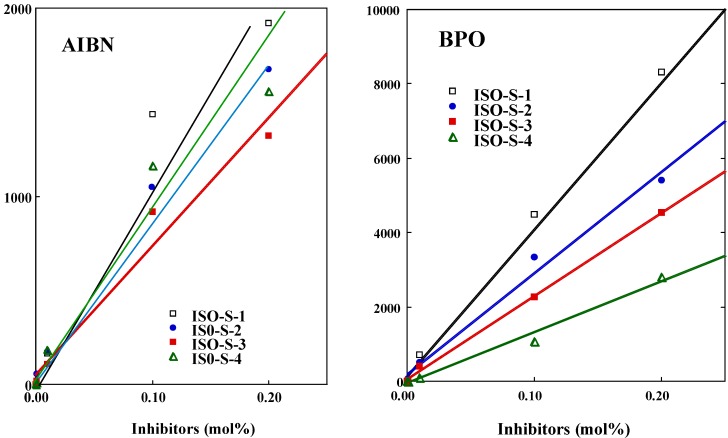
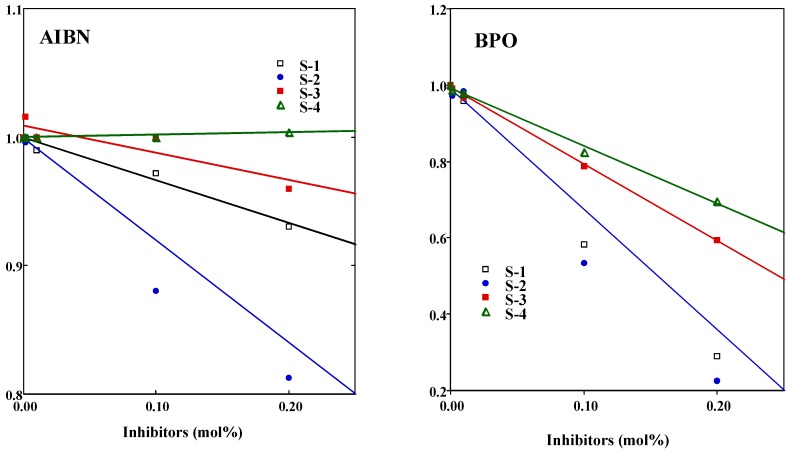
The n value was determined from each slope (Figure 3, see Equation 2). Results are shown in Table 1. For BPO, the n value declined in the order isoeugenol (1.8 )> ISO-S-1 (1.6) > ISO-S-2 (1.2) > ISO-S-3 (0.9) > ISO-S-4 (0.3), whereas for AIBN, the n value of ISO-1, 2, and 4 and isoeugenol were all about 1, while the value for ISO-3 was 0.6. In general, the n value of phenolic compounds is close to 2 [7]. The n value for 2-methoxyphenol is less than 2, due to the strong internal hydrogen bond on the OH and the methoxy group [5] c. It is well known that the one-electron oxidation of phenolic compounds; the n of about 1 leads to dimmers and to polymers (lignin) [8]. In the present study, compounds with the n of about 1 may occur dimerization during induction period.
Figure 3.
A typical plot of the induction period vs the concentration for ISO-S-1, 2, 3, and 4 in the polymerization of MMA initiated by thermal decomposition of AIBN (left panel) and BPO (right panel).
Table 1.
Radical-scavenging activity of the reaction products of isoeugenol (1) with ethanethiol, thiophenol, 2-mercaptothiazoline or 2-mercapto-1-methylimidazole (ISO-S-1 – ISO-S-4) in the polymerization of methyl methacrylate initiated by AIBN and BPO.
| AIBN | BPO | |||
|---|---|---|---|---|
| Compound | Relative n | kinb/kp | Relative n | kinb/kp |
| ISO-S-1 | 0.92 ± 0.05 | 36.43 ± 1.78 | 1.61 ± 0.08 | 8.88 ± 0.44 |
| ISO-S-2 | 0.96 ± 0.04 | 34.81 ± 1.71 | 1.19 ± 0.05 | 11.75 ± 0.58 |
| ISO-S-3 | 0.59 ± 0.02 | 56.63 ± 2.27 | 0.91 ± 0.04 | 15.46 ± 0.76 |
| ISO-S-4 | 1.01 ± 0.05 | 32.86 ± 1.48 | 0.25 ± 0.01 | 55.95 ± 2.79 |
| Isoeugenol | 1.11 ± 0.06 | 30.52 ± 1.36 | 1.75 ± 0.09 | 8.16 ± 0.42 |
n values calculated using Eqn. 2. kinb and kp are the rate constants of inhibition and propagation, respectively. The kinb/kp was calculated using Eqn. 5. MMA, 9.4 mol/L; compounds, 1mM; T=70°C; conversion: 90.3-95.3%; values, the mean ± SD for three different experiments.
The inhibition rate constant, kinh plays a more important role for evaluating the activity, so we investigated this parameter. The relationships between propagation rate (Rp), the initial rate of polymerization and concentration of the ISO-S compounds are shown in Figure 4. Plots of the Rpinh/Rpcon vs concentrations for both initiators decreased linearly as the concentration increased. In particular, ISO-S-2 was strongly reduced in both initiators, followed by the great retardation of the growing MMA radicals. This was possibly due to the strong interaction between the oxidized products of ISO-S-2 with a benzene ring and the growing MMA radicals. For AIBN and BPO, the kinh/kp was calculated using Equation 5.
Figure 4.
A typical plot of the Rpinh/Rpcon vs the concentration for ISO-S-1, 2, 3, and 4.
Results are shown also in Table 1. For AIBN, the kinh/kp value for isoeugenol (31) was the smallest. The kinh/kp value of ISO-S-3 (57) was the greatest, but was about only twice as large as that of other reaction products. For BPO, the kinh/kp declined in the order ISO-S-4 (56) > ISO-S-3 (15) > ISO-S-2 (12) >ISO-S-1 (9) > isoeugenol (8). The kinh of ISO-S-4 was estimated as 4.4 x 104 mol-1s-1, assuming that the kp value for MMA is 797 mol-1s-1 [5c]. Antioxidants having a n value of about 1 or less than 1 may undergo dimerization during the induction period. In contrast, the n of isoeugenol for BPO was about 2, suggesting the formation of vinyl quinone methide derived from a two-electron oxidation of this compound [9]. The radical-scavenging activity of ISO-S-1, 2, 3, and 4 against PhCOO., an oxygen-centered radical derived from BPO, was clearly different from that against R., a carbon-centered radical derived from AIBN. These compounds preferred an oxygen-centered radical rather than a carbon-centered radical.
Conclusions
ISO-S-1, 2, 3, and 4 were obtained by reaction of isoeugenol with compounds having a SH group Their radical-scavenging activity using two methods under nearly anaerobic conditions (n and kinh) was determined using the induction period method. The reaction products showed the greater kinh /kp than that of isoeugenol.
Experimental
General
The chemicals were purchased from Tokyo Kasei Kogyo Ltd. (Tokyo Japan) . NMR spectra were measured in CDCl3 solutions at ambient temperature on a JEOL, JNM-A500 spectrometer (JEOL, Tokyo, Japan). The chemical shifts δ are given in ppm related to tetramethylsilane, TMS as internal standard. Mass spectra were measured on aJMS/700 (JEOL).
General procedure for the synthesis of ISO-S-1, 2, 3, and 4: reaction of isoeugenol (1) with thiols in the presence of Lewis acid
To a solution of 1 (0.5 g, 3 mmol) and ethanethiol (0.76 g, 12 mmol) in dry chloroform (20 mL) trimethylsilyl trifloromethanesulfonate (0.5 mL) was added and the reaction mixture was stirred at room temperature for 0.5 h. After washing with 5% sodium carbonate solution the mixture was evaporated and purified by silicagel chromatography to give an oily product (ISO-S-1) in 66.0% yield. MS; m/z 165 (100%), 226 (M+, C12H18O2S); 1H-NMR (CDCl3) δ (ppm): 6.86 (1H, d, J=1.8 Hz, C2’-H), 6.83 (1H, d, J=8.0 Hz, C5’-H), 6.75 (1H, dd, J=8.0, 1.8 Hz, C6’-H), 3.90 (3H, s, C3’-OCH3), 3.65 (1H, dd, J=8.7, 6.1 Hz, C1-H), 2.3-2.4 (2H, m, SCH2-CH3), 1.78-1.92 (2H, m, C1-CH2CH3), 1.15 (3H, t, J=7.3 Hz, S-CH2CH3), 0.90 (3H, t, J=7.3 Hz, C1-CH2CH3).
Reaction of 1 with thiophenol
According to the general procedure, the reaction of isoeugenol with thiophenol for 1.5 h gave an oily product (ISO-S-2) in 75.0% yield. MS; m/z 165 (100%), 274 (M+, C16H18O2S); 1H-NMR (CDCl3) δ: 7.18-7.26 (5H, m, S-Ph), 6.80 (1H, d, J=8.0 Hz, C5’’-H), 6.74 (1H, d, J=2.1 Hz, C2’-H), 6.72 (1H, dd, J=8.0, 2.1 Hz, C6’-H), 3.97 (1H, dd, J=8.9, 6.1 Hz, C1-CH-), 3.83 (3H, s, C3’-OCH3), 1.80-1.98 (2H, m, C1-CH2CH3), 0.90 (3H, t, J=7.3Hz, C1-CH2CH3).
Reaction of 1 with 2-mercaptothiazoline
According to the general procedure, the reaction of isoeugenol with 2-mercaptothiazoline for 24 h gave an oily product (ISO-S-3) in 36.5% yield. MS; m/z 165 (100%), 283 (M+, C13H17O2NS2); 1H-NMR (CDCl3) δ: 6.96 (1H, d, J=1.7 Hz, C2’-H), 6.89 (1H, d, J=8.2 Hz, C5’-H), 6.86 (1H, dd, J=8.2, 1.7 Hz, C6’-H), 6.10 (1H, m, C1-CH-), 3.88 (3H, s, C3’-OCH3), 3.6-3.9 (2H, m, -S-CH2CH2-N), 3.05-3.24 (2H, m, -S-CH2CH2-N=), 1.88-2.12 (2H, m, C1-CH2CH3), 1.01(3H, t, J=7.3Hz, C1-CH2CH3).
Reaction of 1 with 2-mercapto-1-methylimidazole
According to the general procedure, the reaction of eugenol with 2-mercapto-1-methylimidazole for 24 h gave a crystalline product (ISO-S-4); mp 137-138°C (hexane-AcOEt) in 48.2% yield. MS; m/z 165 (100%), 246 (M+, C14H18O2N2S); 1H-NMR (CDCl3) δ: 6.96 (1H, d, J=1.7Hz, C2’-H), 6.87-6.91 (2H, m, C5’, 6’-H), 6.64 (2H, s, imidazole-CH=CH-), 5.94 (1H, t, J=7.9Hz, C1-CH-), 3.87 (3H, s, C3’-OCH3), 3.62 (3H, s, N-Me), 2.08-2.19 (2H, m, C1-CH2CH3), 0.95 (3H, t, J=7.9Hz, C1-CH2CH3).
DSC measurements
The induction period (IP) and initial rate of polymerization in the presence (Rpinh) or absence (Rpcon) of ISO-S-1, 2, 3, 4 and isoeugenol (1) (Figure 2) were determined by the previously reported method [6]. In brief, the experimental resin consisted of MMA and AIBN (or BPO) with or without additives. AIBN (or BPO) were added at 1.0 mol%, and the additives were used at 0, 0.001, 0.01, 0.02 and 0.1 mol%. Approximately 10 µL of the experimental resin (MMA: 9.12-9.96 mg) was loaded into an aluminum sample container and sealed by applying pressure. The container was placed in a differential scanning calorimeter (model DSC 3100; MAC Science Co., Tokyo, Japan) kept at 70°C, and the thermal changes induced by polymerization were recorded for the appropriate periods. The heat due to polymerization of MMA was 13.0 kcal/mole in this experiment. The conversion of all samples, as calculated from the DSC thermograms, was 91-96%. Polymerization curves were derived from DSC thermograms using the integrated heat evoked by the polymerization of MMA. Polymerization curves break when an inhibitor is consumed (Figure 2). These breaks are sharp and provide a reliable measure of the IP of the inhibitor. The presence of oxygen retards polymerization because oxygen reacts with MMA radicals activated by the initiator and then subsequently produces a non-radical product. Thus, polymerization of the control was slightly inhibited, even though the reaction was carried out in a sealed DSC pan, because the pan contained a small amount of oxygen; approximately 8.128 x 10-8 mole/L since it had been sealed in air. Tangents were drawn to polymerization curves at an early stage in the run. The IP of test compounds was determined from the length of time between the zero point on the abscissa and the point of intersection of tangents drawn to the early stage of polymerization. The IP was calculated from the difference between the induction period of specimens and that of controls. The initial rates of polymerization in the absence (Rpcon) and presence (Rpinh) of natural and synthetic antioxidants were calculated from the slope of the plots of the first linear line of the conversion rate of MMA polymerization (tangent drawn at the early polymerization stage).
Rate of initiation
The induction period method was used to determine the rate of initiation (Ri) due to the thermal decomposition of AIBN or BPO according to Eqn. (1):
| Ri = n [IH]0/[IP] | (1) |
where [IH]0 is the concentration of the inhibitor at time zero and [IP] is the induction period. 2,6-di-tert-butyl-4-methoxyphenol (DTBM) was used to determine Ri, since its stoichiometric factor, n, is known to be 2.00 [7]. In the case of [MMA] = 9.4 M and [AIBN or BPO] = 0.1 M at 70°C, the induction period method using DTBM gave the rate of initiation, Ri, at 70°C. The Ri values of AIBN and BPO were 5.66 x 10-6 Ms-1 and 2.28 x 10-6 Ms-1, respectively.
Measurement of stoichiometric factor (n)
The relative n value in Eqn. (2) can be calculated from the induction period in the presence of inhibitors:
| n = Ri[IP]/[IH] | (2) |
where [IP] is the induction period in the presence of an inhibitor. The number of moles of peroxy radicals trapped by the antioxidant is calculated with respect to 1 mole of inhibitor moiety unit.
Measurement of the inhibition rate constant (kinh)
When Ri is constant, i.e. when new chains are started at a constant rate, a steady-state treatment can be applied and the initial rate of polymerization of MMA is given by Eqn. (3) [6]:
| Rpcon = {kp [MMA] Ri1/2}/(2kt)1/2 | (3) |
where MMA represents methyl methacrylate and kp and kt are the rate constants for chain propagation and termination, respectively.
The kp/(2kt)1/2 rate of polymerization of MMA (9.4 M) by AIBN (1 mol%) and BPO (1 mol%) at 70°C was a constant value, 9.86 x 10-2 M-1/2 s-1/2. The Rpinh rates are determined by Eqn. (4):
| Rpinh = {kp [MMA] Ri} /{n kinh [IH]} | (4) |
in which Rpinh is the initial rate of inhibited polymerization, [MMA], n, [IH] and kp are defined above, and kinh is the rate constant for scavenging (inhibiting) of MMA radicals by an antioxidant. From Eqn. (2) and Eqn. (4), kinh/kp can be calculated (Eqn. 5):
| kinh/kp = [MMA]/{[IP] x [Rpinh]} | (5) |
Footnotes
Sample Availability: Available from the authors.
References
- 1. Van Joost T. H., Siolz E., Van Der Hoek J. C. S. Simultaneous allergy to perfume ingredients. Contact Dermatitis. 1985;12:115–116. doi: 10.1111/j.1600-0536.1985.tb01071.x. White I. R., Johansen J. D., Gimenez Arnau E., Lepoittevin J.-P., Rastogi S., Bruze M., Andersen K. E., Frosch P. J., Goossens A, Menne T. Isoeugenol is an important contact allergen: can it be safely replaced with isoeugenol acetate? Contact Dermatitis. 1999;41:272–275. doi: 10.1111/j.1600-0536.1999.tb06160.x.
- 2.Murakami Y., Shoji M., Hirata A., Tanaka S., Yokoe I., Fujisawa S. Dehydrodiisoeugenol, an isoeugenol dimmer, inhibits lipopolysaccharide-stimulated nuclear factor kappa B activation and cyclooxygenase-2 expression in macrophages. Arch. Biochem. Biophys. 2005;434:326–332. doi: 10.1016/j.abb.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Atsumi T., Fujisawa S., Tonosaki K. A comparative study of the antioxidant/prooxidant activities of eugenol and isoeugenol with various concentrations and oxidation conditions. Toxicol. In Vitro. 2005;19:1025–1033. doi: 10.1016/j.tiv.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Awashi S., Pandya U., Singhal S .S., Lin J. T., Thiviyanathan V., Seifert w. E., Jr., Awashi Y. C., Ansari G. A. Curcumin-glutathione interactions and the role of human glutathione S-transferase P1-1. Chem. Boil. Interact. 2000;128:19–38. doi: 10.1016/S0009-2797(00)00185-X. [DOI] [PubMed] [Google Scholar]
- 5. Fujisawa S., Ishihara M., Kadoma Y. Kinetic evaluation of the reactivity of flavonoids as radical scavengers. SAR QSAR Environ. Res. 2002;13:617–627. doi: 10.1080/1062936021000020071. Fujisawa S., Kadoma Y., Yokoe I. Radical-scavenging activity of butylated hydroxytoluene (BHT) and its metabolites. Chem. Phys. Lipid. 2004;130:189–195. doi: 10.1016/j.chemphyslip.2004.03.005. Fujisawa S., Kadoma Y. Comparative study of the alkyl and peroxy radical scavenging activities of polyphenols. Chemosphere. 2006;62:71–79. doi: 10.1016/j.chemosphere.2005.04.006.
- 6. Kadoma Y., Atsumi T., Okada N., Ishihara M., Yokoe I., Fujisawa S. Radical-scavenging activity of natural methoxyphenols versus synthetic ones using the induction period method. Molecules. 2007;12:130–138. doi: 10.3390/12020130. Kadoma Y., Fujisawa S. Radical-scavenging activity of estrogen and estrogen-like compounds using the induction period method. Int. J. Mol. Sci. 2007;8:295–303.
- 7.Burton G. W., Ingold K. U. Autooxidation of biological molecules.1. The Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidant In Vivo. J. Am. Chem. Soc. 1981;103:6472–6477. doi: 10.1021/ja00411a035. [DOI] [Google Scholar]
- 8.Taylor W. L., Battersby A.R., editors. Oxidative coupling of phenols. Marcel Dekker; New York: 1967. [Google Scholar]
- 9.Zanarotti A. Synthesis and reactivity of vinyl quinone methides. J. Org. Chem. 1985;50:941–945. doi: 10.1021/jo00207a006. [DOI] [Google Scholar]