Abstract
Five novel 3-alkyl-4-phenylacetylamino-4,5-dihydro-1H-1,2,4-triazol-5-ones (2) were synthesized by the reactions of 3-alkyl-4-amino-4,5-dihydro-1H-1,2,4-triazol-5-ones (1) with phenylacetyl chloride and characterized by elemental analyses and IR, 1H-NMR, 13C-NMR and UV spectral data. The newly synthesized compounds 2 were titrated potentiometrically with tetrabutylammonium hydroxide in four non-aqueous solvents such as isopropyl alcohol, tert-butyl alcohol, acetonitrile and N,N-dimethylformamide, and the half-neutralization potential values and the corresponding pKa values were determined for all cases. In addition, these new compounds and five recently reported 3-alkyl-4-(p-methoxybenzoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones (3) were screened for their antioxidant activities.
Keywords: 4,5-Dihydro-1H-1,2,4-triazol-5-ones; Acylation; Acidity; Potentiometric titrations; pKa; Antioxidant
Introduction
Several articles concerning the acylation of some 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives have been published [1,2,3,4,5,6,7]. In addition, 1,2,4-triazole and 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives are reported to show a broad spectrum of biological activities such as antifungal, antimicrobial, hypoglycemic, antihypertensive, analgesic, antiparasitic, hypocholesteremic, antiviral, anti-inflammatory, antioxidant, antitumor and anti-HIV properties [3,8,9,10,11,12].
On the other hand, it is known that 1,2,4-triazole and 4,5-dihydro-1H-1,2,4-triazol-5-one rings have weak acidic properties, so some 1,2,4-triazole and 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives were titrated potentiometrically with tetrabutylammonium hydroxide (TBAH) in non-aqueous solvents, and the pKa values of the compounds were determined [4,5,6,7,11,12,13,14,15,16,17,18].
Furthermore, nowadays, antioxidants have become one of the major areas of scientific research. Antioxidants are extensively studied for their capacity to protect organisms and cells from damage that is induced by oxidative stress. Scientists in many different disciplines have become more interested in new compounds, either synthesized or obtained from natural sources, that could provide active components to prevent or reduce the impact of oxidative stress on cells [19]. Exogenous chemicals and endogenous metabolic processes in human body or in food system might produce highly reactive free radicals, especially oxygen derived radicals, which are capable of oxidizing biomolecules, resulting in cell death and tissue damage. Oxidative damages play a significantly pathological role in human diseases. For example, cancer, emphysema, cirrhosis, atherosclerosis and arthritis have all been correlated with oxidative damage. Also, excessive generation of ROS induced by various stimuli and which exceeds the antioxidant capacity of the organism leads to a variety of pathophysiological processes such as inflammation, diabetes, genotoxicity and cancer [20].
In the present paper, the antioxidant activity of five new 3-alkyl-4-phenylacetylamino-4,5-dihydro-1H-1,2,4-triazol-5-ones 2, which were synthesized by the reactions of 3-alkyl-4-amino-4,5-dihydro-1H-1,2,4-triazol-5-ones 1 with phenylacetyl chloride and five 3-alkyl-4-(p-methoxybenzoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones 3, which were synthesized according to literature methods [21], were determined (Scheme 1) [22,23,24]. Moreover, we also examined the potentiometric titrations of the synthesized type 2 compounds with tetrabutylammonium hydroxide (TBAH) in four non-aqueous solvents (isopropyl alcohol, tert-butyl alcohol, acetonitrile and N,N-dimethylformamide) to determine the corresponding half-neutralization potentials (HNP) and the corresponding pKa values. The data obtained from the potentiometric titrations was interpreted, and the effect of the C-3 substituent in 4,5-dihydro-1H-1,2,4-triazol-5-one ring as well as solvent effects were studied [4,5,6,7,11,12,13,14,15,16,17,18].
Scheme 1.
Results and Discussion
In this study, the structures of five new 3-alkyl-4-phenylacetylamino-4,5-dihydro-1H-1,2,4-triazol-5-ones 2 were identified using elemental analysis and IR, 1H-NMR, 13C-NMR and UV spectral data. In addition, these newly synthesized 2 type compounds were titrated potentiometrically with TBAH in four non-aqueous solvents such as isopropyl alcohol, tert-butyl alcohol, acetonitrile and N,N-dimethyl-formamide. The mV values read in each titration were plotted against TBAH volumes added (mL), and potentiometric titration curves were formed for all the cases. From the titration curves, the HNP values were measured, and the corresponding pKa values were calculated.
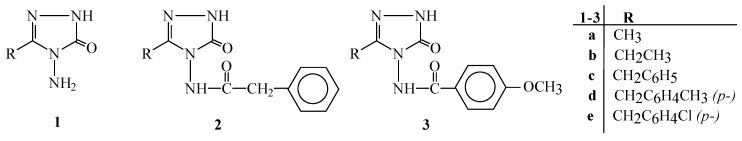
The half-neutralization potential (HNP) values and the corresponding pKa values of compounds 2a-2e, obtained from the potentiometric titrations with 0.05 M TBAH in isopropyl alcohol, tert-butyl alcohol, acetonitrile and N,N-dimethylformamide, are presented in Table 1.
Table 1.
The half-neutralization potentials (HNP) and the corresponding pKa values of compounds 2 in isopropyl alcohol, tert-butyl alcohol, acetonitrile and N,N-dimethyl-formamide.
| Compd | Isopropyl alcohol | Tert-butyl alcohol | N,N-Dimethyl-formamide | Acetonitrile | ||||
|---|---|---|---|---|---|---|---|---|
| HNP (mV) | pKa | HNP(mV) | pKa | HNP(mV) | pKa | HNP(mV) | pKa | |
| 2a | -268 | 11.51 | -386 | 13.44 | -382 | 13.37 | -324 | 12.37 |
| 2b | -290 | 11.84 | -385 | 13.44 | -425 | 14.07 | -370 | 13.15 |
| 2c | -276 | 11.58 | -384 | 13.39 | -403 | 13.68 | -358 | 12.82 |
| 2d | -300 | 11.99 | -378 | 13.20 | -394 | 13.56 | -341 | 12.77 |
| 2e | -296 | 11.81 | -373 | 13.20 | -405 | 13.69 | -379 | 13.35 |
When the dielectric permittivity of the solvents is taken into consideration, the following arrangement in order of decreasing acidity may be expected: N,N-dimethylformamide (ε=36.7) > acetonitrile (ε=36) > isopropyl alcohol (ε=19.4) > tert-butyl alcohol (ε=12). As seen in Table 1, the acidic arrangement for compounds 2b-2d is: isopropyl alcohol > acetonitrile > tert-butyl alcohol > N,N-dimethylformamide, and for compound 2e, it is: isopropyl alcohol > tert-butyl alcohol > acetonitrile > N,N-dimethylformamide, while the order for compound 2a is: isopropyl alcohol > acetonitrile > N,N-dimethylformamide > tert-butyl alcohol (Figure 1). In isopropyl alcohol, all these compounds show the strongest acidic properties, while they show the weakest acidic properties in N,N-dimethylformamide (tert-butyl alcohol for compound 2a). This situation may be attributed to the hydrogen bonding between the negative ions formed and the solvent molecules in the amphiprotic neutral solvents.
Figure 1.
The change of the pKa values with the dielectric constant of the solvent.
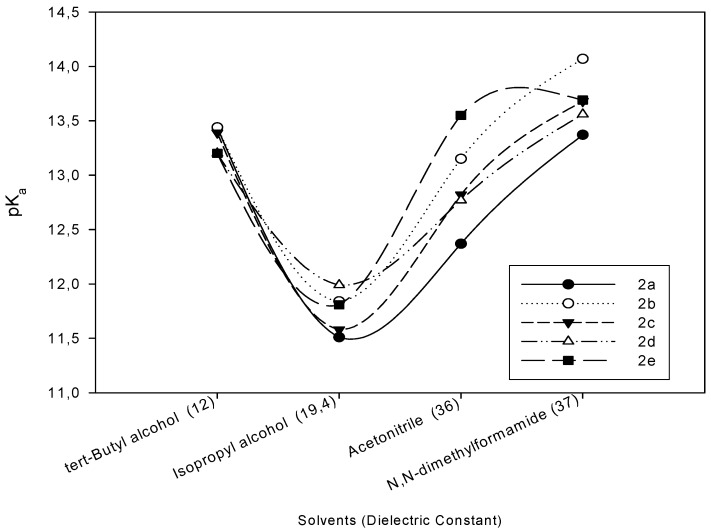
The degree to which a pure solvent ionizes was represented by its autoprotolysis constant, KHS.
| 2HS = H2S+ + S- |
For the above reaction the constant is defined by:
| KHS = [H2S+][S-] |
Autoprotolysis is an acid-base reaction between identical solvent molecules is which some act as an acid and others as a base. Consequently, the extent of an autoprotolysis reaction depends both on the intrinsic acidity and the instrinsic basicity of the solvent. The importance of the autoprotolysis constant in titrations lies in its effect on the completeness of a titration reaction [25]. The change of the pKa values with autoprotolysis constant is given in Figure 2. As it is well known, the acidity of a compound depends on several factors. The two most important ones are the solvent effect and molecular structure [4,5,6,7,11,12,13,14,15,16,17,18]. Table 1 and Figure 1 show that the HNP values and corresponding pKa values obtained from the potentiometric titrations rely on the non-aqueous solvents used and the substituents at C-3, in 4,5-dihydro-1H-1,2,4-triazol-5-one ring.
Figure 2.
The exchange of the pKa values with autoprotolysis constant.
Compounds 2 and 3 were screened for their in-vitro antioxidant activities. Several methods are used to determine antioxidant activities. The methods used in this study are discussed below:
Total reductive capability using the potassium ferricyanide reduction method
The reductive capabilities of compounds are assessed by the extent of conversion of the Fe3+/ ferricyanide complex to the Fe2+/ ferrous form. The reducing powers of the compounds were observed at different concentrations, and results were compared with BHA, BHT and α-tocopherol. The reducing capacity of a compound may serve as a significant indicator for its potential antioxidant activity [26]. The antioxidant activity of putative antioxidant has been attributed to various mechanisms, among which are prevention chain initiation, binding of transition metal ion catalyst, decomposition of peroxides, prevention of continued hydrogen abstraction, reductive capacity and radical scavenging [27]. In this study, all the amounts of the compounds showed lower absorbance than blank. Hence, no activities were observed to reduce metal ions complexes to their lower oxidation state or to take part in any electron transfer reaction. In other words, compounds did not show the ability of electron donor to scavenge free radicals.
DPPH• radical scavenging activity
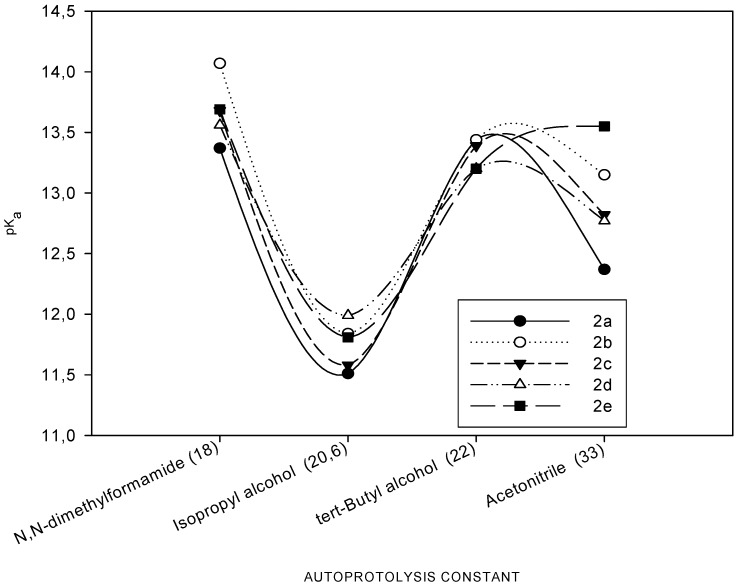
The scavenging the stable DPPH radical model is a widely used method to evaluate antioxidant activities in a relatively short time compared with other methods. The effect of antioxidants on DPPH radical scavenging was thought to be due to their hydrogen donating ability [28]. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule [29]. The reduction capability of DPPH radicals was determined by decrease in its absorbance at 517 nm induced by antioxidants. The absorption maximum of a stable DPPH radical in ethanol was at 517 nm. The decrease in absorbance of DPPH radical was caused by antioxidants, because of reaction between antioxidant molecules and radical, progresses, which result in the scavenging of the radical by hydrogen donation. It is visually noticeable as a discoloration from purple to yellow. Hence, DPPH is usually used as a substrate to evaluate antioxidative activity of antioxidants [30]. BHT and α-toco-pherol were used as a reference to antioxidant compounds. All the compounds tested with this method showed lower absorbance than absorbance of the control reaction and higher absorbance of the standard antioxidant reactions. These results indicate that the newly synthesized compounds showed mild activities as a radical scavenger, indicating that it has moderate activities as hydrogen donors (Figure 3).
Figure 3.
Scavenging effect of compounds 2, 3, BHA and α-tocopherol at different concentrations (50-100-150 µg/mL).
Ferrous ion chelating activity
The chelating effect towards ferrous ions by the compounds and standards was determined according to the method of Dinis [24]. Ferrozine can quantitatively form complexes with Fe2+. In the presence of chelating agents, the complex formation is disrupted with the result that the red colour of the complex is decreased. Measurement of colour reduction therefore allows estimation of the chelating activity of the coexisting chelator [31]. Transition metals have pivotal role in the generation oxygen free radicals in living organism. The ferric iron (Fe3+) is the relatively biologically inactive form of iron. However, it can be reduced to the active Fe2+, depending on condition, particularly pH [32] and oxidized back through Fenton type reactions with the production of hydroxyl radical or Haber-Weiss reactions with superoxide anions. The production of these radicals may lead to lipid peroxidation, protein modification and DNA damage. Chelating agents may not activate metal ions and potentially inhibit the metal-dependent processes [33]. Also, the production of highly active ROS such as O2.-, H2O2, and OH·, are also catalyzed by free iron though Haber-Weiss reactions:
| O·2 + H2O2 → O2 + OH- + OH· |
Among the transition metals is known as the most important lipid oxidation pro-oxidant due to its high reactivity. The ferrous state of iron accelerates lipid oxidation by breaking down the hydrogen and lipid peroxides to reactive free radicals via the Fenton reactions:
| Fe2+ + H2O2 → Fe3+ + OH- + OH. |
Also Fe3+ ion produces radicals from peroxides, although the rate is tenfold less than of Fe2+ ion, which is the most powerful pro-oxidant among various sort of metal ions [34].
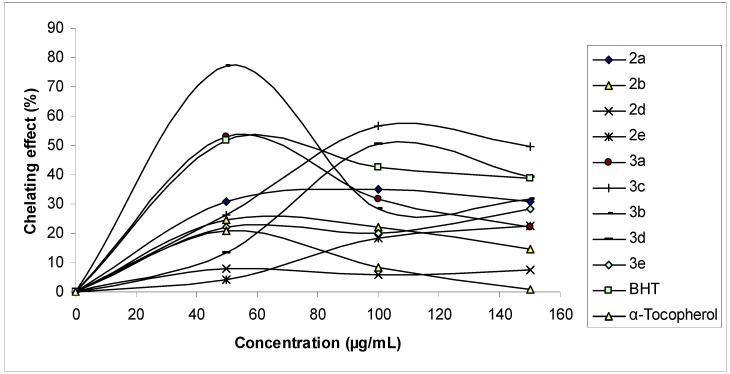
Ferrous ion chelating activities of the compounds, BHT and α-tocopherol are shown in Figure 4. In this study, metal chelating capacity was significant since it reduced the concentrations of the catalyzing transition metal. It was reported that chelating agents that form σ-bonds with a metal are effective as secondary antioxidants because they reduce the redox potential thereby stabilizing the oxidized form of metal ion [35]. The data obtained from Figure 4 reveal that the compounds demonstrate a marked capacity for iron binding, except for 2c, suggesting that their action as peroxidation protector may be related to its iron binding capacity. On the other hand, free iron is known to have low solubility and a chelated iron complex has greater solubility in solution, which can be contributed solely from the ligand. Furthermore, the compound-Fe may also be active, since it can participate in iron-catalyzed reactions.
Figure 4.
Metal chelating effect of different amount of the compounds 2 and 3, BHT and α-tocopherol on ferrous ions.
Experimental
General
Melting points were taken on an Electrothermal 9100 digital melting point apparatus and are uncorrected. IR spectra were registered on a Perkin-Elmer 1600 FTIR spectrometer. 1H-NMR and 13C-NMR spectra were recorded in deuterated dimethyl sulfoxide with TMS as internal standard on a Varian Mercury spectrometer at 200 MHz and 50 MHz, respectively. UV absorption spectra were measured in 10-mm quartz cells between 200 and 400 nm using a Shimadzu UV-1201 spectrophotometer. The starting compounds 1a-e were prepared according to the literature [1,36]. Compounds 3 were obtained through recently reported methods [21].
General method for the preparation of 3-alkyl-4-phenylacetylamino-4,5-dihydro-1H-1,2,4-triazol-5-ones 2
The appropriate 3-alkyl-4-amino-4,5-dihydro-1H-1,2,4-triazol-5-one 1 (0.01 mol) was heated with phenylacetyl chloride (0.01 mol) in n-butyl acetate (40 mL) for 7 hr and then allowed to cool. The crystals formed were filtered. The product was recrystallized from an appropriate solvent to give 2.
3-Methyl-4-phenylacetylamino-4,5-dihydro-1H-1,2,4-triazol-5-one (2a). Yield 85%; mp. 194 °C (EtOH-water, 1:3); Calculated for C11H12N4O2 (232.24): 56.89% C, 5.21% H, 24.12% N; found: 56.74% C, 5.30% H, 23.96% N; 1H-NMR: δ 1.92 (s, 3H, CH3), 3.66 (s, 2H, CH2), 7.08-7.36 (m, 5H, Ar-H), 11.05 (s, 1H, NH), 11.64 (s, 1H, NH); 13C-NMR: δ 10.57 (CH3), 39.94 (CH2), 127.07, 128.67 (2C), 129.27 (2C), 134.96 (aromatic carbons), 145.10 (triazole C3), 152.80 (triazole C5), 170.37 (CO); IR: 3375, 3300 (NH), 1735, 1685 (C=O), 1610 (C=N), 760, 715 (monosubstituted benzenoid ring) cm-1; UV λmax (ε, L·mol-1·cm-1): 264 (3490), 224 (23230) nm.
3-Ethyl-4-phenylacetylamino-4,5-dihydro-1H-1,2,4-triazol-5-one (2b). Yield 87%; mp. 67 °C (EtOH-water, 1:3); 1H-NMR: δ 1.04 (t, 3H, CH3), 2.25 (q, 2H, CH2), 3.66 (s, 2H, CH2), 7.25-7.36 (m, 5H, Ar-H), 11.08 (s, 1H, NH), 11.69 (s, 1H, NH); 13C-NMR: δ 10.05 (CH3), 18.22 (CH3CH2), 40.10 (CH2Ph), 127.16, 128.74 (2C), 129.33 (2C), 134.99 (aromatic carbons), 149.08 (triazole C3), 153.10 (triazole C5), 170.41 (CO); IR: 3500, 3200 (NH), 1720, 1680 (C=O), 1605 (C=N), 740, 705 (monosubstituted benzenoid ring) cm-1; UV λmax (ε, L·mol-1·cm-1): 258 (2140), 222 (22600) nm.
3-Benzyl-4-phenylacetylamino-4,5-dihydro-1H-1,2,4-triazol-5-one (2c). Yield 71%; mp. 104 °C (EtOH-water, 1:3); Calculated for C17H16N4O2 (308.34): 66.22% C, 5.23% H, 18.17% N; found: 65.77% C, 5.01% H, 17.27% N; 1H-NMR: δ 3.65 (s, 2H, CH2), 3.69 (s, 2H, CH2), 7.08-7.35 (m, 10H, Ar-H), 11.08 (s, 1H, NH), 11.87 (s, 1H, NH); 13C-NMR: δ 31.10 (CH2), 40.10 (CH2), 127.20 (2C), 128.77 (2C), 128.82 (2C), 128.96 (2C), 129.43 (2C), 134.97, 135.09 (aromatic carbons), 147.27 (triazole C3), 152.90 (triazole C5), 170.41 (CO); IR: 3400, 3200 (NH), 1735, 1675 (C=O), 1600 (C=N), 750, 710 (monosubstituted benzenoid ring) cm-1; UV λmax (ε, L·mol-1·cm-1): 258 (2290), 221 (22410) nm.
3-(4-Methylbenzyl)-4-phenylacetylamino-4,5-dihydro-1H-1,2,4-triazol-5-one (2d). Yield 80%; mp. 224 °C (EtOH-water, 1:3); 1H-NMR: δ 2.25 (s, 3H, CH3), 3.52 (s, 2H, CH2), 3.61 (s, 2H, CH2), 6.96 (d, A part of AB system, 2H, J=7.94 Hz, Ar-H), 7.08 (d, B part of AB system, 2H, J=7.94 Hz, Ar-H), 7.32 (s, 5H, Ar-H), 11.01 (s, 1H, NH), 11.77 (s, 1H, NH); 13C-NMR: δ 20.83 (CH3), 30.40 (CH2), 39.97 (CH2), 127.07, 128.64 (2C), 128.71 (2C), 129.26 (2C), 129.32 (2C), 131.94, 134.50, 136.10 (aromatic carbons), 146.80 (triazole C3), 153.00 (triazole C5), 170.19 (CO); IR: 3200, 3100 (NH), 1730, 1675 (C=O), 1597 (C=N), 810 (1,4-disubstituted benzenoid ring), 745, 698 (monosubstituted benzenoid ring) cm-1; UV λmax (ε, L·mol-1·cm-1): 257 (1590), 225 (25520) nm.
3-(4-Chlorobenzyl)-4-phenylacetylamino-4,5-dihydro-1H-1,2,4-triazol-5-one (2e). Yield 73%; mp. 195 °C (EtOH-water, 1:3); Calculated for C17H16N4O2Cl (342.78): 59.57% C, 4.41% H, 16.34% N; found: 59.49% C, 4.24% H, 16.20% N; 1H-NMR: δ 3.94 (s, 2H, CH2), 3.60 (s, 2H, CH2), 7.09 (d, A part of AB system, 2H, J=8.55 Hz, Ar-H), 7.19-7.38 (m, 7H, Ar-H), 11.00 (s, 1H, NH), 11.82 (s,1H, NH); 13C-NMR: δ 30.20 (CH2), 39.93 (CH2), 127.07, 128.61 (2C), 128.64 (2C), 129.33 (2C), 130.71 (2C), 131.95, 134.00, 135.10 (aromatic carbons), 146.70 (triazole C3), 152.90 (triazole C5), 170.20 (CO); IR: 3350, 3250 (NH), 1730, 1688 (C=O), 1592 (C=N), 805 (1,4-disubstituted benzenoid ring), 745, 710 (monosubstituted benzenoid ring) cm-1; UV λmax (ε, L·mol-1·cm-1): 258 (3300), 225 (23700) nm.
Potentiometric titrations
In this study, a Jenway 3040-model ion analyzer was used for potentiometric titrations. An Ingold pH electrode was preferred because of the advantage. For each compound that would be titrated, the 0.001 M solution was separately prepared in each non-aqueous solvent. The 0.05 M solution of TBAH in isopropyl alcohol, which is widely used in the titration of acids, was used as titrant. The mV values, that were obtained in pH-meter, were recorded. Finally, the HNP values were determined by drawing the mL (TBAH)-mV graphic.
Antioxidant Activity: Chemicals
Butylated hydroxyltoluene (BHT) was purchased from E. Merck. Ferrous chloride, α-tocopherol, 1,1-diphenyl-2-picryl-hydrazyl (DPPH.), 3-(2-pyridyl)-5,6-bis(phenylsulfonic acid)-1,2,4-triazine (ferrozine), butylated hydroxyanisole (BHA) and trichloracetic acid (TCA) were bought from Sigma (Sigma –Aldrich GmbH, Sternheim, Germany).
Reducing power
The reducing power of the synthesized compounds was determined according to the method of Oyaizu [22]. Different concentrations of the samples (50-250 µg/mL) in DMSO (1 mL) were mixed phosphate buffer (2.5 mL, 0.2 M, pH = 6.6) and potassium ferricyanide (2.5 mL, 1%). The mixture was incubated at 50oC for 20 min. after incubation period; a portion of trichloroacetic acid (2.5 mL, 10%) was added to the mixture, which was then centrifuged for 10 min at 1000 x g. The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCI3 (0.5 mL, 0.1%), and the absorbance was measured at 700 nm in a spectrophometer. Higher absorbance of the reaction mixture indicated greater reducing power.
Free radical scavenging activity
Free radical scavenging activity of compounds was measured by DPPH., using the method of Blois [23]. Briefly, a 0.1 mM solution of DPPH. in ethanol was prepared, and this solution (1 mL) was added to sample solutions in DMSO (3 mL) at different concentrations (50-250 µg/mL). The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. Then the absorbance was measured at 517 nm in a spectrophometer. Lower absorbance of the reaction mixture indicated higher free radical scavenging activity. The DPPH. concentration (mM) in the reaction medium was calculated from the following calibration curve and determined by linear regression (R: 0.997):
| Absorbance = 0.0003 x DPPH. – 0.0174 |
The capability to scavenge the DPPH radical was calculated using the following equation:
| DPPH. scavenging effect (%) = (A0 – A1/A0) x 100 |
Where A0 is the absorbance of the control reaction and A1 is the absorbance in the presence of the samples or standards.
Metal chelating activity
The chelating ferrous ions by the synthesized compounds and standards were estimated by the method of Dinis et al. [24]. Briefly, compounds (50-250 µg/mL) were added to a solution of 2 mM FeCI2 (0.05 mL). The reaction was initiated by the addition of 5 mM ferrozine (0.2 mL) and the mixture was shaken vigorously and left standing at room temperature for 10 min. after the mixture had reached equilibrium, the absorbance of the solution was then measured spectrophotometrically at 562 nm in a spectrophotometer. All test and analyses were run in triplicate and averaged. The percentage of inhibition of ferrozine-Fe2+ complex formation was given by the formula:
| % Inhibition = (A0 – A1/A0) x 100 |
Where A0 is the absorbance of the control, and A1 is the absorbance in the presence of the samples or standards. The control did not contain compound or standard.
Footnotes
Sample Availability: Contact the authors.
References
- 1.Ikizler A. A., Yüksek H. Acetylation of 4-amino-4,5-dihydro-1H-1,2,4-triazol-5-ones. Org. Prep. Proced. Int. 1993;25:99–105. doi: 10.1080/00304949309457935. [DOI] [Google Scholar]
- 2.Ikizler A., Doğan N., Ikizler A. A. The acylation of 4-amino-4,5-dihydro-1H-1,2,4-triazol-5-ones. Rev. Roum. Chim. 1998;43:741–746. [Google Scholar]
- 3.Yüksek H., Demibaş A., Ikizler A., Johansson C. B., Çelik C., Ikizler A. A. Synthesis and antibacterial activities of some 4,5-dihydro-1H-1,2,4-triazol-5-ones. Arzneim.-Forsch./Drug Res. 1997;47:405–409. [PubMed] [Google Scholar]
- 4.Yüksek H., Alkan M., Ocak Z., Bahçeci Ş., Ocak M., Özdemir M. Synthesis and acidic properties of some new potential biologically active 4-acylamino-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives. Indian J. Chem. 2004;43B:1527–1531. [Google Scholar]
- 5.Yüksek H., Ocak Z., Alkan M., Bahçeci Ş., Özdemir M. Synthesis and determination of pKa values of some new 3,4-disubstituted-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives in non-aqueous solvents. Molecules. 2004;9:232–240. doi: 10.3390/90400232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahçeci Ş., Yüksek H., Ocak Z., Azaklı A., Alkan M., Ozdemir M. Synthesis and potentiometric titrations of some new 4-(benzylideneamino)-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives in non-aqueous media. Collect. Czech. Chem. Commun. 2002;67:1215–1222. [Google Scholar]
- 7.Bahçeci Ş., Yüksek H., Ocak Z., Köksal C., Ozdemir M. Synthesis and non-aqueous medium titrations of some new 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives. Acta Chim. Slov. 2002;49:783–794. [Google Scholar]
- 8.Demirbaş N., Uğurluoğlu R. Synthesis and antitumor activities of some 4-(1-naphthylidenamino)-and 4-(1-naphthylmethylamino)-1,2,4-triazol-5-one derivatives. Turk J. Chem. 2004;28:679–690. [Google Scholar]
- 9.Ikizler A. A., Uçar F., Yüksek H., Aytin A., Yasa I., Gezer T. Synthesis and antifungal activity of some new arylidenamino compounds. Acta Pol. Pharm.-Drug Res. 1997;54:135–140. [PubMed] [Google Scholar]
- 10.Bhat A. R., Bhat G. V., Shenoy G. G. Synthesis and in-vitro antimicrobial activity of new 1,2,4-triazoles. J. Pharm. Pharmacol. 2001;53:267–272. doi: 10.1211/0022357011775307. [DOI] [PubMed] [Google Scholar]
- 11.Yüksek H., Küçük M., Alkan M., Bahçeci Ş., Kolaylı S., Ocak Z., Ocak U., Şahinbaş E., Ocak M. Synthesis and andioxidant activities of some new 4-(4-hydroxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives with their acidic properties. Asian J. Chem. 2006;18:539–550. [Google Scholar]
- 12.Yüksek H., Kolaylı S., Küçük M., Yüksek M. O., Ocak U., Şahinbaş E., Sivrikaya E., Ocak M. Synthesis and andioxidant activities of some 4-benzylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives. Indian J. Chem. 2006;45B:715–718. [Google Scholar]
- 13.Ikizler A. A., Şentürk H. B., Ikizler A. pK’a values of some 1,2,4-triazole derivatives in nonaqueous media. Doğa-Tr. J. Chem. 1991;15:345–354. [Chem. Abstr.1992, 116, 173458x] [Google Scholar]
- 14.Ikizler A. A., Ikizler A., Şentürk H. B., Serdar M. The pKa values of some 1,2,4-triazole and 1,2,4-triazolin-5-one derivatives in nonaqueous media. Doğa-Tr. Kimya D. 1988;12:57–66. [Chem. Abstr.1988, 109, 238277q] [Google Scholar]
- 15.Yüksek H., Ocak Z., Özdemir M., Ocak M., Bekar M., Aksoy M. A study on novel 4-heteroarylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-ones. Indian J. Heterocycl. Chem. 2003;13:49–52. [Google Scholar]
- 16.Yüksek H., Üçüncü O., Alkan M., Ocak Z., Bahçeci Ş., Özdemir M. Synthesis and non-aqueous medium titrations of some new 4-benzylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives. Molecules. 2005;10:961–970. doi: 10.3390/10080961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yüksek H., Bahçeci Ş., Ocak Z., Özdemir M., Ocak M., Ermiş B., Mutlu T. Synthesis and determination of acid dissociation constants of some new 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives. Asian J. Chem. 2005;17:195–201. [Google Scholar]
- 18.Yüksek H., Bahçeci Ş., Ocak Z., Alkan M., Ermiş B., Mutlu T., Ocak M., Özdemir M. Synthesis of some 4,5-dihydro-1H-1,2,4-triazol-5-ones. Indian J. Heterocycl. Chem. 2004;13:369–372. [Google Scholar]
- 19.Hussain H. H., Babic G., Durst T., Wright J., Flueraru M., Chichirau A., Chepelev L. L. Development of novel antioxidants: design, synthesis, and reactivity. J. Org. Chem. 2003;68:7023–7032. doi: 10.1021/jo0301090. [DOI] [PubMed] [Google Scholar]
- 20.McClements J., Decker E. A. Lipid oxidation in oil water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food system. J. Food Sci. 2000;65:1270–1282. [Google Scholar]
- 21.Yüksek H., Alkan M., Bahçeci Ş., Çakmak İ., Ocak Z., Baykara H., Aktaş Ö., Ağyel E. Synthesis, determination of pKa values and GIAO NMR calculations of some new 3-alkyl-4-(p-methoxybenzoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones. J. Mol. Struc. 2007 in pres. [Google Scholar]
- 22.Oyaizu M. Studies on products of browning reaction prepared from glucosamine. Japan. Nutri. 1986;44:307–316. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 23.Blois M. S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 24.Dinis T. C. P., Madeira V. M. C., Almeida L. M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 25.Hargis L. G. Analytical Chemistry Principles and Techniques. Prentice-Hall, Inc.; New Jersey: 1988. [Google Scholar]
- 26.Meir S., Kanner J., Akiri B., Hadas S. P. Determination and Involvement of Aqueous Reducing Compounds in Oxidative Defense Systems of Various Senescing Leaves. J. Agri. Food. Chem. 1995;43:1813–1819. doi: 10.1021/jf00055a012. [DOI] [Google Scholar]
- 27.Yildirim A., Mavi A., Kara A. A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agri. Food. Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- 28.Baumann J., Wurn G., Bruchlausen V. Prostaglandin synthetase inhibiting O2- radical scavenging properties of some flavonoids and related phenolic compounds. Naunyn-Schmiedebergs Arch. Pharmacol. 1979;308:R27. [Google Scholar]
- 29.Soares J. R., Dinis T. C. P., Cunha A. P., Ameida L. M. Antioxidant activities of some extracts of Thymus zygis. Free Radical Res. 1997;26:469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- 30.Duh P. D., Tu Y. Y., Yen G. C. Antioxidant activity of water extract of Harng Jyur (Chyrsanthemum morifolium Ramat) Lebn. Wissen. Technol. 1999;32:269. doi: 10.1006/fstl.1999.0548. [DOI] [Google Scholar]
- 31.Yamaguchi F., Ariga T., Yoshimira Y., Nakazawa H. Antioxidative and anti-glycation activity of garcinol from Garcinia indica fruit rind. J. Agri. Food. Chem. 2000;48:180–185. doi: 10.1021/jf990845y. [DOI] [PubMed] [Google Scholar]
- 32.Strlic M., Radovic T., Kolar J., Pihlar B. Anti- and prooxidative properties of gallic acid in fenton-type systems. J. Agri. Food. Chem. 2002;50:6313–6317. doi: 10.1021/jf025636j. [DOI] [PubMed] [Google Scholar]
- 33.Finefrock A. E., Bush A. I., Doraiswamy P. M. Current status of metals as therapeutic targets in Alzheimer's disease. J. Am. Geriatr. Soc. 2003;51:1143–1148. doi: 10.1046/j.1532-5415.2003.51368.x. [DOI] [PubMed] [Google Scholar]
- 34.Çaliş I., Hosny M., Khalifa T., Nishibe S. Secoiridoids from Fraxinus angustifolia. Phytochemistry. 1993;33:1453–1456. [Google Scholar]
- 35.Gordon M. H. Food Antioxidants. Elsevier; London-New York: 1990. pp. 1–18. [Google Scholar]
- 36.Ikizler A. A., Un R. Reactions of ester ethoxycarbonylhydrazones with some amine type compounds. Chim. Acta Turc. 1979;7:269–290. [Chem. Abstr.1991, 94, 15645d] [Google Scholar]