Abstract
The condensation of substituted pyrazine-2-carboxylic acid chlorides with ring-substituted anilines yielded five substituted pyrazine-2-carboxylic acid amides. The synthesis, and analytical, lipophilicity and biological data of the newly synthesized compounds are presented in this paper. The photosynthesis inhibition, antialgal activity and the effect of a series of pyrazine derivatives as abiotic elicitors on the accumulation of flavonoids in a callus culture of Ononis arvensis (L.) were investigated. The most active inhibitor of the oxygen evolution rate in spinach chloroplasts was 6-chloro-pyrazine-2-carboxylic acid (3-iodo-4-methylphenyl)-amide (2, IC50 = 51.0 μmol∙L-1). The highest reduction of chlorophyll content in Chlorella vulgaris was found for 5-tert-butyl-N-(4-chloro-3-methylphenyl)-pyrazine-2-carboxamide (3, IC50 = 44.0 μmol∙L-1). The maximal flavonoid production (about 900%) was reached after a twelve-hour elicitation process with 6-chloropyrazine-2-carboxylic acid (3-iodo-4-methylphenyl)-amide (2).
Keywords: synthesis of pyrazinecarboxamides, photosynthesis inhibiting and antialgal activity, rest-harrow, flavonoid production, culture in vitro, lipophilicity
Introduction
Plants display physiological and morphological responses to a range of physical and chemical factors known as elicitors. These can be both of biotic and abiotic origin. Elicitation-produced stress activates the defensive reactions of plant cells, which result in transcription changes of the genes coding the enzymes influencing secondary metabolite biosynthesis. The mechanism of elicitation in plant cells is intensively discussed. Plant tissue cultures are studied primarily due to their production of many valuable secondary metabolites. The elicitation increased through the production of these secondary metabolites opened their important economical exploitation in biotechnology [1].
The pyrazine ring is a part of many polycyclic compounds of biological or industrial significance. The widespread occurrence of pyrazines in nature, especially in the flavours of many food systems, their effectiveness at very low concentrations as well as the still increasing applications of synthetic pyrazines in the flavour and fragrance industry are responsible for the high interest in these compounds. Pyrazines occur naturally in a wide range of food items, as e.g. in heated bread or meat, baked potatoes and coffee, wherein they are formed from serine and threonine [2].
Pyrazine is a weaker base than pyridine, due to the induction effect of the second nitrogen. Certain pyrazines, especially dihydropyrazines, are essential for all forms of life. Pyrazine derivatives have been used as antioxidants. These compounds have shown important therapeutic applications, for example a high antimycobacterial activity [2,3,4,5,6]. Furthermore, a simple pyrazine compound, 3-amino-6-chloro-pyrazine-6-carboxylic acid, has shown anti-auxin behaviour [7].
Plant tissue and cell cultures provide model systems for the study of various molecular, physiological, organism and genetic problems. These systems have been used in the study of herbicides and other xenobiotics [8]. Herbicides are generally considered as growth inhibitors, thus their different inhibitory responses have been studied in various culture systems.
A callus tissue, particularly that of tobacco, was used in examination of the inhibitory activity of various compounds on the cytokinine production [9,10]. It presents a bioassay suitable for substances inhibiting cell division, because the differentiation is minimized and the cell division is the primary morphological process. Response of the tobacco callus on the presence of 11 different dinitroanilines was examined. According to the values of fresh and dry weight, dinitramine was the most active growth inhibitor [11].
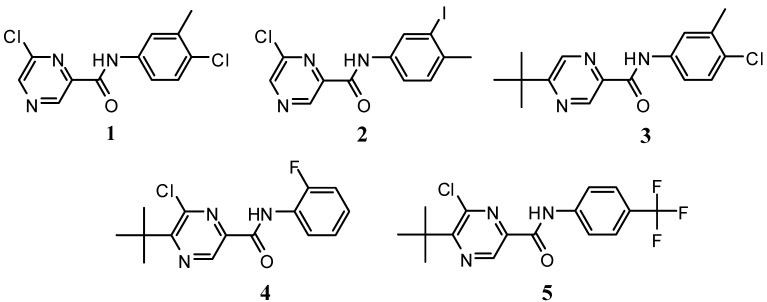
On the basis of the results of our previous studies [4,12,13,14], novel pyrazine derivatives (Figure 1) were designed and prepared and their herbicidal and abiotic elicitation activity was evaluated. The aim of this work is the synthesis of the title compounds, evaluation of their photosynthesis-inhibitory and antialgal activity and quantification of their influence on the flavonoid production.
Figure 1.
Novel pyrazinecarboxylic acid amides 1-5 designed and prepared for their herbicide and abiotic elicitation activity evaluation.
Results and Discussion
The majority of the compounds involved in this study inhibited the photosynthetic electron transport in spinach chloroplasts. From the obtained data [3,4,14] it can be concluded that the inhibitory activity of investigated compounds on the oxygen evolution rate (OER) depends on their lipophilicity as well as on the electron accepting or withdrawing effects of the substituents. Therefore we designed in preference the compounds with the lipophilic and/or electron-withdrawing substituents in the benzene moiety (3-CH3-4-Cl, 3-I-4-CH3, 3-CH3-4-Cl, 2-F, 4-CF3). However, the values of their inhibitory activities were rather low. At higher applied concentrations, the solubility of some compounds in the suspension of spinach chloroplasts was limited. The most effective inhibitor was 6-chloro-N-(3-iodo-4-methylphenyl)-pyrazine-2-carboxamide (2, IC50 = 51 μmol∙L-1).
All investigated compounds reduced the chlorophyll content in Chlorella vulgaris. The IC50 values (see Table 1), related to reduction of chlorophyll content in C. vulgaris, were determined for all the compounds and the highest inhibitory effect was detected for 5-tert-butyl-N-(4-chloro-3-methylphenyl)-pyrazine-2-carboxamide (3, IC50 = 44 μmol∙L-1).
Table 1.
Oxygen evolution rate (OER) inhibition in spinach chloroplasts (IC50), reduction of chlorophyll content in C. vulgaris (IC50), maximal flavonoid content (% / hours) after elicitation and without elicitation (%), and calculated lipophilicity (log P, Clog P) of compounds 1-5 in comparison with the herbicide diurone (DCMU).
| Comp. | OER inhibition Spinacia oleracea IC50 [mmol/L] | Chlorophyll reduction Chlorella vulgaris IC50 [mmol/L] | Maximal flavonoid content (%) after elicitation/hours | Flavonoid content (%) without elicitation | Lipophilicity Log P / CLog P |
| 1 | 0.595 | 0.080 | 0.0987 / 6 | 0.0557 | 2.53 / 3.43369 |
| 2 | 0.051 | a | 0.5072 / 12 | 0.0557 | 3.33 / 3.54369 |
| 3 | 0.190 | 0.044 | 0.1007 / 48 | 0.0557 | 3.76 / 4.53404 |
| 4 | 0.069 | 0.089 | 0.0759 / 6 | 0.0557 | 3.78 / 3.59069 |
| 5 | 0.184 | a | 0.1839 / 168 | 0.0557 | 4.54 / 4.95436 |
| DCMU | 0.0019 | 0.0073 | - | - | 2.76 / 2.69124 |
a: IC50 values for the compounds 2 and 5 were not obtained due their low solubility in the medium.
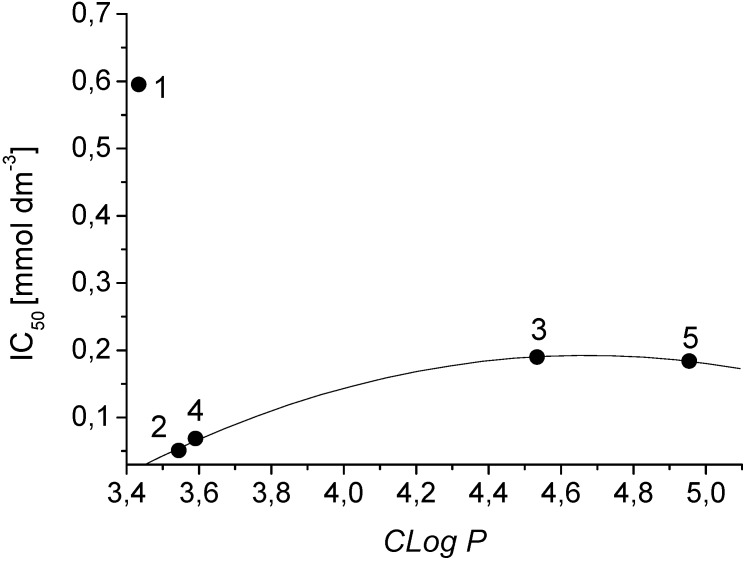
Figure 2 presents the dependence of the IC50 values of the studied compounds related to the inhibition of OER (detected in the suspension of spinach chloroplasts) on the compound lipophilicity. The correlation between the IC50 values and the lipophilicity parameters shows a quasi-parabolic course for compounds with CLog P from 3.54369 to 4.95436. The low inhibitory activity of compound 1 (despite of its relatively low lipophilicity, CLog P = 3.43369) could be caused by its insufficient aqueous solubility.
Figure 2.
Dependence of inhibition of oxygen evolution rate in the suspension of spinach chloroplasts (IC50 values) on the lipophilicity (Clog P) in the series of studied compounds 1-5.
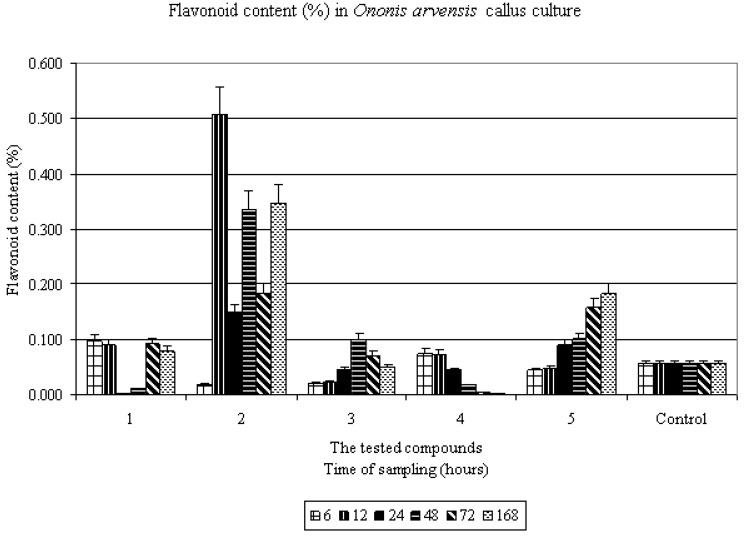
All tested compounds were able to influence the flavonoid content in Ononis arvensis L. culture in vitro, see Table 1. Flavonoid production was increased after 6, 12, 72 and 168-hour elicitation with compound 1. The maximal flavonoid production (about 900%) was reached after twelve-hour elicitation with 6-chloro-N-(3-iodo-4-methylphenyl)-pyrazine-2-carboxamide (2). High levels of flavonoid content were also observed after 48 and 72-hour elicitation with the same compound. Compound 3 increased the flavonoid accumulation only after 48 and 72-hour exposure to this elicitor. Increased flavonoid production was found also after 6 and 12-hour exposure to compound 4. Compound 5 amplified the flavonoid content after 24-hour elicitation process, while the highest level of flavonoids was reached in the case of 168-hour application.
If we compare the elicitation effects on the flavonoid production in a callus culture of Ononis arvensis L., we can conclude that iodo derivative 2 is the most active compound (enhancement about 900%), see Figure 3. Our results confirm the findings that these compounds are able to influence (increase) the secondary metabolite (flavonoids) production. The substituted amides of pyrazinecarboxylic acids also intensely increased the flavonolignan content in Silybum marianum culture in vitro [13].
Figure 3.
Abiotic elicitation activity evaluations of prepared compounds 1-5 and Control (without elicitors).
Our research is focused on binuclear analogues with the -CONH- bridge, which can form centro symmetric dimer pairs with the peptidic carboxamido group of a peptide, needed for binding to the receptor site, possibly by forming of hydrogen bond [14]. All substituted amides of pyrazinecarboxylic acid studied can be interpreted as the more lipophilic aza-analogues of nicotinamide. Therefore, the new goal of our research is the study of the site of action of the active abiotic elicitors.
Experimental
General
All organic solvents used for the synthesis were of analytical grade. The solvents were dried and freshly distilled under argon atmosphere. The reactions were monitored and the purity of the products was checked by TLC (Silufol UV 254, Kavalier Votice, Czech Republic) using petroleum ether/EtOAc (9:1) as developing solvents. The plates were visualized using UV light (254 nm). Melting points (uncorrected) were determined on Boetius PHMK 05 (VEB Kombinat Nagema, Radebeul, Germany). Elemental analyses were performed on the automatic microanalyser CHNS-O CE instrument (FISONS EA 1110, Milano, Italy). Infrared spectra were recorded in Nicolet Impact 400 spectrometer in KBr pellets. 1H- and 13C-NMR spectra were recorded on a Varian Mercury – Vx BB 300 (Varian, Palo Alto CA, USA), operating at 299.95 MHz for 1H- and 75.43 MHz for 13C-, in CDCl3 solutions at ambient temperature. The chemical shifts δ are given in ppm related to tetramethylsilane (TMS) as an internal standard. The coupling constants (J) are reported in Hz.
General procedure for pyrazinecarboxamide synthesis.
A mixture of acid, i.e. pyrazine-2-carboxylic, 6-chloropyrazine-2-carboxylic [15], 5-tert-butylpyrazine-2-carboxylic [16] or 5-tert-butyl-6-chloropyrazine-2-carboxylic [5] acids, respectively, (50.0 mmol) and thionyl chloride (5.5 mL, 75.0 mmol) in dry toluene (20 mL) was refluxed for about 1 h. Excess of thionyl chloride was removed by repeated evaporation with dry toluene in vacuo. The crude acyl chloride dissolved in dry acetone (50 mL) was added dropwise to a stirred solution of the corresponding substituted amine (50.0 mmol) in 50 mL of dry pyridine at room temperature. After the addition was complete, stirring continued for another 30 min. The reaction mixture was then poured into 100 mL of cold water and the crude amide was collected and recrystallised from aqueous ethanol.
6-Chloro-N-(4-chloro-3-methylphenyl)-pyrazine-2-carboxamide (1). Yield 47%; Anal. Calcd. for C12H9Cl2N3O (282.1): 51.09% C, 3.22% H, 14.89% N; Found: 50.81% C, 3.11% H, 14.64% N; Mp 137 °C; Log P: 2.53; Clog P: 3.43369; TLC: RF = 0.82; IR cm-1: 3343 (N-H), 2926 (methyl), 1690 (C=O), 1589 (phenyl), 1541 (N-H), 1377, 1172, 1144, and 1009 (pyrazine); 1H-NMR δ: 9.38 (1H, s, H3), 9.34 (1H, bs, NH), 8.81 (1H, s, H5), 7.66 (1H, d, J=2.6 Hz, H2´), 7.54 (1H, dd, J=8.5 Hz, J=2.6 Hz, H6´), 7.34 (1H, d, J=8.5 Hz, H5´), and 2.40 (3H, s, CH3); 13C-NMR δ: 159.3, 147.6, 147.4, 143.8, 142.2, 137.0, 135.3, 130.4, 129.6, 122.2, 118.7, and 20.2.
6-Chloro-N-(3-iodo-4-methylphenyl)-pyrazine-2-carboxamide (2). Yield 83%; Anal. Calcd. for C12H9ClIN3O (373.6): 38.58% C, 2.43% H, 11.25% N; Found: 38.80% C, 2.62% H, 11.37% N. Mp 173.4-174.5 °C; Log P: 3.33; Clog P: 3.54369; TLC: RF = 0.81. IR cm-1: 3321 (N-H), 2975 (methyl), 1667 (C=O), 1598 (phenyl), 1530 (N-H), 1371, 1300, 1167, 1118, and 1012 (pyrazine); 1H-NMR δ: 9.38 (1H, s, H3), 9.32 (1H, bs, NH), 8.81 (1H, s, H5), 8.21 (1H, d, J=2.2 Hz, H2´), 7.68 (1H, dd, J=8.2 Hz, J=2.2 Hz, H6´), and 7.24 (1H, d, J=8.2 Hz, H5´), 2.42 (3H, s, CH3); 13C-NMR δ: 159.2, 147.6, 147.4, 143.7, 142.2, 138.3, 135.3, 129.9, 129.7, 119.8, 100.8, and 27.5
5-tert-Butyl-N-(4-chloro-3-methylphenyl)-pyrazine-2-carboxamide (3). Yield 86%; Anal. Calcd. for C16H18ClN3O (303.8): 63.26% C, 5.97% H, 13.83% N; Found: 63.44% C, 6.11% H, 13.54% N; Mp 122 °C; Log P: 3.76; Clog P: 4.53404; TLC: RF = 0.94; IR cm-1: 3425 (N-H), 2965, 2933, 2921, 2869 (tert-butyl, methyl), 1688 (C=O), 1587 (phenyl), 1519 (N-H), 1407, 1278, and 1143 (pyrazine); 1H-NMR δ: 9.60 (1H, bs, NH), 9.38 (1H, d, J=1.5 Hz, H3), 8.61 (1H, d, J=1.5 Hz, H6), 7.69 (1H, d, J=2.5 Hz, H2´), 7.52 (1H, dd, J=8.5 Hz, J=2.5 Hz, H6´), 7.32 (1H, d, J=8.5 Hz, H5´), 2.39 (3H, s, CH3), and 1.45 (1H, bs, OH); 13C-NMR δ: 167.8, 161.0, 142.9, 141.2, 139.0, 136.9, 135.9, 129.8, 129.5, 122.0, 118.4, 37.1, 29.7, and 20.2.
5-tert-Butyl-6-chloro-N-(2-fluorophenyl)-pyrazine-2-carboxamide (4). Yield 71%; Anal. Calcd. for C15H15ClFN3O (307.7): 58.54% C, 4.91% H, 13.65% N; Found: 58.57% C, 4.94% H, 13.57% N; Mp 81.7 °C; Log P: 3.78; Clog P: 3.59069; TLC: RF = 0.95; IR cm-1: 3385 (N-H), 2990, 2978, 2967 (tert-butyl), 1699 (C=O), 1618 (phenyl), 1532 (N-H), 1259, 1149, and 1057 (pyrazine); 1H-NMR δ: 9.64 (1H, bs, NH), 9.23 (1H, d, J=1.4 Hz, H3), 8.50 (1H, d, J=1.4 Hz, H6´), 7.22 (1H, m, H3´), 7.20 (1H, m, H5´), 7.08 (1H, m, H4´), and 1.55 (9H, s, CH3); 13C-NMR δ: 164.8, 159.9, 154.5, 151.2, 145.9, 140.8, 125.7 (d, J=10.0 Hz), 125.1 (d, J=3.5 Hz), 124.6 (d, J=3.5 Hz), 121.5, and 115.1 (d, J=18.9 Hz).
5-tert-Butyl-6-chloro-N-(4-trifluoromethylphenyl)-pyrazine-2-carboxamide (5). Yield 67%; Anal. Calcd. for C16H15ClF3N3O (357.8): 53.72% C, 4.23% H, 11.75% N; Found: 53.70% C, 4.34% H, 11.67% N. Mp 81.7 °C; Log P: 4.54; Clog P: 4.95436; TLC: RF = 0.95; IR cm-1: 3385 (N-H), 2990, 2978, 2967, 2936, 2873 (tert-butyl, trifluoromethyl), 1709 (C=O), 1618 (phenyl), 1538 (N-H), 1328, 1161, 1122, and 1069 (pyrazine); 1H-NMR δ: 9.51 (1H, bs, NH), 9.28 (1H, s, H3), 7.94-7.86 (2H, m, AA´, BB´, H2´, H6´), 7.70-7.62 (2H, m, AA´, BB´, H3´, H5´), and 1.56 (9H, s, CH3); 13C-NMR δ: 168.3, 161.4, 143.1, 140.9, 140.4, 139.1, 126.4 (q, J=3.8 Hz), 126.3 (q, J=32.7 Hz), 124.1 (q, J=271.4 Hz), 119.4, 37.1, and 29.7.
Lipophilicity calculations
Log P, i. e. the logarithm of the partition coefficient P for n-octanol/water, and CLog P values (the logarithm of n-octanol/water partition coefficient P based on established chemical interactions) were calculated using the programs CS ChemOffice Ultra ver. 10.0 (CambridgeSoft, Cambridge, MA, U.S.A.) The results are shown in Table 1 and in Figure 2.
Herbicidal activities
Study of the inhibition of oxygen evolution rate in spinach chloroplasts.
Chloroplasts were prepared by the procedure of Walker from spinach (Spinacia oleracea L.) [17]. The inhibition of photosynthetic electron transport (PET) in spinach chloroplasts was determined spectrophotometrically (Kontron Uvikon 800, Kontron, Muenchen, Germany) using an artificial electron acceptor 2,6-dichlorophenol-indophenol (DCIPP) according to Kralova et al. [18] and the rate of photosynthetic electron transport was monitored as a photo-reduction of DCPIP. The measurements were carried out in a phosphate buffer (0.02 mol·L-1, pH 7.2) containing sucrose (0.4 mol·L-1), MgCl2 (0.005 mol·L-1) and NaCl (0.015 mol·L-1). The chlorophyll content was 30 mg/L in these experiments and the samples were irradiated (~100 W/m2) from a 10 cm distance with a halogen lamp (250 W) using a 4 cm water filter to prevent warming of the samples (suspension temperature 22 °C). The studied compounds were dissolved in DMSO due to their limited water solubility. The applied DMSO concentration (up to 4%) did not affect the photochemical activity in spinach chloroplasts (PET). The inhibitory efficiency of the studied compounds was expressed as the IC50 values, i.e. molar concentration of the compounds causing 50% decrease in the oxygen evolution relative to the untreated control. The comparable IC50 value for a selective herbicide 3-(3,4-dichlorophenyl)-1,1-dimethylurea, (diurone, DCMU) was about 1.9 μmol/L. [19]. The results are summarised in Table 1.
Reduction of chlorophyll content in the green algaeChlorella vulgaris Beij.
The green algae C. vulgaris Beij. was cultivated statically at room temperature according to Kralova et al. [20] (photoperiod 16 h light/8 h dark; photosynthetic active radiation 80 μmol/m2∙s, pH 7.2). The effect of the compounds on algal chlorophyll (Chl) content was determined after 7-day cultivation in the presence of the tested compounds. The Chl content in the algal suspension was determined spectrophotometrically (Kontron Uvikon 800, Kontron, München, Germany) after extraction into methanol according to Wellburn [21]. The Chl content in the suspensions at the beginning of the cultivation was 0.01 mg·L-1. The applied compound concentrations were as follows: 0.83, 4.2, 8.3, 25, 50, 75, and 100 μmol·L-1. Due to the low solubility of the studied compounds in water, these were dissolved in DMSO. DMSO concentration in the algal suspensions did not exceed 0.25 % and the control samples contained the same amount of DMSO as the suspensions treated with the tested compounds. The antialgal activity of the compounds was expressed as the IC50 (the concentration of the inhibitor causing a 50% decrease in content of chlorophyll as compared with the control sample) or as a percentage of the control determined for the studied concentration range (0.83 - 100 μmol·L-1) with the corresponding standard deviation (S.D.). The comparable IC50 value for a selective herbicide DCMU was about 7.3 μmol·L-1 [21]. The results are summarised in Table 1.
Abiotic elicitacion
Study of the substituted pyrazinecarboxylic acids on flavonoid production in vitro cultures.
Callus culture was derived from the seeds of a germinating plant Ononis arvensis. L. (10th-20th) passage. Calluses were added to the Murashige-Skoog medium (MS) [22] containing α-naphtylacetic acid (α-NAA) as a growth regulator in a concentration of 5.4x10-5 mol·L-1. Cultivation was performed on paper bridges in Erlenmeyer flasks for 35 days. These cultures were incubated in growth chambers at 26 °C under a 16-hour photoperiod. The abiotic elicitors/concentrations used were: 6-chloro-N-(4-chloro-3-methylphenyl)-pyrazine-2-carboxamide (1)/1.06 x 10-3 mol∙L-1; 6-chloro-N-(3-iodo-4-methylphenyl)-pyrazine-2-carboxamide (2)/0.53 x 10-3 mol∙L-1; 5-tert-butyl-N-(4-chloro-3-methyl-phenyl)-pyrazine-2-carboxamide (3)/0.98 x 10-3 mol∙L-1; 5-tert-butyl-6-chloro-N-(2-fluorophenyl)-pyrazine-2-carboxamide (4)/1.02 mol∙L-1 and 5-tert-butyl-6-chloro-N-(4-trifluoromethylphenyl)-pyrazine-2-carboxamide (5)/0.84 mol∙L-1, respectively. These elicitors were added to the callus culture on the 35th day of cultivation. 12; 24; 48; 72 and 168 hours after elicitor application, the calluses were taken off, dried and the content of flavonoids was determined. Simultaneously, the control (without elicitors) was run after 24 and 168 hours.
Evaluation of flavonoid Stock solution [23].
Powdered calluses (0.600 g) and a solution of hexamethylenetetramine (1 mL, 5 g∙L-1) were placed in a 100 mL round-bottomed flask and acetone (20 mL) and hydrochloric acid (2 mL) were added. The mixture was boiled under reflux condenser for 30 min. The liquid was filtered through a plug of absorbent cotton into a flask. The extraction was repeated twice for 10 min. After cooling and filtration the combined acetone extracts were diluted in a 100.0 mL volumetric flask with acetone. The solution (20.0 mL) was introduced into separating funnel, water (20.0 mL) was added and the mixture was shaken with one 15 mL aliquot and then three 10 ml aliquots ethyl acetate. The ethyl acetate extracts were combined in a separating funnel, then washed with two portions of water (50 mL each) and filtered through anhydrous sodium sulphate (10 g) into a volumetric flask and diluted to 50.0 mL with ethyl acetate.
Test solution.
To stock solution (10.0 mL) was added aluminium chloride reagent (1 mL) and the mixture was diluted to 25.0 mL with a 5% v/v solution of glacial acetic acid in methanol.
Compensation solution.
Stock solution (10.0 mL) was diluted to 25.0 mL with a 5% v/v solution of glacial acetic acid in methanol. The absorbance of the test solution was measured after 30 min, by comparison with the compensation solution at 425 nm. The percentage content of flavonoids was calculated as isoquercitroside from the expression: (A x 1.25)/m; i.e. taking the specific absorbance of isoquercitroside to be 500, and where A = absorbance at 425 nm; m = mass of the substance to be examined in grams. All experimental analyses were carried out on a minimum of three independent samples for each elicitation period and three replicates of each sample were assayed. Statistical significance was calculated using Student’s T-test for unpaired data (P≤0.05). The results are summarised in Table 1.
Acknowledgments
This study was supported by the Ministry of Education of the Czech Republic (MSM 0021620822), and by the Slovak Scientific Grant Agency VEGA (No. 1/0089/03). The authors thank to co-workers from the Faculty of Pharmacy in Hradec Kralove, namely to Dr. J. Kunes for the interpretation of NMR spectra, to Ms. V. Hronova for technical assistance, to Ms. Iva Vencovska for recording IR spectra.
Footnotes
Sample Availability: Samples of the compounds 1-5 are available from authors.
References
- 1.Angelova Z., Georgiev S., Roos W. Elicitation of plants. Biotechnol. Biotechnol. Eq. 2006;20:72–83. doi: 10.1080/13102818.2006.10817345. [DOI] [Google Scholar]
- 1.Dolezal M. Biological active pyrazines of natural and synthetic origin. Chem. Listy. 2006;100:959–966. [Google Scholar]
- 2.Dolezal M., Palek L., Vinsova J., Buchta V., Jampilek J., Kralova K. Substituted Pyrazinecarboxamides; Synthesis and Their Biological Evaluation. Molecules. 2006;11:242–256. doi: 10.3390/11040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolezal M., Cmedlova P., Palek L., Vinsova J., Kunes J., Buchta V., Jampilek J., Kralova K. Synthesis and antimycobacterial evaluation of substituted pyrazinecarboxamides. Eur. J. Med. Chem. 2008;43 doi: 10.1016/j.ejmech.2007.07.013. in press. [DOI] [PubMed] [Google Scholar]
- 4.Janin Y. L. Antituberculosis drugs: Ten years of research. Bioorg. Med. Chem. 2007;15:2479–2513. doi: 10.1016/j.bmc.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Tripathi R. P., Tewari N., Dwivedi N., Tiwari V. K. Fighting tuberculosis: An old disease with new challenges. Med. Res. Rev. 2005;25:93–131. doi: 10.1002/med.20017. [DOI] [PubMed] [Google Scholar]
- 6.Ricci D., Maggiali C.A., Ronchini F., Tirillini B., Fraternale D. Phytochemistry. 1991;30:2821–2824. doi: 10.1016/S0031-9422(00)98205-0. [DOI] [Google Scholar]
- 7.Camper N. D., McDonald S. K. Tissue and cell cultures as model system in herbicide research. Rev. Weed Sci. 1989;4:169–190. [Google Scholar]
- 8.Linsmaier E., Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 1965;18:100–127. doi: 10.1111/j.1399-3054.1965.tb06874.x. [DOI] [Google Scholar]
- 9.Schmitz R. V., Skoog F., Hecht S. M., Leonard N. J. Comparison of zeatin indoleacetate with zeatin and indoleacetic acid in tobacco bioassay. Plant. Physiol. 1972;50:114–116. doi: 10.1104/pp.50.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huffman J. B., Camper N. D. Growth inhibition in tobacco (Nicotiana tabacum) callus by 2,6-dinitroaniline herbicides and protection by D-α-tocopherol acetate. Weed Sci. 1978;26:527–530. [Google Scholar]
- 11.Tumova L., Ostrozlik P. Ononis arvensis in vitro - Abiotic elicitation. Cesk. Slov. Farm. 2002;51:173–176. [PubMed] [Google Scholar]
- 12.Tumova L., Gallova K., Rimakova J., Dolezal M., Tuma J. The effect of substituted amides of pyrazine-2-carboxylic acids on flavolignan production in Silybum marianum culture in vitro. Acta Physiol. Plant. 2005;27:357–362. [Google Scholar]
- 13.Dolezal M., Kralova K., Sersen F., Miletin M. The site of action of some anilides of pyrazine-2-carboxylic acids in the photosynthetic apparatus. Folia Pharm. Univ. Carol. 2001;26:13–20. [Google Scholar]
- 14.Abe Y., Shigeta Y., Uchimaru F., Okada S., Ozasayama E. 69 12,898. Japanese Patent. 1969 [Chem. Abstr. 1969, 71, 112979y]
- 15.Dolezal M., Hartl J., Miletin M., Machacek M., Kralova K. Chem. Pap. 1999;53:126–128. [Google Scholar]
- 16.Walker D. A. In: Methods in Enzymology Part C. Colowick S.P., Kaplan N.O., editors. Vol. 69. Academic Press; New York: 1980. pp. 94–104. [Google Scholar]
- 17.Kralova K., Sersen F., Sidoova E. Photosynthesis inhibition produced by 2-alkylthio-6-R-benzothiazoles. Chem. Pap. 1992;46:348–350. [Google Scholar]
- 18.Fedke C. Biochemistry and Physiology of Herbicide Action. Springer Verlag; Berlin-Heidelberg-New York: 1982. [Google Scholar]
- 19.Kralova K., Sersen F., Melnik M. Inhibition of photosynthesis in Chlorella vulgaris by Cu(II) complexes with biologically active ligands. J. Trace Microprobe Techn. 1998;16:491–500. [Google Scholar]
- 20.Wellburn A. R. The spectral determination of chlorophyll-A and chlorophyll-B, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant. Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- 21.Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–479. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 22.Czech Pharmacopea 1997. Grada; Prague: 1997. p. 1491. [Google Scholar]