Abstract
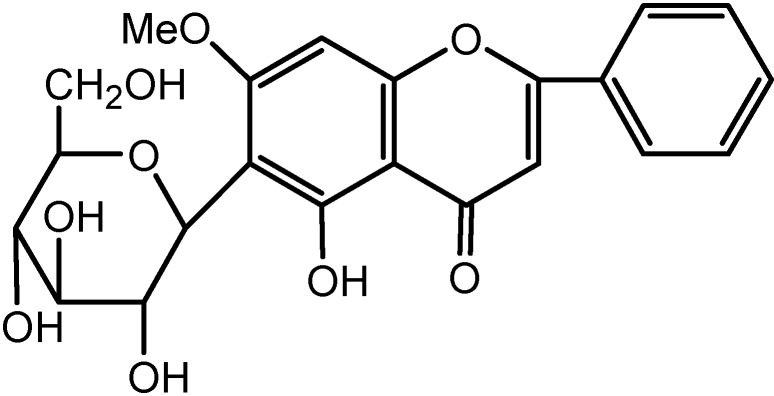
A novel flavonoid C-glycoside, 5-hydroxy-7-methoxy-6-C-glycosylflavone (1), was isolated from the aerial part of Sphaeranthus indicus. Its structure was elucidated by spectroscopic methods.
Keywords: Sphaeranthus indicus, flavonoid C-glycoside, 5-hydroxy-7-methoxy-6-C-glycosylflavone.
Introduction
Sphaeranthus indicus L. is a multi-branched herb with round purple flowers that grows plentifully in rice fields [1,2] and is distributed throughout India, Ceylon, Malay, China and Africa. It used indigenously in the Indian system of traditional medicine as a remedy for various ailments, being used as a tonic, laxative, digestive, anthelmintic, and the treatment of insanity, tuberculosis, diseases of the spleen, anaemia, bronchitis, elephantiasis, pain of the uterus and vagina, piles, asthma, leucoderma and hemicrania. Almost every part of the plant is useful. Leaves of the plant are eaten as a pot-herb and have anxiolytic [3], macrofilaricidal [4], antimicrobial [5], and insecticidal activities [6]. Earlier work on the aerial parts of this plant revealed it to be quite rich in essential oils, glucosides and eudesmanoids [7], along with some uncharacterized sesquiterpenes, phenolic glycosides, and sesquiterpene lactones [8,9].
A systematic phytochemical study of the chemical constituents of the plant led us to isolate a new flavonoid C-glycoside compound 1, together with eight known compounds, namely n-pentacosan, hentriacontane, n-triacontanol, β-sitosterol, stigmasterol, β-D-glucoside of β-sitosterol, sphaeranthine and a phenolic glycoside (C22H26O12). In this paper we report the isolation and structure elucidation of 5-hydroxy-7-methoxy-6-C-glycosylflavone (1) from the aerial parts of the plant.
Results and Discussion
Compound 1 was obtained as pale yellow needles, m.p. 230-232oC. Elemental analysis of compound 1 is consistent with the molecular formula C22H22O9 (calcd. 430.409). Its IR spectrum indicated the presence of hydroxyl (3403 cm-1) and carbonyl (1645 cm-1) groups. Bands in the 1650-1050 cm-1 range are typical of a flavone skeleton. The UV spectrum exhibited absorption maxima at 260 nm (band II) and 300 nm (band I), that are characteristic absorption bands of a flavone skeleton [10]. No shift in band I of compound 1 was observed with the addition of AlCl3/HCl, suggesting formation of a hydroxy-keto complex at the 5-OH and the absence of an O-dihydroxyl grouping in the B ring [10,11]. The fragment m/z 105 (15%) and 102 (18%) in the mass spectrum supports the unsubstituted nature of the flavonoid ring B.
Figure 1.
Structure of Compound 1.
The 1H-NMR spectrum exhibited a flavonoid pattern and showed signals at δ 6.65 (1H, s), 6.51 (1H, br), typical of the flavone skeleton protons at C-3 and C-8. Chemical shifts of 7.90 (d, H2’, H6’) and 7.51 (m, H3’, H4’and H5’) suggested that there is no substitution on the B ring of the flavonoid. The signal at δ 12.80 ppm was assigned to the C-5 hydroxyl. The observed singlet at 3.91 (s, -OCH3) is evidence for one methoxy group. Signals in the 13C-NMR spectrum just below δ 77 ppm indicated the presence of a glucose moiety [12]. The signal at δ 5.84 ppm was assigned to the anomeric proton (H-1”) with a coupling constant (J = 10 Hz) indicating a β-configuration [13], sugar proton signals at δ 4.47, multiple attributed to the H-3”, H-4” and H-5” respectively. The position of the C-glucose moiety on the C-6 position was based on the above evidence and comparison with the values in literature for the analogous compound isoorientin [14].
Experimental
General
Melting points were measured in open capillary tubes on a Buchi 530 apparatus and are uncorrected. NMR spectra were obtained on a JEOL AL300 FT-NMR spectrometer (300 MHz for 1H, 75.45 MHz for 13C) in CDCl3 solutions with tetramethylsilane as an internal reference. EI-MS data was obtained on a JEOL JMS D-300 instrument. UV spectra were recorded on a Cary-14 instrument. IR spectra were recorded on Perkin Elmer 257 infrared spectrometer.
Plant material and product isolation
Aerial parts of plant were collected in September 2006 in a suburb of Varanasi, India. The plant identification was verified by Professor N. K. Dubey, Departement of Botany, Faculty of Science, Banara Hindu University, Varanasi, India. A specimen sample of the plant material has been preserved in our laboratory no. 39, Department of Chemistry, Faculty of Science, Banaras Hindu University, Varanasi, India. The dried plant material (3 Kg) was first extracted with petroleum ether (5 L) for 24 h and then with MeOH (5 L) for 38 h using a Soxhlet apparatus. After concentration of the methanolic extracts (0.5 g), the residues were extracted with CHCl3 (2 L x 3), EtOAc (3 L x 3) and EtOH (3 L x 2) respectively. The EtOH extract (20.5 g) was chromatographed on silica gel (200-300 mesh; 600 g) and eluted with EtOAc-MeOH-H2O (4:1:0.1,), after rechromatography with MeOH, MeOH-H2O (1:1) eluates gave pale yellow compound 1 (35 mg).
Compound 1: UV-Visible λmax (nm) MeOH: 252, 260, 300; MeOH+MeONa: 246, 270; MeOH+AlCl3 250, 272, 335, 380 nm; IR νmax (KBr, cm-1) 3403, 1645; 1H-NMR: 12.80 (s, HO-5), 7.90 (d, J = 6.5, H-2’, H-6’), 7.51 (m, H-3’, H-4’, H-5’), 6.65 (s, H-3), 6.51(s, H-8), 5.84 (d, J = 10, H-1”), 5.20 (t, J = 8.6, H-2”), 4.47 (m, H-3”, 4”, 5”), 4.46 (d, J = 9.6, H-6”), 3.39 (s, -OCH3) ppm; 13C-NMR: 182.9 (C-4), 164.7 (C-5), 160.5 (C-2), 160.2 (C-7), 157.9 (C-9), 139.9 (C-4’), 130.1 (C-1’), 130 (C-3’, C-5’), 127.2 (C-2’, C-6’), 110 (C-6), 105.5 (C-3), 104.7 (C-10), 90 (C-8), 83.4 (C-5”), 80.7 (C-3”), 75.3 (C-1”), 72.5 (C-2”), 72.0 (C-4”), 63.0 (C-6”), 56.2 (C-7-OMe); MS m/z (rel. int.): 430 [M+] (60%), 429 (10%), 415 (15%), 105 (15%), 102 (18%).
Acknowledgments
Department of Chemistry, Faculty of Science, Banaras Hindu University, Varanasi, India, is sincerely acknowledged for providing the spectral facilities, including 1H-NMR and 13C-NMR. Central Drug Research Institute, Lucknow, India, is also acknowledged for mass spectroscopy of the samples.
Footnotes
Sample availability: Contact the Author.
References
- 1.Kirtikar K.R., Basu B.D. Indian Medicinal Plants. Lalit Mohan Basu; Allahabad, India: 1935. p. 1346. II. [Google Scholar]
- 2.Sadaf F., Saleem R., Ahmed M., Ahmad S. I., Zafar N. Healing potential of cream containing extract of Sphaeranthus indicus on dermal wounds in Guinea pigs. J. Ethnopharmacol. 2006;107:161–163. doi: 10.1016/j.jep.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Ambavade S. D., Mhetre N. A., Tate V. D., Bodhankar S. L. Pharmacological evaluation of the extracts of Sphaeranthus indicus flowers on anxiolytic activity in mice. Indian J. Pharmacol. 2006;38:254–259. doi: 10.4103/0253-7613.27021. [DOI] [Google Scholar]
- 4.Nisha M., Kalyanasundaram M., Paily K. P., Abidha, Vanamail P., Balaraman K. In vitro screening of medicinal plant extracts for macrofilaricidal activity. Parasitol. Res. 2007;100:575–579. doi: 10.1007/s00436-006-0294-9. 3. [DOI] [PubMed] [Google Scholar]
- 5.Ram A. J., Bhakshu L. M., Raju R. R. V. In vitro antimicrobial activity of certain medicinal plants from Eastern Ghats, India, used for skin diseases. J. Ethnopharmacol. 2004;90:353–357. doi: 10.1016/j.jep.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan D., Nadarajan L. Wet land weeds Sphaeranthus indicus Asteraceae (Linn.) a potential green pesticide for managing Angoumois grain moth Sitotroga cerealella (Oliv.) Resist. Pest Manag. 2006;16:21–25. 1. [Google Scholar]
- 7.Pujar P. P., Sawaikar D. D., Rojatkar S. R., Nagasampagi B. A. Eudesmanoids from Sphaeranthus indicus. Fitoterapia. 2000;3:264–268. doi: 10.1016/s0367-326x(99)00157-4. [DOI] [PubMed] [Google Scholar]
- 8.Gogte M. G., Ananthasubramanian L., Nargund K. S., Bhattacharya S. C. Some interesting sesquiterpenoids from Sphaeranthus indicus Linn. Indian J. Chem. 1986;25:233–238. [Google Scholar]
- 9.Sohoni J. S., Rojatkar S. R., Kulkarhni M. M., Dhaneshwar H. N., Tavale S. S., Gururow T. N., Nagasampagi B. A. A new eudesmenolide & 2-hydroxycostic acid from Sphaeranthus indicus linn. X-Ray molecular structure of 4α, 5α-epoxy-7α-hydroxyeudesmanolide. J. Chem. Soc.Perkin Trans I. 1988:157–160. [Google Scholar]
- 10.Markham K. R. Techniques of Flavonoids Identification. Academic Press; London: 1982. pp. 36–51. Chapter 3. [Google Scholar]
- 11.Mabry T. J., Markham K. R., Thomas M. B. The Systematic Identification of Flavonoids. Springer; Berlin: 1970. pp. 35–250. [Google Scholar]
- 12.Markham K. R., Ternai B., Stanly R., Geiger H., Mabry T.J. Carbon-13 NMR studies of flavonoids- III. Tetrahedron. 1978;34:1389–1397. doi: 10.1016/0040-4020(78)88336-7. [DOI] [Google Scholar]
- 13.Harbone J. B. In: The Flavonoids. Advances in Research. Jay M., editor. Chapman and Hall; London, New York: 1986. pp. 57–87. Chapter 3. [Google Scholar]
- 14.Komatsu M., Tomimori T., Makiguchi Y. Studies on the constituents of Swertia japonica, II. Isolation and structure of new flavonoid, swertia japonin. Chem. Pharm. Bull. 1967;15:1567–1572. doi: 10.1248/cpb.15.1567. [DOI] [PubMed] [Google Scholar]