Abstract
Since early work in the 1980s, the bile acids have become well established as building blocks for supramolecular chemistry. The author’s laboratory has specialised in converting cholic acid, the archetypal bile acid, into macrocyclic and acyclic receptors for anions and carbohydrates. This review highlights the synthetic aspects of this work, especially the use of modern synthetic methodology to perform less obvious structural transformations.
Keywords: Supramolecular chemistry, steroids, anion recognition, carbohydrate recognition, stereoselective synthesis
Introduction
Supramolecular chemistry involves the translation of molecular structure into function. A first requirement is that structures should be predictable, and this can be difficult for flexible systems (even when, as with proteins, a single preferred structure may exist). Rigid subunits are therefore valuable assets for supramolecular design. In the mid-1980s we surveyed the area and realised that the range of units employed was quite limited, hardly extending beyond the aromatic ring. On the other hand, it seemed that Nature produced a variety of alternatives, often aliphatic and almost always chiral. Some were available cheaply in substantial quantities, and thus realistic candidates for exploitation.
Perhaps the most obvious were the steroids, with their extended rigid polycyclic frameworks. Of these, the bile acids were exceptionally attractive. Firstly they possessed high levels of functionality, distributed fairly evenly around the steroidal framework. Secondly, the functional groups could be differentiated and transformed in various ways, developed during the golden age of steroid chemistry. Finally, they were readily available, the least expensive being cholic acid (1) at ca. €0.5/g (current prices). Fortunately, cholic acid was also the most functionalised, and therefore the most interesting and versatile of these starting materials.
Although a few studies had employed bile acids in micellar systems [1,2], there had been no attempts to exploit them as building blocks for preorganised 3D architectures. Beginning around 1985, we therefore embarked on a programme of design and synthesis aimed at creating supramolecular systems from cholic acid. Others have followed similar paths, and the bile acids have become well-established, standard components for supramolecular chemistry [3,4]. Our own efforts have focused on the construction of carbohydrate receptors, the recognition of inorganic anions and the enantioselective recognition of carboxylates. This work has been reviewed previously from the supramolecular viewpoint (i.e. concentrating on the binding and recognition properties) [5,6], but less attention has been paid to synthetic aspects. In the course of the programme, a variety of new transformations have been performed on 1, and a range of steroidal intermediates have been generated. Some of these compounds and procedures could well find application outside our own programme. This brief account summarises a number of our synthetic sequences, highlighting the less usual, “nonclassical” conversions which we have uncovered or developed. As well as introducing the methodology, and hopefully stimulating its use, the stories illustrate the close relationship between design and synthesis. Supramolecular design must always be performed with an eye on synthesis; molecules must be accessible to be useful. Although supramolecular chemists are often conservative in this regard, modern methodology can be powerfully liberating. In the following schemes there are several cases where new synthetic methodology, often stereoselective, was critical to the design process.
Macrocyclic Architectures - Cholaphanes and Cyclocholamides
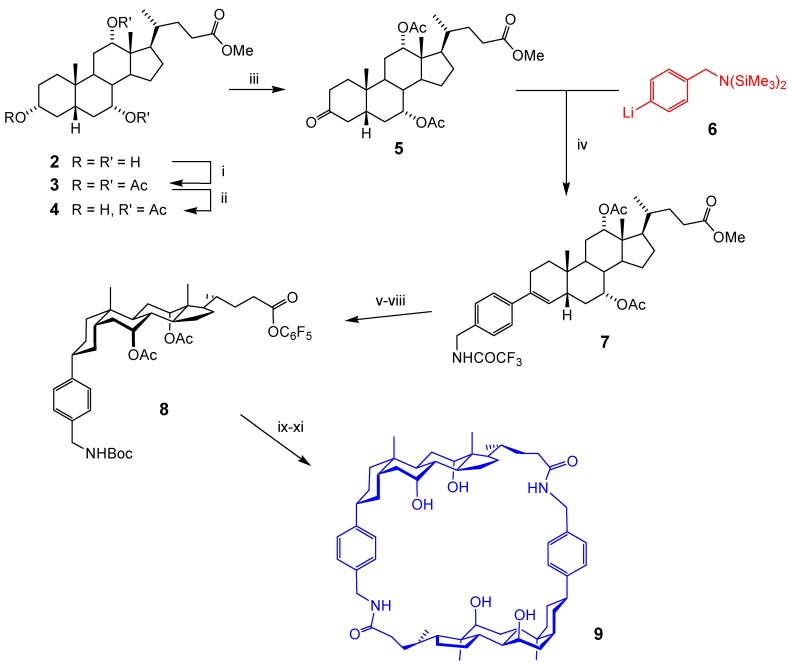
A distinctive characteristic of (most) bile acids is the cis junction between rings A and B of the steroid nucleus. This imparts a curved profile, and suggests the possibility of enclosed structures through macrocyclisation. By placing a spacer in the 3α position of 1, one can generate intermediates in which the curvature is accentuated and which, on cyclodimerisation, give molecules with substantial cavities. Spacers directly attached to the steroid carbon need not add flexibility to the system, which can be quite nicely preorganised. In accord with this rationale our first target was 9, dubbed a “cholaphane” because of the presence of both steroids and aromatic rings in a macrocyclic structure. Macrocycle 9 consists of two rigid units, extending from the benzylic CH2 groups to ring D of the steroid nucleus, connected by short flexible linker units. The cavity is able to accept medium-sized polar substrates, and is furnished with several inward directed polar groups.
The synthesis of 9 is summarised in Scheme 1 [7]. Following classical methods [8,9], methyl cholate (2) was first acetylated to 3 then selectively deacetylated at the equatorial C3-oxygen (giving 4). Oxidation to ketone 5 set up the key step, the introduction of the aromatic spacer. The methodology for this transformation needed to satisfy several criteria. Firstly it must be chemoselective, attacking the ketone carbonyl and not the three ester groups. Secondly, it needed to be stereoselective, leading to an equatorial orientation for the spacer. Thirdly, the spacer required a masked -NH2 group, compatible with the reaction conditions.
Scheme 1.
Reagents and conditions: i) Ac2O, Et3N, DMAP; ii) MeOH, HCl; iii) pyridinium chlorochromate; iv) MnI2, Et2O then TFAA, TFA; v) H2, Pd/C; vi) NaOH, MeOH, THF; vii) (Boc)2O, Pri2NEt, THF; viii) DCC, C6F5OH; ix) TFA; x) DMAP.TFA, CHCl3, DMF, K2HPO4, high dilution; xi) NaOH, H2O, THF, MeOH.
The reagent chosen was an organomanganese species [10] derived from organolithium 6. Organomanganese reagents are unusual in reacting smoothly with ketones while ignoring esters, even over long periods. The addition was not stereoselective, but this did not matter because work-up with TFA/TFAA caused elimination to alkene 7 (incidentally replacing N(SiMe3)2 with NHCOCF3). The stereochemistry was then controlled by hydrogenating the double bond from the less-hindered, convex face of the steroid. To set up the cyclisation, the N-protecting group was changed to Boc and the ester OMe converted to OC6F5, giving 8. Removal of Boc with acid, followed by addition to mild base at high dilution, allowed cyclodimerisation to take place. Finally O-deacetylation gave tetrahydroxy-cholaphane 9. As discussed elsewhere [5], this molecule found use as a carbohydrate receptor, binding monosaccharides in chloroform with good affinities and selectivities (including enantioselectivities).
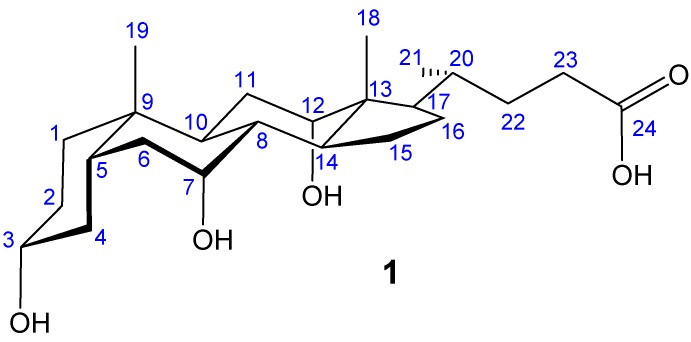
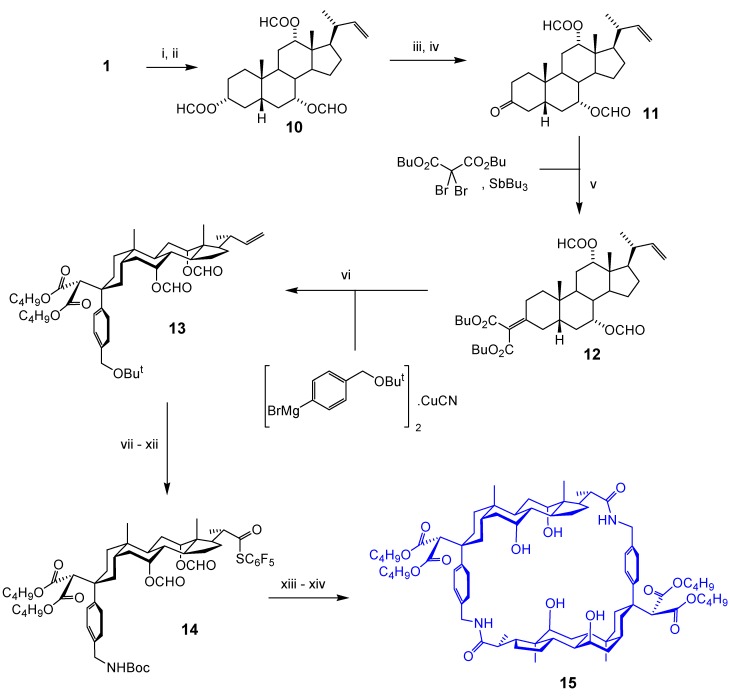
Cholaphane 9 does have some degree of flexibility, and is thus not perfectly preorganised for carbohydrate recognition. This flexibility resides mainly in the linkages derived from the steroidal side chain. If the side chain could be shortened by removal of C22 and C23 (see Figure 1), the derived macrocycle would have very little conformational freedom. Unfortunately, rigidity often correlates with insolubility, so a “tetra-nor” analogue of 9 might be little use as a receptor. However, if flexibility can be reintroduced in externally-directed substituents, both solubility and preorganisation might be possible. This thinking underpinned the design of our second series of cholaphanes, epitomised by 15 (Scheme 2) [11]. To access 15, we first protected the secondary hydroxyl groups of cholic acid by conversion to formate, then performed an oxidative decarboxylation to give alkene 10. Although just one side-chain carbon had been lost, the second was primed for removal through oxidative cleavage of the C=C unit. Steroid 10 could thus be seen as a masked derivative of 22,23-bis-norcholic acid.
Figure 1.
Cholic acid 1 with numbering system (blue).
Scheme 2.
Reagents and conditions: i) HCO2H, HClO4 (cat.), Ac2O; ii) Pb(OAc)4, Cu(OAc)2 cat., py, C6H6; iii) NaOAc·3H2O, MeOH; iv) pyridinium chlorochromate, CH2Cl2, SiO2; v) THF, 60 - 65 oC; vi) THF, -60 oC; vii) TFA, CH2Cl2, 50 oC, then NH3 aq., Et2O; viii) MsCl, i-Pr2NEt, CH2Cl2, -14 - 0 oC, then (Me2N)2CNH2+N3-, 0 - 30 oC; ix) Ph3P, THF, H2O, 60 oC, then (Boc)2O; x) N-methyl-morpholine-N-oxide, OsO4 (cat.), py, Bu4NOAc; xi) NaIO4, CH3CN, H2O, then NaClO2, H2NSO3H, CH3CN, H2O; xii) C6F5SH, DCC, CH2Cl2; xiii) TFA, CH2Cl2, then DMAP, CH2Cl2; xiv) K2CO3, THF, MeOH, H2O, 60 oC.
Alkene 10 was deformylated at position 3 then oxidised to give ketone 11. To introduce the spacer, we planned to perform a Knoevenagel-type reaction to generate a malonylidene derivative, and then an equatorial-selective conjugate addition. The malonyl unit in the product would end up on the outside of the macrocycle, moderating the solubility. To ensure good solubility in chloroform, the design featured dibutyl malonyl units as shown. Unfortunately the plan contained an unexpected flaw: the Knoevenagel reaction between 11 and dibutyl malonate proved impossible under the conditions tried. However, the literature contained some references to “forcing Knoevenagel” reactions, driven by redox transformations [12]. A method employing antimony(III) and dibromomalonate circumvented the problem, giving malonylidene derivative 12 in good yield [13]. The spacer was added as a higher-order cuprate to give 13, and a series of functional group interconversions then gave 14. Finally N-deprotection, cyclisation and deacylation (as for 8) gave 15. One difference from 8 is worth noting: the monomer for cyclodimerisation is activated as a pentafluorophenylthio ester [14], as opposed to the more common pentafluorophenyl ester. Shortening the side chain increases steric hindrance at the acyl carbon, and the reactivity of the thioester compensates for this effect.
After solving these problems, and executing such a long sequence, the properties of 15 were somewhat disappointing. Despite the greater rigidity, and promising molecular modelling results, the affinities for carbohydrates were slightly less than those of 9. We did however obtain a crystal structure of 15, the first of a cholaphane receptor [11].
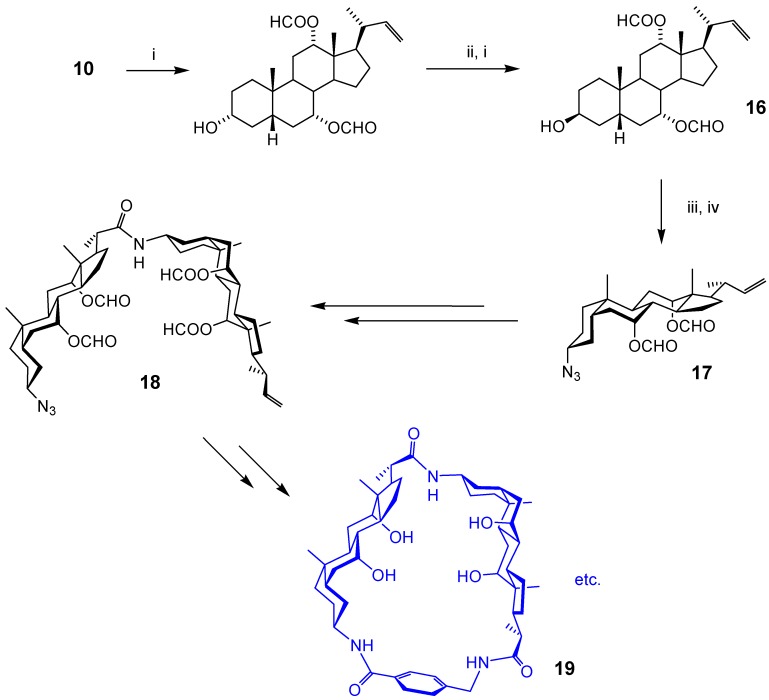
A disadvantage of cholaphanes is that each new example requires a separate (and probably lengthy) synthesis. In search of a “variation-friendly” system, we developed the sequence in Scheme 3 [15]. Alkene intermediate 10 was deprotected as in Scheme 2, but then subjected to a Mitsunobu inversion to give alcohol 16. Conversion to azide 17 was straightforward, and this could now serve as a protected bis-nor-cholic amino acid. A sequence of deprotections and coupling gave linear dimer 18, and this could then be cyclised with a range of amino acid spacers to give, for example, 19. This system allowed us to demonstrate “cavity tuning”, in that some variants (including 19) bound carbohydrates while others did not (although none, unfortunately, possessed outstanding affinities) [15b]. As aromatic rings are not necessary components of these macrocycles (the third unit can easily be aliphatic), we felt they were better termed “cyclocholamides” than “cholaphanes”.
Scheme 3.
Reagents and conditions: i) NaOH, MeOH; ii) Ph3P, HCO2H, DEAD, THF; iii) MeSO2Cl, py, Pri2NEt; iv) NaN3, DMPU.
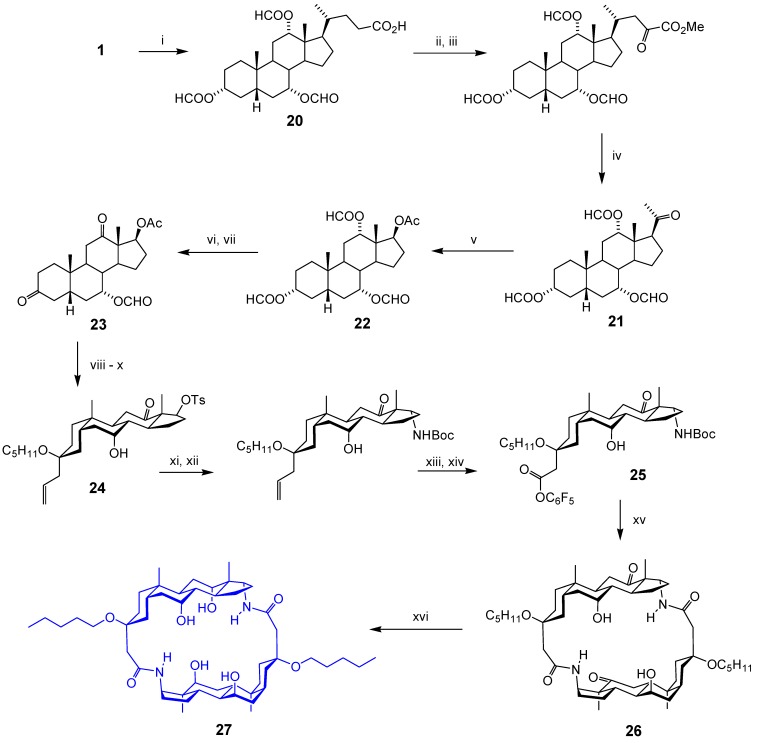
The systems described thus far were designed to bind polar organic molecules, principally carbohydrates. The final macrocycle in this section was aimed at a much smaller target, the chloride anion. To create an appropriately-sized cavity, it was necessary to “prune” the starting material more vigorously than before, and also to use a smaller spacer. Our design process led us to structure 27, with a rigid framework, a small (chloride-sized) cavity and a solubilising pentyloxy substituent. The synthesis of 27 is summarised in Scheme 4 [16]. The first challenge was to remove the entire bile acid side-chain and replace it with an α-directed NH2 group. The secondary hydroxyls of 1 were protected as formate, and triester 20 was then degraded to ketone 21 though a sequence due to Barton [17]. Baeyer-Villiger oxidation [18] of 21 gave acetate 22, which was selectively deprotected in positions 7 and 12 (see below) and oxidised to diketone 23. A silyl-modified Sakurai reaction [19] then introduced both spacer and solubilising groups, with excellent regio- and stereoselectivity. Hydrolysis of both esters was followed by selective tosylation at position 17, to give 24. Displacement of tosyl with azide, reduction to amine, protection as Boc, oxidative cleavage of the alkene and activation of the ester gave 25. Finally cyclisation gave diketone 26 and catalytic hydrogenation (stereoselectively from the exterior of the macrocycle) gave the target 27.
Scheme 4.
Reagents and conditions: i) HCO2H, cat. HClO4; ii) SOCl2, py, DCM, then MeOH; iii) RuCl3, NaIO2, MeCN, H2O, CCl4; iv) DABCO, bipy, Cu(OAc)2, DMF, air; v) mCPBA, DCE; vi) K2CO3, THF, H2O, MeOH; vii) pyridinium chlorochromate, CH2Cl2; viii) Me3SiCH2CH=CH2, Me3SiOC5H11, Me3SiOTf (cat.), CH2Cl2, -40 oC; ix) NaOMe, MeOH; x), TsCl, pyridine; xi) NaN3, DMPU, 130 oC, 48 h; xii) Zn, AcOH, then (BOC)2O, CH2Cl2, Et3N; xiii) O3, CH2Cl2, then H2O2; xiv) C6F5OH, DCC; xv) TFA, CH2Cl2, then DMAP, Et3N, CH2Cl2 (2 mL per mg of substrate); xvi) H2, PtO2, EtOAc, AcOH.
The sequence in Scheme 4 contains one oddity, the generation of a carbonyl group at C12 and then its reduction as the final step. This proved necessary because any α substituent at C12 prevented the azide displacement at C17. Oxidation cleared the way for azide approach and, fortunately, caused no serious problems during the remainder of the synthesis.
Macrocycle 27 proved a successful receptor of halide anions, showing good affinities and selectivities for its day [16]. However the length of the synthesis, and the difficulties encountered, discouraged further work in this direction. Instead, we conceived of a new approach to anion recognition, employing acyclic structures based on just one steroidal unit. The synthetic challenges involved are discussed in the next section.
Acyclic Scaffolds - the Cholapod Architecture
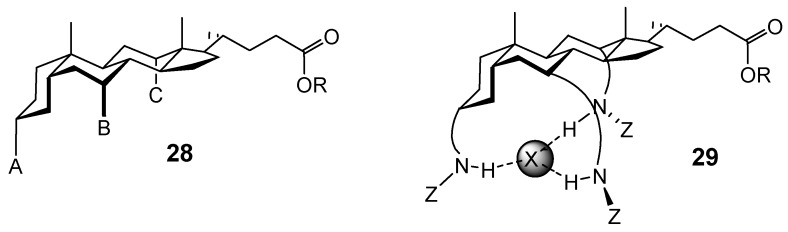
Cholaphanes and cyclocholamides have the advantage of enclosing their substrates, presenting binding functionality on all sides. However variation, either of binding groups or of solubilising substituents, is not straightforward. An alternative approach is to use a single molecule of bile acid to create a podand-type architecture (“cholapod”), as in 28. The binding site is formed by “legs” A-C, while the solubility can be controlled by ester group R. Early systems of this type were reported by Kahne [20], and especially by W. C. Still, who realised that receptors of this type could be varied combinatorially [21]. We were interested in anion recognition, and therefore in versions where A-C contain H-bond donors (see 29). Their number and positions could be varied, and also their donor strength (for example, by adjusting Z).
Figure 2.
Although it is possible to make cholapods by straightforward derivatisations of cholic acid (1), the array of three hydroxyl groups is not ideal for the purpose. Esterification is the obvious method, but it is slow, hard to perform in sequence and does not give especially useful products. However, if one or more hydroxyls can be replaced by amino groups, the resulting scaffolds are much more attractive. The amino groups are readily convertible into amides, ureas, sulfonamides and guanidinium groups, all with useful recognition properties. Sequential derivatisation is also easier. In mixed amino/hydroxy scaffolds the amino groups will react first, and where two or three amines are present they can be differentially protected (see below).
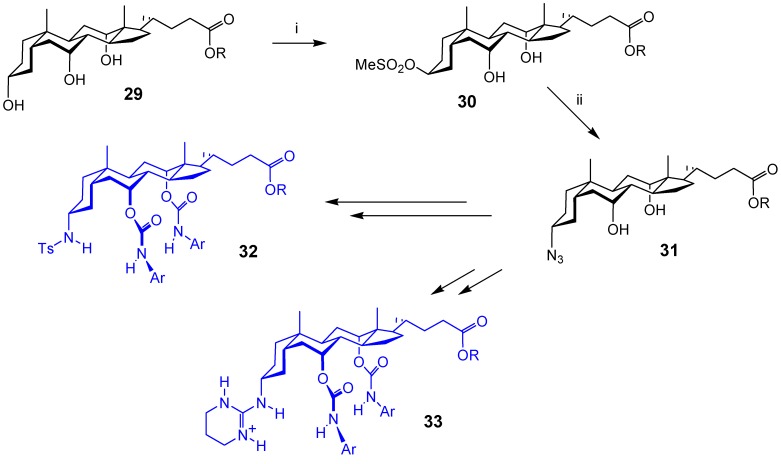
We began with the relatively straightforward conversion of the equatorial 3α-OH to 3α-N3 (i.e. masked 3α-NH2). This had previously been accomplished for intermediate 17 (Scheme 3), but the 4-step sequence via a conventional Mitsunobu reaction (formate nucleophile) seemed long-winded. We reasoned that a Mitsunobu reaction in which the nucleophile was also a good leaving group might simplify the process. The leaving group could be displaced directly by azide, allowing a 2-step -OH → -N3 conversion with net retention of configuration. In fact it turned out that methanesulfonate anion can act as nucleophile in some cases. Treatment of 3α-hydroxycholanoates such as 29 (Scheme 5) with Ph3P/DEAD/Me3SO3H/DMAP gave methanesulfonate esters such as 30 [22,23]. As a bonus, the 7α,12α-OH groups in 29 remained untouched, and did not require protection. Displacement of methanesulfonate with azide anion gave 3α-azido products such as 31. These intermediates could be converted into anion receptors such as 32 [24], and enantioselective carboxylate receptors such as 33 [25].
Scheme 5.
Reagents and conditions: i) Ph3P, DEAD, Me3SO3H, DMAP; ii) NaN3, DMF.
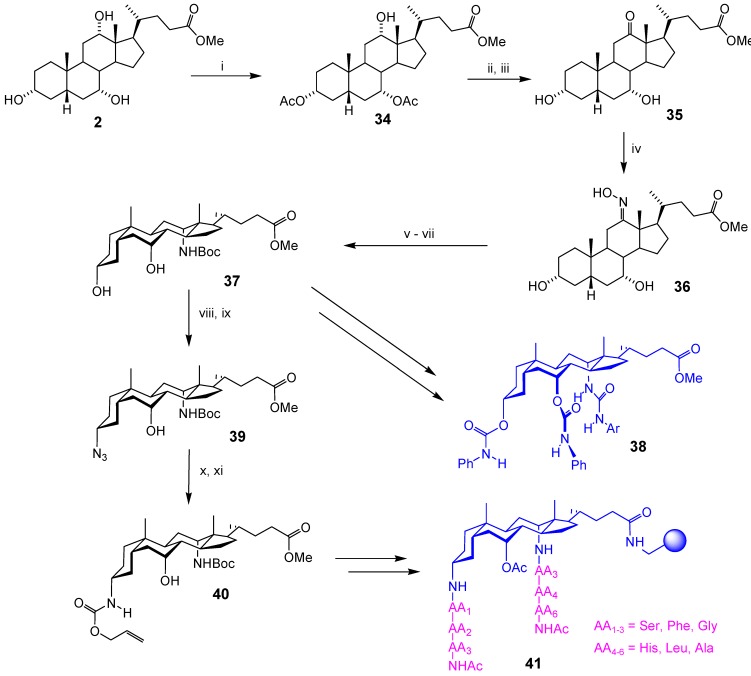
The next task was the conversion of the axial 7α,12α-OH groups to amines. Nucleophilic displacement at these positions is inefficient due to steric hindrance. Oxidation/reductive amination can serve as an alternative [26], although stereocontrol is not guaranteed. However, we found that the Pt-catalysed hydrogenation of 7- or 12-oximes gave excellent stereoselectivity in favour of axial products. The initial products (hydroxylamines) were only slowly converted to amines, but the problem could be solved by a two-stage reduction method, involving catalytic hydrogenation followed by treatment with Zn. Scheme 6 shows how the method was applied in the synthesis of two scaffold types, the N-protected 12α-aminodiol 37 and the bis-protected 3α,12α-diamino-7α-hydroxycholanoates 35 and 36 [27]. The first step, 3,7-bisacetylation of methyl cholate (2) to give 34, is a classical method for differentiating between the 7α and 12α hydroxyl groups (both of which are axial) [28]. Oxidation gave ketone 35, and this was then converted via oxime 36 to 37. Scaffold 37 was used, for example, to prepare enantioselective carboxylate receptor 38 [29]. The methanesulfonate-Mitsunobu method (Scheme 5) could then be used to convert 37 to azide 39, and thence (if desired) to allyloxycarbonyl-protected 40. Both 39 and 40 possess differential N-protection, capable of sequential demasking. In the case of 40, this was exploited in the construction of polymer-bound combinatorial library 41 [30].
Scheme 6.
Reagents and conditions: i) Ac2O, py, CH2Cl2; ii) K2CrO4, AcOH; iii) NaOMe, MeOH; iv) H2NOH.HCl, NaOAc, MeOH; v) H2, PtO2 (~1 mol%), AcOH, 7 d; vi) Zn, AcOH; vii) (Boc)2O, THF, aq. KOH; viii) Ph3P, DEAD, MeSO3H, THF-toluene; ix) NaN3, THF-DMPU; x) H2, Pd/C, EtOAc-MeOH; xi) allyl chloroformate, i-Pr2NEt, CH2Cl2, NaHCO3 aq.
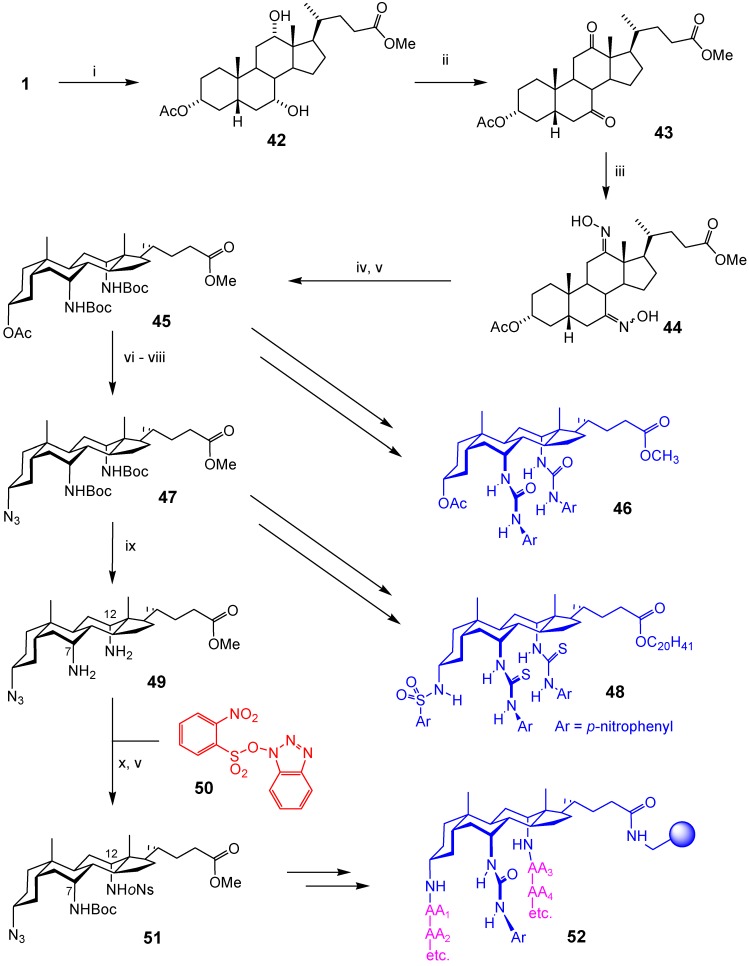
The most versatile and useful scaffolds were obtained by applying the oximation-reduction method simultaneously in positions 7 and 12, giving diamino derivatives. As shown in Scheme 7, treatment of cholic acid with methyl acetate gave 42, protecting both carboxyl and 3α-OH in a single operation [31]. Oxidation to ketone 43, oximation to 44 and hydrogenation/Zn reduction gave the corresponding diamine, which was protected as Boc to give 45 [32,33]. This sequence is carried out on a large scale to make 20 g batches of 45, which underpins much of our current work. Scaffold 45 may be converted to bis-urea anion receptors 46 [33]. Alternatively, the methanesulfonate-Mitsunobu method (Scheme 5) may be used to introduce a 3α-azido group, giving (protected) triaminoscaffold 47 [34]. This may then be converted to further anion receptors, such as sulfonamido-bisthiourea 48 [35].
Scheme 7.
Reagents and conditions: i) MeOAc, p-TsOH∙H2O (cat.); ii) Ca(ClO)2, KBr (cat.), AcOH/H2O; iii) NH2OH∙HCl, NaOAc, MeOH; iv) H2, PtO2, AcOH, r.t., 3 days, then Zn, AcOH, r.t., 24 h; v) Boc2O, NaHCO3, THF/H2O; vi) NaOH, MeOH (dry); vii) MsOH, PPh3, DEAD, DMAP, THF; viii) NaN3, DMF; ix) TFA, DCM then NaHCO3 aq.; x) THF.
These cholapods are unique in showing very high anion affinities (up to 1011 m-1 for chloride in chloroform) while maintaining compatibility with non-polar media (such as the interior of bilayer membranes) [6]. As a result they can act as transmembrane anion carriers, being the first neutral organic molecules to show this property [33,36,37]. There is a realistic prospect that they might show useful biological activity, a rare outcome for a supramolecular research programme.
Scaffold 47 would be even more versatile if all three positions were differentially protected, so that the nitrogens could be revealed in sequence. This was not so easily achieved, because the 7α and 12α positions are both axial and subject to similar degrees of steric hindrance. However they are not identical, and differentiation proved possible by careful choice of reagent. Treatment of diamine 49 with o-nitrosulfonyl (oNs) derivative 50 gave high levels of regioselectivity, in favour of the 12α-protected derivative. Protection of the remaining amino group as Boc gave scaffold 51 [23]. The oNs group can be removed with thiolate [38], Boc with acid and azide with reduction, so that scaffold 51 is ideal for the preparation of asymmetrical cholapods. For example, it has been used to make combinatorial libraries of form 52, for screening as enantioselective receptors.
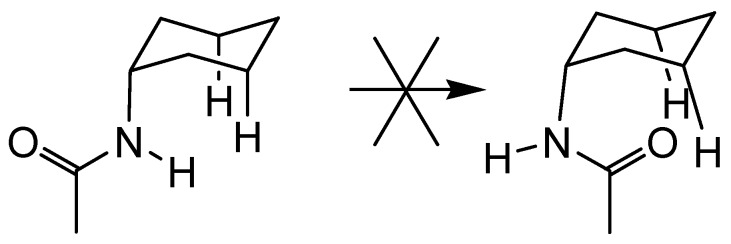
Finally, a useful feature of cholapods such as 46 and 48 is the axial disposition of the 7α and 12α C-N bonds. As shown in Figure 3, this restricts rotation about the bonds such that the NH groups are inwardly directed, preorganised to act as H-bond donors. The 3α position in standard cholapods does not possess this advantage, being equatorial as a result of the cis-AB ring junction. However, analogues derived from the all-trans allocholanoyl framework would have three axial binding units. An allocholanoyl scaffold had been used previously by Still, but only with two functionalised positions. We therefore undertook the preparation of 56, the triamino-analogue of methyl allocholate.
Figure 3.
Restricted rotation about axial C-N bonds in cholapod receptors.
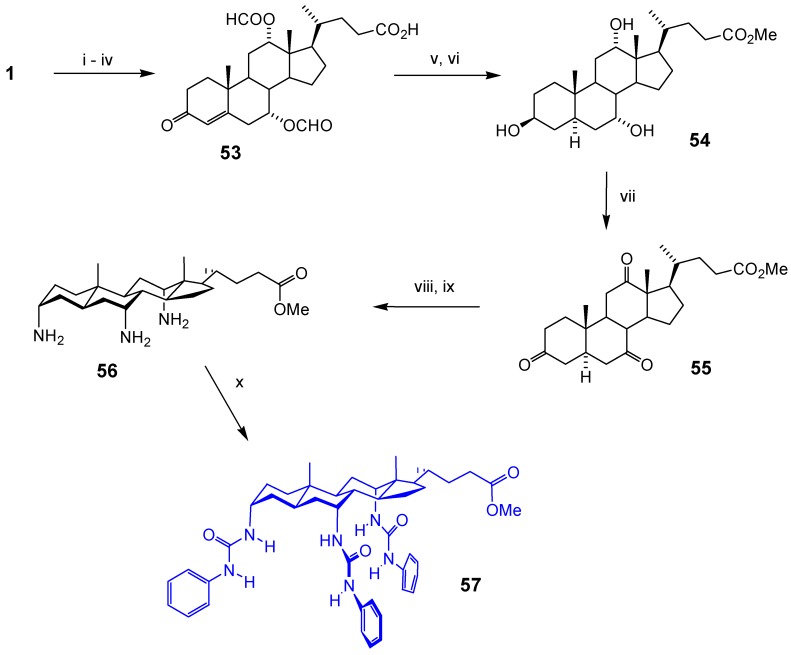
As shown in Scheme 8 [39], the first steps involved triformylation of cholic acid 1, selective deformylation at position 3 and oxidation to enone 53. Reduction with Li/NH3/ButOH gave triol 54 [40], which was oxidised to triketone 55. Oximation and hydrogenation/Zn reduction gave triaxial triamine 56 with good stereoselectivity at all three centres. The amino groups could be protected as Boc or reacted directly with phenyl isocyanate to give anion receptor 57. The preorganisation of all three urea groups was reflected in higher affinities, relative to a cholanoyl analogue [39].
Scheme 8.
Reagents and conditions: i) HCO2H, HClO3 cat., Ac2O; ii) NaOH, acetone; iii) N-bromo-succinimide, t-butanol; iv) semicarbazide hydrochloride, NaHCO3, t-butanol, then pyruvic acid, H2O; v) NaOH aq.; vi) Li, NH3, THF, t-butanol, then MeOH, H2SO4; vii) Ca(OCl)2, AcOH; viii) H2NOH.HCl, NaOAc, MeOH; ix) H2, Pt cat, AcOH, then Zn, AcOH; x) PhNCO, THF.
Conclusions
This account has highlighted a number of sequences in which cholic acid, the archetypal bile acid, has been “sculpted” into synthetic receptors. It is not an exhaustive account of our work, and omits a great many useful contributions from other laboratories. Nonetheless, it illustrates the value of bile acids in supramolecular chemistry, especially when allied to modern synthetic methodology. There are few other readily-available scaffolds which are comparably large, preorganised and chemically versatile. Although they have been quite widely used, there is ample potential for new applications based on less familiar (or novel) derivatives. This article, hopefully, is not the end of the story.
Acknowledgments
The author thanks the coworkers and collaborators who have made this programme possible, especially the synthetic chemists whose work has been highlighted above. Thanks are also due to the funding agencies who have supported the work, including the Irish science agencies Eolas and Forbairt, the UK research councils BBSRC and EPSRC, and the European Commission.
References and Notes
- 1.Menger F. M., McCreery M. J. Kinetic characterisation of bile salt micelles. J. Am. Chem. Soc. 1974;96:121–126. doi: 10.1021/ja00808a020. [DOI] [PubMed] [Google Scholar]
- 2.McKenna J., McKenna J. M., Thornthwaite D. W. Bis-steroids as potential enzyme models: Perylene solubilisation and dye spectral changes with aqueous solutions of some derivatives of conessine and cholic acid. J. Chem. Soc., Chem. Commun. 1977:809–811. doi: 10.1039/c39770000809. [DOI] [Google Scholar]
- 3.(a) Davis A. P. Cholaphanes et al.; Steroids as structural components in molecular engineering. Chem. Soc. Rev. 1993;22:243–253. [Google Scholar]; (b) Davis A. P., Bonar-Law R. P., Sanders J. K. M. In: Comprehensive Supramolecular Chemistry. Murakami Y., editor. Vol. 4. Pergamon; Oxford: 1996. pp. 257–286. (Supramolecular Reactivity and Transport: Bioorganic Systems) [Google Scholar]; (c) Li Y. X., Dias J. R. Dimeric and oligomeric steroids. Chem. Rev. 1997;97:283–304. doi: 10.1021/cr9600565. [DOI] [PubMed] [Google Scholar]; (d) Tamminen J., Kolehmainen E. Bile acids as building blocks of supramolecular hosts. Molecules. 2001;6:21–46. [Google Scholar]; (e) Virtanen E., Kolehmainen E. Use of bile acids in pharmacological and supramolecular applications. Eur. J. Org. Chem. 2004:3385–3399. [Google Scholar]
- 4.We estimate that at least 40 groups have contributed to the area.
- 5.Davis A. P., Wareham R. S. Carbohydrate recognition through noncovalent interactions: A challenge for biomimetic and supramolecular chemistry. Angew. Chem., Int. Ed. 1999;38:2978–2996. doi: 10.1002/(SICI)1521-3773(19991018)38:20<2978::AID-ANIE2978>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 6.(a) Davis A. P., Joos J.-B. Steroids as organising elements in anion receptors. Coord. Chem. Rev. 2003;240:143–156. doi: 10.1016/S0010-8545(03)00020-1. [DOI] [Google Scholar]; (b) Davis A. P. Anion binding and transport by steroid-based receptors. Coord. Chem. Rev. 2006;250:2939–2951. [Google Scholar]
- 7.(a) Bonar-Law R. P., Davis A. P. Synthesis of steroidal cyclodimers from cholic acid; A molecular framework with potential for recognition and catalysis. J. Chem. Soc., Chem. Commun. 1989:1050–1052. doi: 10.1039/c39890001050. [DOI] [Google Scholar]; (b) Bonar-Law R. P., Davis A. P. Cholic acid as an architectural component in molecular recognition chemistry. Synthesis of the first cholaphanes. Tetrahedron. 1993;49:9829–9844. [Google Scholar]
- 8.Höfle G., Steglich W., Vorbruggen H. 4-Dialkylaminopyridines as acylation catalysts. 4. 4- Dialkylaminopyridines as highly active acylation catalysts. Angew. Chem., Int. Ed. Engl. 1978;17:569–583. doi: 10.1002/anie.197805691. [DOI] [Google Scholar]
- 9.Dias J. R., Ramachandra R. Studies directed toward synthesis of quassinoids. 3. Selective hydrolysis of 3-alpha-acetate functional-group of cholic-acid derivatives. Synth. Commun. 1977;7:293–297. doi: 10.1080/00397917708050750. [DOI] [Google Scholar]
- 10.Cahiez G., Normant J. F. Reactivity of organomanganese(ii) reagents - reaction with carbonyl- compounds, selective preparation of ketols from keto-aldehydes. Tetrahedron Lett. 1977:3383–3384. doi: 10.1016/S0040-4039(01)83245-7. [DOI] [Google Scholar]
- 11.Bhattarai K. M., Davis A. P., Perry J. J., Walter C. J., Menzer S., Williams D. J. A new generation of "cholaphanes": Steroid-derived macrocyclic hosts with enhanced solubility and controlled flexibility. J. Org. Chem. 1997;62:8463–8473. doi: 10.1021/jo971272w. [DOI] [PubMed] [Google Scholar]
- 12.(a) Liao Y., Huang Y. Z. A novel olefination of diazo-compounds with carbonyl-compounds mediated by tributylstibine and catalytic amount of Cu(I)I. Tetrahedron Lett. 1990;31:5897–5900. doi: 10.1016/S0040-4039(00)97988-7. [DOI] [Google Scholar]; (b) Zhou Z. L., Shi L. L., Huang Y. Z. The synthetic application of elementoorganic compounds of 15th and 16th groups. 89. A novel olefination of carbonyl-compounds with dibromomalonate promoted by dibutyl telluride. Synth. Commun. 1991;21:1027–1037. [Google Scholar]
- 13.Davis A. P., Bhattarai K. M. Antimony-based “forcing Knoevenagel” methodology for the conversion of ketones into alkylidenemalonates. Tetrahedron. 1995;51:8033–8042. doi: 10.1016/0040-4020(95)00418-8. [DOI] [Google Scholar]
- 14.Davis A. P., Walsh J. J. Amide bond formation via pentafluorothiophenyl active esters. Tetrahedron Lett. 1994;35:4865–4868. doi: 10.1016/S0040-4039(00)76989-9. [DOI] [Google Scholar]
- 15.(a) Davis A. P., Walsh J. J. Steroid-based receptors with tunable cavities; Stepwise and direct syntheses of a C-3-symmetrical prototype. Chem. Commun. 1996:449–451. [Google Scholar]; (b) Davis A. P., Menzer S., Walsh J. J., Williams D. J. Steroid-based receptors with tunable cavities; A series of polyhydroxylated macrocycles of varying size and flexibility. Chem. Commun. 1996:453–455. [Google Scholar]
- 16.Davis A. P., Gilmer J. F., Perry J. J. A steroid-based cryptand for halide anions. Angew. Chem., Int. Ed. Engl. 1996;35:1312–1315. doi: 10.1002/anie.199613121. [DOI] [Google Scholar]
- 17.Barton D. H. R., Wozniak J., Zard S. Z. A short and efficient degradation of the bile-acid side- chain - some novel reactions of sulfines and alpha-ketoesters. Tetrahedron. 1989;45:3741–3754. doi: 10.1016/S0040-4020(01)89235-8. [DOI] [Google Scholar]
- 18.Dias J. R., Ramachandra R. Studies directed toward synthesis of quassinoids. 4. D-Ring cleavage of cholic-acid derivatives. Org. Prep. Proc. Int. 1977;9:109–115. doi: 10.1080/00304947709356866. [DOI] [Google Scholar]
- 19.Mekhalfia A., Markó I. E. The silyl modified Sakurai (SMS) reaction. An efficient and versatile one-pot synthesis of homoallylic ethers. Tetrahedron Lett. 1991;32:4779–4782. doi: 10.1016/S0040-4039(00)92306-2. [DOI] [Google Scholar]
- 20.Cheng Y., Ho D. M., Gottlieb C. R., Kahne D., Bruck M. A. Facial amphiphiles. J. Am. Chem. Soc. 1992;114:7319–7320. doi: 10.1021/ja00044a067. [DOI] [Google Scholar]
- 21.Boyce R., Li G., Nestler H. P., Suenaga T., Still W. C. Peptidosteroidal receptors for opioid peptides. Sequence-selective binding using a synthetic receptor library. J. Am. Chem. Soc. 1994;116:7955–7956. doi: 10.1021/ja00096a086. [DOI] [Google Scholar]
- 22.Davis A. P., Dresen S., Lawless L. J. Mitsunobu reactions with methanesulfonic acid; The replacement of equatorial hydroxyl groups by azide with net retention of configuration. Tetrahedron Lett. 1997;38:4305–4308. doi: 10.1016/S0040-4039(97)00886-1. [DOI] [Google Scholar]
- 23.del Amo V., Siracusa L., Markidis T., Baragaña B., Bhattarai K. M., Galobardes M., Naredo G., Pérez-Payán M. N., Davis A. P. Differentially-protected steroidal triamines; Scaffolds with potential for medicinal, supramolecular, and combinatorial chemistry. Org. Biomol. Chem. 2004;2:3320–3328. doi: 10.1039/b412298d. [DOI] [PubMed] [Google Scholar]
- 24.(a) Davis A. P., Perry J. J., Williams R. P. Anion recognition by tripodal receptors derived from cholic acid. J. Am. Chem. Soc. 1997;119:1793–1794. doi: 10.1021/ja9629930. [DOI] [Google Scholar]; (b) Ayling A. J., Broderick S., Clare J. P., Davis A. P., Pérez-Payán M. N., Lahtinen M., Nissinen M. J., Rissanen K. An extraction-based assay for neutral anionophores: The measurement of high binding constants to steroidal receptors in a nonpolar solvent. Chem. Eur. J. 2002;8:2197–2203. doi: 10.1002/1521-3765(20020503)8:9<2197::AID-CHEM2197>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 25.(a) Lawless L. J., Blackburn A. G., Ayling A. J., Pérez-Payán M. N., Davis A. P. Steroidal guanidines as enantioselective receptors for N-acyl alpha-amino acids. Part 1. 3 alpha-Guanylated carbamates derived from cholic acid. J. Chem. Soc., Perkin Trans. 1. 2001:1329–1341. [Google Scholar]; (b) Baragaña B., Blackburn A. G., Breccia P., Davis A. P., de Mendoza J., Padron-Carrillo J. M., Prados P., Riedner J., de Vries J. G. Enantioselective transport by a steroidal guanidinium receptor. Chem. Eur. J. 2002;8:2931–2936. doi: 10.1002/1521-3765(20020703)8:13<2931::AID-CHEM2931>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 26.For an example, see Hsieh H.-P., Muller J. G., Burrows C. J. Structural effects in novel steroidal polyamine-DNA binding. J. Am. Chem. Soc. 1994;116:12077–12078. doi: 10.1021/ja00105a068.
- 27.Barry J. F., Davis A. P., Pérez-Payán M. N., Elsegood M. R. J., Jackson R. F. W., Gennari C., Piarulli U., Gude M. A trifunctional steroid-based scaffold for combinatorial chemistry. Tetrahedron Lett. 1999;40:2849–2852. doi: 10.1016/S0040-4039(99)00309-3. [DOI] [Google Scholar]
- 28.Fieser L. F., Rajagopalan S. Oxidation of Steroids. III. Selective oxidations and acylations in the bile acid series. J. Am. Chem. Soc. 1950;72:5530–5536. doi: 10.1021/ja01168a046. [DOI] [Google Scholar]
- 29.Siracusa L., Hurley F. M., Dresen S., Lawless L. J., Pérez-Payán M. N., Davis A. P. Steroidal ureas as enantioselective receptors for an N-acetyl alpha-amino carboxylate. Org. Lett. 2002;4:4639–4642. doi: 10.1021/ol027009l. [DOI] [PubMed] [Google Scholar]
- 30.De Muynck H., Madder A., Farcy N., De Clercq P. J., Pérez-Payán M. N., Öhberg L. M., Davis A. P. Application of combinatorial procedures in the search for serine-protease-like activity with focus on the acyl transfer step. Angew. Chem., Int. Ed. 2000;39:145–148. doi: 10.1002/(SICI)1521-3773(20000103)39:1<145::AID-ANIE145>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 31.Kuhajda K., Kandrac J., Cirin-Novta V., Miljkovic D. One-pot esterification and selective 3 alpha-acetylation of cholic and deoxycholic acid. Coll. Czech. Chem. Comm. 1996;61:1073–1076. doi: 10.1135/cccc19961073. [DOI] [Google Scholar]
- 32.Davis A. P., Pérez-Payán M. N. The "triamino-analogue" of methyl cholate; A practical, large- scale synthesis. Synlett. 1999:991–993. [Google Scholar]
- 33.Koulov A. V., Lambert T. N., Shukla R., Jain M., Boon J. M., Smith B. D., Li H. Y., Sheppard D. N., Joos J. B., Clare J. P., Davis A. P. Chloride transport across vesicle and cell membranes by steroid-based receptors. Angew. Chem., Int. Ed. 2003;42:4931–4933. doi: 10.1002/anie.200351957. [DOI] [PubMed] [Google Scholar]
- 34.For a complementary approach to a triamino steroidal scaffold see Li C. H., Rehman A., Dalley N. K., Savage P. B. Short syntheses of triamine derivatives of cholic acid. Tetrahedron Lett. 1999;40:1861–1864. doi: 10.1016/S0040-4039(99)00076-3.
- 35.(a) Ayling A. J., Pérez-Payán M. N., Davis A. P. New "cholapod" anionophores; High-affinity halide receptors derived from cholic acid. J. Am. Chem. Soc. 2001;123:12716–12717. doi: 10.1021/ja016796z. [DOI] [PubMed] [Google Scholar]; (b) Clare J. P., Ayling A. J., Joos J. B., Sisson A. L., Magro G., Pérez-Payán M. N., Lambert T. N., Shukla R., Smith B. D., Davis A. P. Substrate discrimination by cholapod anion receptors: Geometric effects and the "affinity-selectivity principle". J. Am. Chem. Soc. 2005;127:10739–10746. doi: 10.1021/ja0524144. [DOI] [PubMed] [Google Scholar]
- 36.McNally B. A., Koulov A. V., Smith B. D., Joos J. B., Davis A. P. A fluorescent assay for chloride transport; Iidentification of a synthetic anionophore with improved activity. Chem. Commun. 2005:1087–1089. doi: 10.1039/b414589e. [DOI] [PubMed] [Google Scholar]
- 37.Davis A. P., Sheppard D. N., Smith B. D. Development of synthetic membrane transporters for anions. Chem. Soc. Rev. 2007;36:348–357. doi: 10.1039/b512651g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuyama T., Jow C.-K., Cheung M. 2- nd 4-Nitrobenzenesulfonamides: Exceptionally versatile means for preparation of secondary amines and protection of amines. Tetrahedron Lett. 1995;36:6373–6374. doi: 10.1016/0040-4039(95)01316-A. [DOI] [Google Scholar]
- 39.Bhattarai K. M., del Amo V., Magro G., Sisson A. L., Joos J. B., Charmant J. P. H., Kantacha A., Davis A. P. The "triamino-analogue" of methyl allocholate; A rigid, functionalised scaffold for supramolecular chemistry. Chem. Commun. 2006:2335–2337. doi: 10.1039/b602415g. [DOI] [PubMed] [Google Scholar]
- 40.Kallner A. A method of synthesis of allocholanoic acids - bile acids and steroids 182. Acta Chem. Scand. 1967;21:322–328. doi: 10.3891/acta.chem.scand.21-0322. [DOI] [PubMed] [Google Scholar]