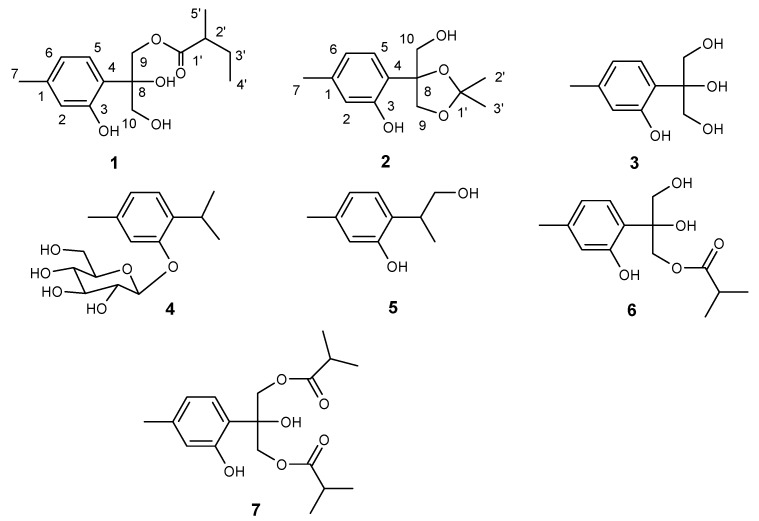
Abstract
Two new monoterpenoids, 8,10-dihydroxy-9(2)-methylbutyryloxythymol (1) and 10-hydroxy-8,9-dioxyisopropylidene-thymol (2), together with five known thymol derivatives: 8,9,10-trihydroxythymol (3), thymol-β-glucopyranoside (4), 9-hydroxy- thymol (5), 8,10-dihydroxy-9-isobutyryloxythymol (6), and 8-hydroxy-9,10-diisobutyryl- oxythymol (7), were isolated from Centipeda minima. Their structures were identified by means of spectroscopic analyses. Interestingly, compound 2 is not an extraction artifact according to a close HPLC examination of material after extraction by analytical MeOH at ambient temperature. The antibacterial activities of compounds 1-7 were evaluated against eight microbial strains by the agar dilution method.
Keywords: Centipeda minima, Compositae, thymol derivatives, antibacterial activity
Introduction
The loss of quality and safety of food largely results from microorganisms. The use of antimicrobials and antiseptics is a common alternative to control bacteria in food [1]. However, the widespread use of antibiotics in human medicine and agriculture has caused serious problem of bacterial resistance [2]. Therefore, plant derived antimicrobial agents with high potency and low mammalian toxicity, useful for food preservation and human health, have gained special interest in recent decades [3,4,5]. China is a country well known for its utilization of traditional medicine; for centuries, indigenous people have been using herbal medicines to treat various diseases, including a wide range of infectious ones. Interestingly, there is not much documented data on microbial resistance. This indicates that traditional Chinese medicine is a potential source for discovering promising antibacterial substances. Centipeda minima (L.) (Compositae) is found spread throughout China, Japan, Korea, India, Malaysia, and Oceania [6], and is a commonly used Chinese folk medicine for colds, nasal allergy, diarrhea, malaria and asthma [7]. Previous studies on C. minima revealed that its flavonoids, sesquiterpene lactones and amides could block histamine release, and therefore contribute to its anti-allergy effects [8,9]. Sesquiterpenoids were also found to have antagonistic activities for platelet activating factor and antibacterial activities [10,11]. During our search for new antimicrobial components from traditional Chinese medicine, two new monoterpenoids, 8,10-dihydroxy- 9(2)-methylbutyryloxythymol (1), 10-hydroxy-8,9-dioxyisopropylidenethymol (2), along with five known thymol derivatives: 8,9,10-trihydroxythymol (3), thymol-β-glucopyranoside (4), 9-hydroxy- thymol (5), 8,10-dihydroxy-9-isobutyryloxythymol (6), and 8-hydroxy-9,10-diisobutyryloxythymol (7) were isolated from the whole plants of C. minima (Figure 1). In this paper, we report the isolation and structural identification of the new compounds, and the antibacterial properties of compounds 1-7.
Figure 1.
Structures of compounds 1-7.
Results and Discussion
Compound 1 was obtained as a colorless oil. Its molecular formula C15H22O5 was deduced on the basis of HRESIMS showing a peak at m/z 305.1363 [M+Na]+ (calcd. C15H22O5Na, 305.1364). The 1H-NMR spectrum exhibited a set of signals characteristic of a 1,3,4-trisubstituted phenyl moiety. The 13C-NMR data (Table 1) were very similar to those of 6, except for two methyls at δC 11.4 and 16.4, one methylene at δC 26.6, and one methine at δC 41.0 present in 1, instead of two symmetrical methyl groups at δC 19.1, and one methine at δC 34.4 seen in 6, which in conjunction with the 1H-1H COSY interactions of aliphatic protons, suggesting the existence of a CH3-CH2-CH-CH3 segment in 1. The HMBC correlations of H-2’, H-3’, H-5’ and H-9 all correlated with a carbonyl carbon at δC 177.9 established the linkage of C-1’and C-9 (δC 67.3). The absolute stereochemistry at C-8 and C-2’ still remained obscure. Thus, the structure of 1 was determined to be 8,10-dihydroxy-9(2)-methyl- butyryloxythymol.
Table 1.
1H- and 13C-NMR spectra data for compounds 1 and 2a.
| position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J=Hz) | δC | δH (J=Hz) | δC | |
| 1 | 139.9 | 139.5 | ||
| 2 | 6.72 br. s | 118.5 | 6.57 br. s | 117.1 |
| 3 | 156.4 | 154.6 | ||
| 4 | 119.6 | 127.3 | ||
| 5 | 6.94 d (7.9) | 126.3 | 7.32 d (7.8) | 128.5 |
| 6 | 6.67 br. d (7.9) | 120.5 | 6.62 br. d (7.8) | 120.9 |
| 7 | 2.29 s | 20.9 | 2.23 s | 21.1 |
| 8 | 78.9 | 86.5 | ||
| 9a9b | 4.54 d (12.0)4.46 d (12.0) | 67.3 | 4.40 d (9.0)4.16 d (9.0) | 72.3 |
| 10a10b | 3.90 d (12.0)3.81 d (12.0) | 65.9 | 3.73 br. d (11.5)3.60 br. d (11.5) | 67.3 |
| 1’ | 177.9 | 110.6 | ||
| 2’ | 2.40 m | 41.0 | 1.27 s | 25.9 |
| 3’a3’b | 1.62 m1.51 m | 26.6 | 1.52 s | 27.2 |
| 4’ | 0.84 m | 11.4 | ||
| 5’ | 1.11 d (7.7) | 16.4 | ||
a The spectra were recorded in CDCl3 (400 MHz for 1H, 100 MHz for 13C).
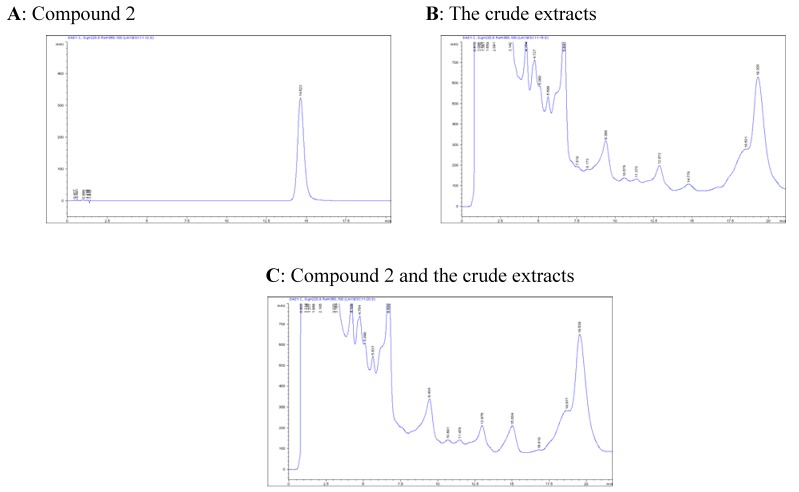
The molecular composition of 2 was assigned as C13H18O4 by HRESIMS (peak at m/z 261.1108 [M+Na]+ (calcd. C13H18O4Na 261.1102), featuring five degrees of unsaturation. The resemblance of 13C-NMR spectra between 2 and 3 suggest they are analogues. However, two additional methyl signals (δC 25.9, 27.2) and one sp3 quaternary carbon (δC 110.6) were observed in the 13C-NMR spectrum of 2, in conjunction with one more unsaturation and the 40 Da larger mw of 2 than 3, disclosing the presence of an isopropyl moiety. This was further confirmed by the observed HMBC interactions of H-9, H-2’, and H-3’ with C-1’. The cross peaks of Ha-9 with Ha-10 and Hb-10, and Hb-9 with H-2’ observed in the ROESY spectrum indicated that hydroxymethyl group, Ha-9, and H-3’ were at the same face of the five-membered ring. The structure of 2 suggests an extraction artifact, however, the unambiguous detection of 2 by a close HPLC analysis in the raw material after extracting with analytical methanol and concentrating at ambient temperature suggested that 2 is indeed a new natural product (Figure 2). Therefore, the structure of 2 was assigned as 10-hydroxy-8,9-dioxy- isopropylidenethymol.
Figure 2.
HPLC check of compound 2 in the plant a
a HPLC conditions: column: LiChrospher 100 RP-18e (125 x 4 mm, 5 μm), mobile phase: 22% acetonitrile aqueous, flow rate: l mL/min, detection: 220 nm; A peak at tR14.8 min of the chromatogram of the crude extracts bears the same UV/DAD absorption and retention time as the reference compound; The proportion of peeks at 13.0 and 14.8 min is reversal when mixing reference with crude extracts.
The five known monoterpenoids were identified as 8,9,10-trihydroxythymol (3) [12], thymol-β-glucopyranoside (4) [13], 9-hydroxythymol (5) [14], 8,10-dihydroxy-9-isobutyryloxy- thymol (6) [15] and 8-hydroxy-9,10-diisobutyryloxythymol (7) [15], respectively, by comparison of their spectroscopic data (1H-, 13C-NMR and MS) with those reported in the literature. Thymol derivatives have been isolated from other Compositae species [16,17,18,19,20], with compounds 3-7 being thymol derivatives were isolated from C. minima for the first time.
Antibacterial tests revealed that all the agents tested exhibited antibacterial effects against all the bacteria investigated (Table 2). At the MIC of 6.25 μg/mL, compound 2 was found to be most effective against B. subtilis, compound 3 against S. typhimurium, and compound 7 against S. aureus, S. flexneri, and S. paratyphi-B. The MIC value of 2 against S. aureus is 12.5 μg/mL comparable with that of cefradine. All the tested bacteria were less sensitive to compound 1 with the MIC larger than 100 μg/mL. Thymol, as a component of volatile oil in many plants, has been proved to possess antimicrobial activities [21], the antibacterial activities of compounds 2-7 were probably resulted from their structural similarities with thymol. The results implied that C. minima could be a potential source for searching natural antibacterial substances applied for food preservation.
Table 2.
Antibacterial activity of compounds 1-7 (MIC a values, μg/mL).
| Pathogen | 1 | 2 | 3 | 4 | 5 | 6 | 7 | standards b | |
|---|---|---|---|---|---|---|---|---|---|
| Reference strains | |||||||||
| Staphylococcus aureus CMCC26001 | >100 | 12.5 | >100 | >100 | >100 | 100 | 6.25 | 15 | 7.5 |
| Escherichia coli CMCC44103 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 7.5 | 7.5 |
| Salmonella typhimurium CMCC80087 | >100 | >100 | 6.25 | 25 | 50 | 25 | >100 | 7.5 | 7.5 |
| Shigella flexneri CMCC51335 | >100 | 12.5 | >100 | >100 | >100 | >100 | 6.25 | 3.25 | 3.25 |
| Clinically isolated strains | |||||||||
| Staphylococcus epidermidis | >100 | 25 | >100 | 50 | >100 | 100 | 50 | 3.25 | 7.5 |
| Bacillus subtilis | >100 | 6.25 | >100 | >100 | >100 | 50 | >100 | 3.25 | 3.25 |
| Salmonella paratyphi-A | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 3.25 | 3.25 |
| Salmonella paratyphi-B | >100 | >100 | >100 | >100 | >100 | >100 | 6.25 | 3.25 | 3.25 |
a Minimum inhibition concentration; b Left column is for cefradine and the right is for gentamycin.
Experimental
General
Melting points were obtained on an XRC-1 micromelting apparatus. Optical rotations were determined on a JASCO-20C digital polarimeter. UV spectra were recorded on a Shimadzu UV-2401PC spectrophotometer. IR spectra were obtained with a Bruker Tensor 27 FT-IR spectrophotometer with KBr pellets. 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra were recorded on a Bruker AM-400 spectrometer with TMS as an internal reference. 2D NMR spectra were measured with a DRX-500 spectrometer. EIMS (70 eV) were recorded on a VG Auto Spec-3000 spectrometer. ESIMS and HRESIMS were carried our with an API QSTAR Pulsar 1 spectrometer. Silica gel (200-300 mesh and 10-40 μm) for column chromatography and GF254 for TLC were obtained from Qingdao Marine Chemical Factory, Qingdao, People’s Republic of China. Sephadex LH-20 was obtained from Amersham Pharmacia Biotech, Sweden. RP-18 silica gel (40-63 μm) used for open column chromatography was purchased from Daiso Co., Japan. Diaion HP20 and MCI gel CHP 20P (75-150 μm) were obtained from Mitsubishikasei, Tokyo, Japan. Fractions were monitored by TLC and spots were visualized after spraying with 10% H2SO4 in ethanol or anisaldehyde reagent followed by heating.
Plant Material
The whole plants of C. minima were purchased from Yunnan Corporation of Materia Medica, Yunnan Province, P. R. China, and identified by Mr. H. Y. Sun at Yunnan Corporation of Materia Medica. A voucher specimen (No. CHYX0159) was deposited at the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, P. R. China.
Extraction and Isolation
Dried and powdered plant materials of C. minima (10 kg) were extracted three times with 95% EtOH under reflux and the combined solvent was evaporated in vacuo. The extracts was suspended in water and successively partitioned with petroleum ether, EtOAc and n-BuOH, respectively. The EtOAc extracts (170 g) were subjected to column chromatography (CC) over silica gel (200-300 mesh) and eluted with CHCl2-MeOH (8:1) to give fractions 1-4. Fraction 3 (70 g) was submitted to CC on silica gel, eluting with CHCl3-MeOH (11:1) to give fractions 3.1-3.4. Fraction 3.3 (13 g) was chromatographed on MCI gel CHP 20P eluted with MeOH-H2O (9:1) followed by gel filtration on Sephadex LH-20 (MeOH) and repeated vacuo liquid chromatography (VLC) to yield 3 (22 mg) and 4 (52 mg). Fraction 2 (30 g) was chromatographed on Diaion HP20 (95% EtOH) to decolor, the eluents were then subjected to CC on silica gel, eluting with CHCl3-MeOH (1:0-5:1) to give fractions 2.1-2.8. Fraction 2.2 (10 g) was passed through MCI gel CHP 20P eluted with MeOH-H2O (9:1) to decolor, and then subjected to Sephadex LH-20 (MeOH), RP-18 (MeOH-H2O, 50:50-100:0) and repeated VLC to yield 1 (44 mg), 2 (13 mg), 5 (28 mg), 6 (66 mg), and 7 ( 90 mg).
8,10-Dihydroxy-9(2)-methylbutyryloxythymol (1). Colorless oil; [α]D20.8 +13.46o (c 0.52, CHCl3); UV (CHCl3) λmax (log ε) nm : 278 (3.34), 238(3.08); IR (KBr) νmax: 3417, 1717, 1578, 1462 cm-1. ESIMS m/z: 305 [M+Na]+; HRESIMS m/z: 305.1363 [M+Na]+ (calcd. C15H22O5Na 305.1364, error = -0.635 ppm); 1H- and 13C-NMR data, see Table 1.
10-Hydroxy-8,9-dioxyisopropylidene-thymol (2). Colorless crystal; mp 156-157oC; [α]D20.5 +9.36o (c 0.29, MeOH); UV (MeOH) λmax (log ε) nm: 282 (3.32), 275(3.33), 219 (3.87), 203 (4.36); IR (KBr) νmax: 3405, 1617, 1588, 1458 cm-1; ESIMS m/z: 261 [M+Na]+; HRESIMS m/z: 261.1108 [M+Na]+ (calcd for C13H18O4Na 261.1102, error = 1.9953 ppm); 1H- and 13C-NMR data, see Table 1.
Antibacterial Assay
Antibacterial properties were tested by agar dilution method [22]. The bacterial strains employed were Staphylococcus aureus CMCC26001 (CMCC, National Center for Medical Culture Collections, Beijing, China), Escherichia coli CMCC44103, Salmonella typhimurium CMCC80087, and Shigella flexneri CMCC51335, and the following clinically isolated strains: Staphylococcus epidermidis, Bacillus subtilis, Salmonella paratyphi-A, Salmonella paratyphi-B. Cefradine and gentamycin were used as reference standards, plates containing only MHA medium and 1% DMSO in MHA medium served as negative and solvent controls. The tests were performed in triplicate and repeated once.
Acknowledgements
This work was financially supported by Natural Science Foundation of Yunnan (2006B0041Q) and “Xi-Bu-Zhi-Guang” Project from the Chinese Academy of Sciences. We thank Mrs. Y. Zhang and C. J. Zhang at Department of Microbiology and immunology, Kunming Medical College, for their assistance in antimicrobial assays.
Footnotes
Sample Availability: Samples of compounds 4 and 6 are available from the authors.
References and Notes
- 1.Tauxe R. V. Emerging foodborne diseases: An evolving public health challenge. Dairy Food Environm. Sanitation. 1990;17:788–795. doi: 10.3201/eid0304.970403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beović B. The issue of antimicrobial resistance in human medicine. Int. J. Food Microbiol. 2006;112:280–287. doi: 10.1016/j.ijfoodmicro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Smid E. J., Gorris L. G. M. Natural antimicrobials for food preservation. In: Rahman M. S., editor. Handbook of Food Preservation. New York; Marcel Dekker: 1999. pp. 285–308. [Google Scholar]
- 4.Essawi T., Srour M. Screening of some Palestinian medicinal plants for antibacterial activity. J. Ethnopharmacol. 2000;70:343–349. doi: 10.1016/S0378-8741(99)00187-7. [DOI] [PubMed] [Google Scholar]
- 5.Reische D. W., Lillard D. A., Eintenmiller R. R. Antioxidants in food lipids. In: Ahoh C. C., Min D. B., editors. Chemistry, Nutrition and Biotechnology. New York; Marcel Dekker: 1998. pp. 423–448. [Google Scholar]
- 6.Shi Z., Fu G. X. Flora of China. no. 1. Vol. 76. Science Publishing House; Beijing, People’s Republic of China: 1983. pp. 132–133. [Google Scholar]
- 7.Jiangsu New Medical College . Zhongyao Dacidian (Dictionary of Traditional Chinese Medicine) Shanghai Science and Technology Publishing House; Shanghai, People’s Republic of China: 1992. [Google Scholar]
- 8.Wu J. B., Chun T. T., Ebizuka Y., Sankawa U. Biologically active constituents of Centipeda minima: Isolation of a new plenolin ester and the anti-allergy activity of sesquiterpene lactones. Chem. Pharm. Bull. 1985;33:4091–4094. doi: 10.1248/cpb.33.4091. [DOI] [PubMed] [Google Scholar]
- 9.Wu J. B., Chun T. T., Ebizuka Y., Sankawa U. Biologically active constituents of Centipeda minima: Sesquiterpenes of potential anti-allergy activity. Chem. Pharm. Bull. 1991;39:3272–3275. doi: 10.1248/cpb.39.3272. [DOI] [PubMed] [Google Scholar]
- 10.Iwakami S., Wu J. B., Ebizuka Y., Sankawa U. Platelet activating factor (PAF) antagonists contained in medicinal plants: Lignans and sesquiterpenes. Chem. Pharm. Bull. 1992;40:1196–1198. doi: 10.1248/cpb.40.1196. [DOI] [PubMed] [Google Scholar]
- 11.Taylor R. S. L., Towers G. H. N. Antibacterial constituents of the Nepalese medicinal herb, Centipeda minima. Phytochemistry. 1998;47:631–634. doi: 10.1016/S0031-9422(97)00534-7. [DOI] [PubMed] [Google Scholar]
- 12.Bohlmann F., Grenz M., Jakupovic J., King R. M., Robinson H. Four heliangolides and other sesquiterpenes from Brasilia sickii. Phytochemistry. 1983;22:1213–1218. doi: 10.1016/0031-9422(83)80224-6. [DOI] [Google Scholar]
- 13.Ahmed A. A., Jakupovic J. Sesqui- and monoterpenes from Jasonia montana. Phytochemistry. 1990;29:3658–3661. doi: 10.1016/0031-9422(90)85296-R. [DOI] [Google Scholar]
- 14.Passreiter C. M., Matthiesen U., Willuhn G. 10-Acetoxy-9-chloro-8,9-dehydrothymol and further thymol derivatives from Arnica sachalinensis. Phytochemistry. 1998;49:777–781. doi: 10.1016/S0031-9422(98)00242-8. [DOI] [Google Scholar]
- 15.Gonzalez A. G., Barrera J. B., Rosas F. E., Hernandez A. Y., Espineira J., Joseph-Nathan P. Thymol derivatives from Schizogyne glaberrima. Phytochemistry. 1986;25:2889–2891. doi: 10.1016/S0031-9422(00)83761-9. [DOI] [Google Scholar]
- 16.Gonzalez A. G., Bermejo J. Sesquiterpene lactones and other constituents from Allagopappus species. J. Nat. Prod. 1995;3:432–437. doi: 10.1021/np50117a014. [DOI] [Google Scholar]
- 17.Uchiyama T., Miyase T., Ueno A., Usmanghani K. Terpene and lignan glycosides from Pluchea indica. Phytochemistry. 1991;2:655–657. doi: 10.1016/0031-9422(91)83746-8. [DOI] [Google Scholar]
- 18.Shi Y. P., Guo W., Yang C., Jia Z. J. Two new aromatic monoterpene derivatives from Carpesium lipskyi. Planta Med. 1998;64:671–672. doi: 10.1055/s-2006-957550. [DOI] [PubMed] [Google Scholar]
- 19.Tori M., Ohara Y., Nakashima K., Sono M. Thymol dervivatives from Eupatorium fortunei. J. Nat. Prod. 2001;64:1048–1051. doi: 10.1021/np0101191. [DOI] [PubMed] [Google Scholar]
- 20.Marco J. A., Sanz-Cervera J. F., Manglano E. Chlorinated thymol derivatives from Inula Crithmoides. Phytochemistry. 1993;4:875–878. doi: 10.1016/0031-9422(93)85294-2. [DOI] [Google Scholar]
- 21.Yoshida T., Mori K., He G. X. Inulavosin, a new thymol dimmer with piscicidal activity from Inula nervosa. Heterocycles. 1995;41:1923–1926. doi: 10.3987/COM-95-7148. [DOI] [Google Scholar]
- 22.Baker C. N., Banerjee S. N., Tenover F. C. Evolution of Alamar colorimetric MIC method for antimicrobial susceptibility testing of Gram-negative bacteria. J. Clinic Microbiol. 1994;32:1261–1267. doi: 10.1128/jcm.32.5.1261-1267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]