Abstract
Two new groups of cholane-peptoid hybrid macrocycles were produced by implementing novel combinations of the MiB methodology. Steroid-based hybrid macrolactams including heterocycle and aryl moieties were obtained by utilizing cholanic dicarboxylic acids and diamines in a bidirectional double Ugi-Four-Component (Ugi-4CR) based macrocyclization protocol. Alternatively, N-substituted cyclocholamides were produced from a cholanic pseudo-amino acid by an Ugi-4CR-based cyclooligomerization approach. Both types of macrocycles are steroid-peptoid hybrid macrocycles containing exocyclic peptidic chains. These novel frameworks are a result of the use of bile acids bifunctionalized with carboxylic and amino functionalities as bifunctional building blocks of the Ugi-MiB approach.
Keywords: Bile acids, macrocycles, steroids, peptoids, Ugi reaction, multicomponent reactions
Introduction
Macrocycles incorporating the cholanic skeleton have proven successful as host compounds in molecular and ion pair recognition studies [1,2,3,4]. It is known that the appropriate binding of a guest to a synthetic receptor is not only a result of the presence of the proper recognition elements, but also of their relative disposition in space and the three-dimensional shape of the overall interaction [5]. In this sense, molecules such as steroids with well-defined, preorganized architectures are amenable to the design of macrocyclic hosts wherein conformational freedom can be kept under close control [1,2,3,4]. The cholane skeleton of bile acids is very suitable for receptor design as its curved geometry allows positioning of multiple binding functionalities directed towards the concave side.
Different types of bile acid-based macrocycles have been reported to be useful for the encapsulation of metal cations, anions or biologically relevant molecules such as sugars and amino acids. These macrocycles can be integrated into two main types of compounds, i.e., the oligomeric and the hybrid steroidal macrocycles. The oligomeric steroidal macrocycles include the well-known cyclocholates [3,6,7,8,9] and other analogous cyclocholamide skeletons [3,10,11]. On the other hand, the hybrid steroidal macrocycles are those wherein the steroidal nucleus alternates with another type of chemical moiety, thus forming hybrid skeletons. Examples of this widely explored type of macrocycles are the well-known cholaphanes [1], as well as other steroid-aryl [3,4,12], steroid-peptide [13,14] and steroid-polyether hybrids [3,4,15].
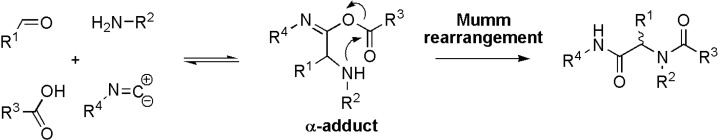
We have recently developed a new macrocyclization approach termed multiple multicomponent macrocyclizations including bifunctional building blocks (MiBs) [16,17,18]. The Ugi-version of this methodology (i.e. Ugi-MiB) is based on the performance of multiple Ugi four-component reactions (Ugi-4CR) to afford the macrocyclic scaffolds in one pot. The Ugi-4CR is the condensation of an oxo compound, a primary amine, a carboxylic acid and an isocyanide (Scheme 1) to afford an N-substituted dipeptide backbone (i.e., peptoid) [19]. The Ugi-MiB approach has been employed to produce the first cholaphanes including the peptoid backbone in their structures [16]. The biarylether moiety and natural α-amino acids have also been incorporated into the cholaphane frameworks as Ugi-reactive building blocks [20]. It should be mentioned that all previously obtained peptoid-containing cholaphanes have been produced by bidirectional Ugi-MiBs, that is, the use of building blocks bifunctionalized with the same Ugi-reactive group. Furthermore, only the diacid/diisocyanide and the diamine/diisocyanide combinations of bifunctional building blocks have been explored for the synthesis of steroid-based macrocycles [17,20,21].
Scheme 1.
Schematic representation of the Ugi four-component reaction (Ugi-4CR).
Herein we report on the first use of the diacid/diamine combination of bidirectional Ugi-MiBs to assemble a new type of steroid-peptoid hybrid macrocycles, as well as the first example of the use of an unidirectional Ugi-MiB approach to obtain N-substituted cyclocholamides with a natural cyclopeptide-like uniform N- to C-terminus directionality.
Results and Discussion
The use of different combinations of bifunctional building blocks to assemble otherwise structurally similar macrocycles is an important feature to achieve easy variation of macrocycle sizes and their flexibility [17,18]. The variation of these combinations not only affords a dissimilar number of endo versus exocyclic amide bonds, but also enables a tunable differentiation of the peptoid N→C directionality [17]. The diacid/diamine combination herein explored produces macrocycles containing one fully exocyclic amide bond at each Ugi-peptoid backbone formed. Accordingly, the resulting compounds are macrocyclic N-substituted dilactams presenting appended peptidic functionalities derived from the oxo and isocyanide components of the Ugi-4CRs.
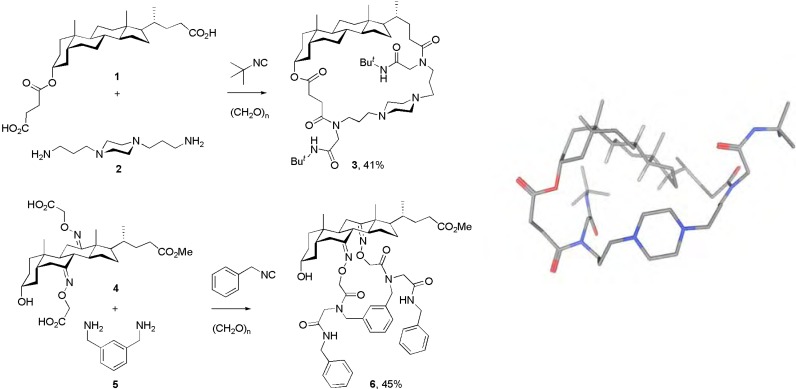
Scheme 2 illustrates two examples of the synthesis of cholane-peptoid hybrid macrocycles based on the diacid/diamine combination of bidirectional Ugi-MiBs. Typical pseudo-dilution conditions were employed in all macrocyclizations, which consist in the slow addition (via syringe pump) of the diacid and the isocyanide to a stirring solution of the preformed diimine (c = 4 mM). Under these conditions, previously optimized in our laboratory, the smallest macrocycle is usually formed unless folding or strain factors (also on the stage of the cyclic Mumm intermediate) shift the system towards higher oligomeric macrocycles. Also, acyclic oligomers are sometimes detected in the crude product, but they are easily separated from the cyclized products due to their quite different chromatographic behavior.
Scheme 2.
Synthesis of cholane-based hybrid macrolactams by bidirectional double Ugi-4CR-based macrocyclizations (Ugi-MiBs). Examples of the diacid/diamine combination. The figure on the right side shows one calculated minimized structure of macrocycle 3 without guest or protonation. For calculation method details see the Experimental section.
The synthesis of macrocycle 3 comprises the use of the long and flexible diamine 2 as counterpart of the steroidal diacid 1. This diamine is able to span the distance of the cholanic skeleton, thus the macrocycle formation is accomplished based on only two Ugi-4CRs, one for the connection of the bifunctional building blocks, and the other one for the ring closure. A similar case was employed for the synthesis of macrocycle 6, wherein the shorter diamine 5 was allowed to react with the steroidal diacid 4. In this latter compound the two carboxylic functionalities are closer and directed towards the same side, due to the E geometry of both O-(carboxymethyl)-oximino groups. These features allow the formation of the smallest macrocycle (6), also based on only two Ugi-4CRs.
It should be noticed that the use of paraformaldehyde is preferred only to avoid diastereoisomer formation, and other aldehydes usually react at least equally well. Also the isocyanide component can be easily changed without affecting the macrocyclization product distribution. This applies not only to commercially available isocyanides but also to derivatives bearing further functionalities desired to be attached to the macrocyclic core. Of course, less reactive isocyanides will afford lower yields or require longer reaction times, as usual.
Macrocycle libraries of peptoid-based macrocycles have been previously produced in a combinatorial fashion by utilizing both the diacid/diisocyanide and the diamine/diisocyanide combination of Ugi-MiBs [21]. The use of several different monofunctional components in the same reaction pot then affords macrocycles presenting the same macrocyclic skeletons but different appended functionalities. The benefit of this original combinatorial method – in contrast to parallel synthesis – is the one-pot incorporation of different appendages at each peptoid moiety within the same macrocycle. Accordingly, we decided to produce a combinatorial MiB approach by using the diacid/diamine combination focused on steroid-based macrocycles.
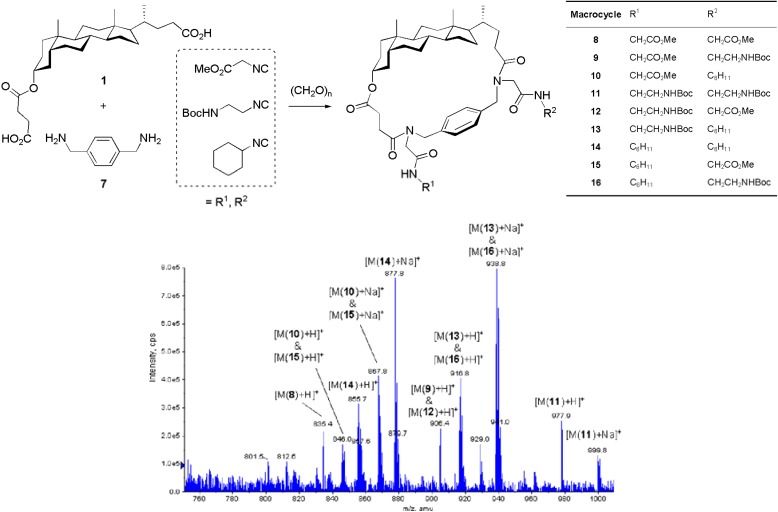
Scheme 3 depicts the production of a small combinatorial library of steroid-peptoid-aryl hybrid macrocycles by employing diacid 1 and diamine 7 as bifunctional building blocks and three different isocyanides as the source of appendage diversity. Due to the unsymmetric nature of the cholanic skeleton, the final macrocyclic frameworks lack a plane of symmetry thus producing nine different macrocycles, i.e., three equally disubstituted bislactams and three pairs of regio-isomeric bislactams. As shown in the ESI-MS spectrum, all possible macrocycles 8-16 were found in the crude library, although in different amounts. This was also proved by HPLC-MS analysis, wherein the peaks corresponding to macrocycles formed from methyl isocyanoacetate are smaller than those lacking this isonitrile. This result may not only be due to the lower reactivity of methyl isocyanoacetate, but also to its propensity to undergo side reactions such as nucleophilic attack to the ester functionality and carbanion-mediated condensations [19,22]
Scheme 3.
Mixed library of macrocycles produced by combinatorial Ugi-MiB. Variation of the isocyanide component in a diacid/diamine combination. Expanded region of the ESI-MS spectrum (positive mode) of the crude library.
Aside from the use of equally bifunctionalized building blocks to accomplish the Ugi-4CR-based macrocyclizations (bidirectional MiBs), there is also the possibility to employ building blocks differently bifunctionalized with two Ugi-reactive groups. This allows obtaining macrocycles via an unidirectional double Ugi-4CR-based macrocyclization (unidirectional MiB). With unidirectional we mean that the two resulting peptoid backbones have N→C directionalities running clockwise (or counterclockwise), in contrast to the bidirectional approach wherein the peptoid chains run clockwise and counterclockwise in the same molecule. Unidirectional Ugi-MiB has been previously employed to cyclize oligopeptides [23], but their use to assemble steroid-based macrocycle has not been reported.
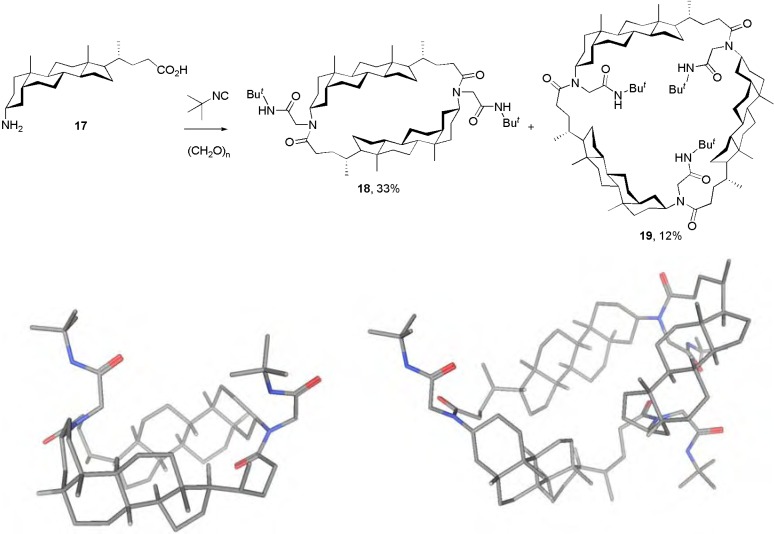
As depicted in Scheme 4 (top), an unidirectional Ugi-MiB approach can be implemented easily by utilizing the lithocholic acid derivative 17 functionalized with an amino group at C-3. This compound was readily prepared from methyl lithocholate according to reported procedures [13,24]. The 3α-OH is replaced by an azido group with retention of configuration, followed by ester hydrolysis and azide reduction. Upon reaction of this amino acid building block with an oxo component and an isocyanide under dilution conditions, a mixture of the cyclodimer 18 and the cyclotrimer 19 were obtained in 33% and 12% yield, respectively. This procedure relies on the performance of double and threefold Ugi-4CR-based macrocyclizations that furnish the dimeric and trimeric N-substituted cyclocholamides 18 and 19, respectively. The corresponding tetramer was formed only in traces as detected by ESI-MS, but it could not be isolated by column chromatography.
Scheme 4.
Synthesis of N-substituted cyclocholamides by unidirectional Ugi-MiB and their calculated minimzed structures (for calculation details see the Experimental section).
Cyclocholamides 18 and 19 are analogs of varied oligomeric cholane-based macrolactams previously reported by other groups [3,10,11]. However, the N-substitution introduces new structural features compared to the classical cyclocholamides and this may be seen as negative or positive depending on the desired application. For example, while the important hydrogen bond donor capability of the amide is lost, the presence of an exocyclic peptide-like chain can be used to incorporate additional functionalities of relevance for, e.g., molecular recognition. If equipped with appropriate recognition motifs (e.g., axially-oriented hydroxy groups), this new class of cyclocholamides may behave as suitable synthetic receptors of important biomolecules such as carbohydrates [25]. Molecular modelling (Scheme 3, bottom) shows that the compounds, especially 19, can produce sufficiently large interiors to accomodate small molecules and that several minimized conformations can be adopted (increasing in number from 18 < 19 < 3). However, even in the larger members, the N-tert-butyl-acetamide side chains do not like to fold into the interior of these compounds but rather act as additional walls on the side, whereas in the more flexible macrocycle 3 with a very polar non-steroidal half (Scheme 2), one tert.-butyl group has enough freedom to bend toward the lipophilic steroid moiety, temporarily closing one side of the ring.
Conclusions
In conclusion, two new groups of steroid-peptoid hybrid macrocycles have been produced by utilizing previously unexplored combinations of the MiB strategy. Hybrid macrolactams were obtained by using the diacid/diamine combination of bidirectional Ugi-MiBs, implemented with the use of cholanic dicarboxylic acids and simple diamines as building blocks. Similarily, N-substituted cyclocholamides were achieved by an unidirectional Ugi-MiB approach based also on the acid/amine combination. This latter approach relies on a cyclooligomerization process accomplished via multiple Ugi-4CRs that serve for both growing the acyclic intermediates and the macrocyclic ring closure.
Experimental
General
Melting points were determined on a Leica DM LS2 apparatus and are uncorrected. 1H-NMR and 13C-NMR spectra were recorded in CDCl3 on a Varian Mercury 300 spectrometer at 300.2 MHz and 75.5 MHz, respectively. Chemical shifts (δ) are reported in ppm relative to the TMS (1H-NMR) and to the solvent signal (13C-NMR). IR spectra were obtained on a Bruker FT-IR spectrometer. High resolution ESI mass spectra were obtained from a Bruker Apex III Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer equipped with an Infinity™ cell, a 7.0 Tesla superconducting magnet, an RF-only hexapole ion guide and an external electrospray ion source (Agilent, off axis spray). ESI-MS was recorded on a Finnigan TSQ 7000, LC-Tech Ultra Plus pumps, Linear UV-VIS 200 detector, Sepserve Ultrasep ES RP-18 5μm 1×100 mm column, flow 70µL min-1. Flash column chromatography was carried out using Merck silica gel 60 (0.015-0.040 nm) and analytical thin layer chromatography (TLC) was performed using Merck silica gel 60 F254 aluminium sheets. The solid compounds were recrystallized from selected solvents for the melting point measurements. All commercially available chemicals were used without further purification. Diacids 1 and 4 were obtained as described in references 26 and 20, respectively. For the macrocycle modelling and figures the software "Molecular Operating Environment 2007.07 MOE, 2007.07 release" (http://www.chemcomp.com/, Chemical Computing Group, Montreal, Quebec, Canada H3A 2R7) was used, applying the MMFF94x force field according to Halgren [27]. The pictures show the respective lowest energy conformation from a stochastic conformational search performed with the above mentioned program and force field with the following (standard) parameters: Chiral conversion, allow bond rotation, mode: bias-30, Cartesian perturbation (delta: 0.0001), Cartesian minimization (RMS gradient: 0.001), energy cutoff: 7, failure limit 20, RMS tolerance: 0.1, conformation limit: 10.000, iteration limit: 10.000, MM iteration limit: 10.000, with added chiral constraints, calculating force field partial charges.
General procedure for the double Ugi-4CR-based macrocyclizations of the diacid/diamine combination
A solution of the diamine (1 mmol) and paraformaldehyde (2 mmol) in MeOH (250 mL) was stirred for 1 h at room temperature. Two solutions, one of the diacid (1 mmol) and another of the isocyanide (2 mmol) in 20 mL of MeOH each, are simultaneously and slowly added to the reaction mixture using syringe pumps (flow rate 0.5 mLh-1). After the addition is complete, the reaction mixture is stirred for 8 h and concentrated under reduced pressure to give a crude product, which is purified by flash column chromatography on silica.
Macrocycle 3: Diacid 1 (476 mg, 1 mmol), diamine 2 (200 mg, 1 mmol), paraformaldehyde (60 mg, 2 mmol), and tert-butylisocyanide (0.23 mL, 2 mmol) were reacted according to the general macrocyclization procedure. Flash column chromatography purification (CH2Cl2/MeOH 15:1) afforded macrocycle 3 (355 mg, 41%) as a white solid. Rf = 0.43 (CH2Cl2/MeOH 10:1). m.p. (from MeOH): 217-219 °C. 1H-NMR: δ = 6.64 (s, 1H, NH); 6.21 (s, 1H, NH); 4.79 (m, 1H, H-3β); 4.11 (d, 1H, J=14.6 Hz, CH2); 3.83 (m, 2H, CH2); 3.72 (d, 1H, J=14.4 Hz, CH2); 3.68-3.61 (m, 2H, CH2); 3.52 (t, 2H, J=7.0 Hz, CH2); 3.39-3.33 (m, 2H, CH2); 1.31 (s, 9H, (CH3)3C); 1.30 (s, 9H, (CH3)3C); 1.00 (d, 3H, J=6.8 Hz, CH3-21); 0.92 (s, 3H, CH3-19); 0.67 (s, 3H, CH3-18). 13C-NMR: δ = 175.8, 172.2, 170.4, 168.9, 168.4, 73.8, 56.7, 54.6, 53.1, 52.9, 51.8, 46.6, 41.5, 40.6, 40.0, 35.6, 34.7, 34.4, 34.3, 33.6, 32.0, 31.7, 30.1, 28.6, 28.5, 27.8, 27.5, 26.7, 26.6, 26.4, 26.2, 24.3, 24.0, 23.2, 20.7, 18.7, 11.9. HRMS (ESI-FT-ICR) m/z: 867.6662 [M+H]+; calcd. for C50H87O6N6: 867.6681.
Macrocycle 6: Diacid 4 (564 mg, 1 mmol), diamine 5 (136 mg, 1 mmol), paraformaldehyde (60 mg, 2 mmol), and benzylisocyanide (0.23 mL, 2 mmol) were reacted according to the general macrocyclization procedure. Flash column chromatography purification (CH2Cl2/MeOH 20:1) afforded macrocycle 6 (431 mg, 45%) as a white solid. Rf = 0.71 (CH2Cl2/MeOH 15:1). m.p. (from EtOAc): 175-177 °C. 1H-NMR: δ = 7.66 (d, 1H, J=7.2 Hz, CH-Ph), 7.58 (t, 1H, J=6.4 Hz, NH); 7.44 (t, 1H, J=6.2 Hz, NH); 7.38-7.18 (m, 12H); 6.80 (s, 1H, CH); 5.69-5.64 (m, 2H); 5.00-4.94 (m, 2H); 4.74 (d, 1H, J=14.2 Hz, CH2), 4.67 (m, 2H, CH2), 4.54 (d, 2H, J=14.4 Hz, CH2), 4.43 (dd, 2H, J=14.6/5.8 Hz, CH2), 4.24 (m, 2H, CH2), 3.67 (s, 3H, CH3O); 3.48 (m, 1H, H-3β); 1.04 (s, 3H, CH3-19); 0.92 (d, 3H, J=6.8 Hz, CH3-21); 0.80 (s, 3H, CH3-18). 13C-NMR: δ = 174.3, 169.6, 169.1, 168.7, 168.4, 166.3, 166.1, 162.0, 138.1, 137.3, 136.4, 134.7, 130.8, 130.5, 129.5, 128.7, 128.5, 127.9, 127.8, 127.7, 127.6, 127.3, 77.2, 74.3, 73.6, 69.7, 53.7, 53.5, 51.5, 49.7, 48.5, 48.2, 45.7, 45.2, 43.7, 43.4, 41.8, 37.8, 35.6, 35.5, 34.5, 31.8, 30.6, 30.0, 28.0, 27.6, 25.0, 22.4, 20.6, 19.5, 12.5. HRMS (ESI-FT-ICR) m/z: 981.5089 [M+Na]+; calcd. for C55H70O9NaN6: 981.5091.
Combinatorial double Ugi-4CR-based macrocyclization
Diacid 1 (47.6 mg, 0.1 mmol), diamine 7 (13.6 mg, 0.1 mmol), paraformaldehyde (6 mg, 0.2 mmol), and a mixture of methyl isocyanoacetate (6 μL, 0.07 mmol), tert-butyl 2-isocyanoethyl-carbamate (12 mg, 0.07 mmol), and cyclohexylisocyanide (8 μL, 0.07 mmol) were reacted according to the general macrocyclization procedure. The crude library was analyzed by ESI-MS and HPLC (Nucleosil 50 column, 5 μm 4×250 mm, CH2Cl2/MeOH 96: 4, flow rate: 1 mLmin-1, 254 nm).
Procedure for the unidirectional double Ugi-4CR-based macrocyclization
Two solutions, one of the steroidal amino acid 17 (375 mg, 1 mmol) and another of tert-butylisocyanide (0.23 mL, 2 mmol) in 20 mL of MeOH each, were simultaneously and slowly added to a stirred suspension of paraformaldehyde (60 mg, 2 mmol) in MeOH (100 mL) using syringe pumps (flow rate 0.5 mLh-1). After the addition was completed, the reaction mixture was stirred for 8 h and concentrated under reduced pressure to give a crude product. Flash column chromatography purification on silica with n-hexane/EtOAc 10:1 furnished macrocycles 18 (155 mg, 33%) and 19 (56.5 mg, 12%).
Macrocycle 18: Rf = 0.38 (n-hexane/EtOAc 5:1). White solid. m.p. (from CH2Cl2/MeOH): 288-290 °C. 1H-NMR: δ = 6.60-6.54 (m, 2H, NH); 4.16-4.12 (m, 2H, CH2); 3.86-3.81 (m, 2H, CH2); 3.47 (br. m, 2H, H-3β); 1.31 (s, 9H, (CH3)3C); 1.30 (s, 9H, (CH3)3C); 0.93-0.92 (m, 6H, CH3-21); 0.89 (s, 6H, CH3-19); 0.65 (s, 6H, CH3-18). 13C-NMR: δ = 169.4, 168.5, 70.6, 56.3, 54.5, 52.5, 45.6, 45.1, 42.5, 41.4, 40.8, 39.3, 35.9, 35.4, 34.8, 34.6, 32.2, 31.4, 28.7, 28.1, 28.0, 27.4, 26.8, 26.7, 24.4, 23.2, 20.6, 19.7, 12.1; HRMS (ESI-FT-ICR) m/z: 963.7651 [M+Na]+; calcd. for C60H100O4NaN4: 963.7646.
Macrocycle 19: Rf = 0.31 (n-hexane/EtOAc 5:1). White solid. m.p. (from EtOAc): 257-259 °C; 1H- NMR: δ = 6.61-6.57 (m, 3H, NH); 4.08-4.00 (m, 6H, CH2); 3.61 (br. m, 3H, H-3β); 1.30 (s, 27H, (CH3)3C); 0.96 (d, 9H, CH3-21); 0.91 (s, 9H, CH3-19); 0.66 (s, 9H, CH3-18); 13C-NMR: δ = 169.7, 168.6, 70.4, 56.6, 54.6, 45.1, 44.8, 42.6, 41.8, 40.6, 40.2, 35.8, 35.3, 35.0, 34.5, 32.4, 30.7, 29.9, 28.2, 28.1, 27.2, 26.6, 26.5, 24.3, 23.5, 20.8, 18.6, 12.0; HRMS (ESI-FT-ICR) m/z: 1434.1519 [M+Na]+; calcd. for C90H150O6NaN6: 1434.1516.
Acknowledgements
We gratefully acknowledge the Deutsche Forschungsgemeinschaft for financial support (Grant: GRK-894). We thank Robert Klein for his contribution to macrocycle modelling and graphics as part of his student research program.
Footnotes
Sample Availability: Contact the author.
References
- 1.Davis A.P. Cholaphanes et al.; Steroids as Structural Components in Molecular Engineering. Chem. Soc. Rev. 1993;22:243–253. doi: 10.1039/cs9932200243. [DOI] [Google Scholar]
- 2.Walliman P., Marti T., Fürer A., Diederich F. Steroids in Molecular Recognition. Chem. Rev. 1997;97:1567–1608. doi: 10.1021/cr960373b. [DOI] [PubMed] [Google Scholar]
- 3.Tamminen J., Kolehmainen E. Bile Acids as Building Blocks of Supramolecular Hosts. Molecules. 2001;6:21–46. doi: 10.3390/60100021. [DOI] [Google Scholar]
- 4.Virtanen E., Kolehmainen E. Use of Bile Acids in Pharmacological and Supramolecular Applications. Eur. J. Org. Chem. 2004:3385–3399. doi: 10.1002/ejoc.200300699. [DOI] [Google Scholar]
- 5.Lehn J.-M. Supramolecular Chemistry: Concepts and Perspectives. Wiley-VCH; Weinheim: 1995. [Google Scholar]
- 6.Dias J.R., Paskal R.A., Morrill J., Holder A.J., Gao H., Barnes C. Remarkable Structures of Cyclotri(deoxycholate) and Cyclotetra(24-norcholate) Acetate Esters. J. Am. Chem. Soc. 2002;124:4647–4652. doi: 10.1021/ja011797c. [DOI] [PubMed] [Google Scholar]
- 7.Gao H., Dias J.R. Cyclocholates with 12-Oxo and 7,12-Oxo Groups. Eur. J. Org. Chem. 1998:719–724. doi: 10.1002/(SICI)1099-0690(199804)1998:4<719::AID-EJOC719>3.0.CO;2-F. [DOI] [Google Scholar]
- 8.Brady P.A., Bonar-Law R.P., Rowan S.J., Suckling C.J., Sanders J.K.M. ‘Living’ macrolactonization: Thermodynamically-controlled cyclization and interconversion of oligocholates. Chem. Commun. 1996:319–320. [Google Scholar]
- 9.Bonar-Law R.P., Sanders J.K.M. Cyclocholates: Synthesis and Ion Binding. Tetrahedron Lett. 1992;33:2071–2074. doi: 10.1016/0040-4039(92)88145-U. [DOI] [Google Scholar]
- 10.Feigel M., Ladberg R., Winter M., Bläaser D., Boese R. Synthesis and Structure of Macrolactams of 3α-Aminodeoxycholanic Acid. Eur. J. Org. Chem. 2006:371–377. [Google Scholar]
- 11.Davis A.P., Walsh J.J. Steroid-based receptors with tunable cavities; stepwise and direct synthesis of a C3-symmetrical prototype. Chem. Commun. 1996:449–451. doi: 10.1039/cc9960000449. [DOI] [Google Scholar]
- 12.Sisson A.L., Clare J.P., Davis A.P. Contra-Hofmeister anion extraction by cyclosteroidal receptors. Chem. Commun. 2005:5263–5265. doi: 10.1039/b510768g. [DOI] [PubMed] [Google Scholar]
- 13.Albert D., Feigel M.A. Steroidal Cyclopeptide, Synthesis and Shape of the Cavity. Tetrahedron Lett. 1994;35:565–568. doi: 10.1016/S0040-4039(00)75839-4. [DOI] [Google Scholar]
- 14.Albert D., Feigel M. β-Loop, γ-Loop, and Helical Peptide Conformations in Cyclopeptides Containing a Steroidal Pseudo-Amino Acid. Helv. Chim. Acta. 1997;80:2168–2181. doi: 10.1002/hlca.19970800716. [DOI] [Google Scholar]
- 15.Maitra U., Bag B.G. Synthesis and Cation Binding Properties of a Novel “Chola-Crown”. J. Org. Chem. 1994;59:6114–6115. doi: 10.1021/jo00099a055. [DOI] [Google Scholar]
- 16.Voigt B., Rivera D.G. Diversity Oriented One-pot Synthesis of Complex Macrocycles: Very Large Steroid-Peptoid Hybrids via Multiple Multicomponent Reactions Including Bifunctional Building Blocks (MiBs) Angew. Chem. Int. Ed. 2005;44:4785–4790. doi: 10.1002/anie.200500019. [DOI] [PubMed] [Google Scholar]
- 17.Rivera D.G., Wessjohann L.A. Supramolecular Compounds by Multiple Multicomponent Macrocyclizations: Peptoid-based Cryptands, Cages and Cryptophanes. J. Am. Chem. Soc. 2006;128:7122–7123. doi: 10.1021/ja060720r. [DOI] [PubMed] [Google Scholar]
- 18.Wessjohann L.A., Ruijter E. Macrocycles rapidly produced by multiple multicomponent reactions including bifunctional building blocks (MiBs) Mol. Diversity. 2005;9:159–169. doi: 10.1007/s11030-005-1313-y. [DOI] [PubMed] [Google Scholar]
- 19.Dömling A., Ugi I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 20.Wessjohann L.A., Rivera D.G., Coll F. Steroid-Biarylether Hybrid Macrocycles with High Skeletal and Side Chain Variability by Multiple Multicomponent Macrocyclization including Bifunctional Building Blocks (MiBs) J. Org. Chem. 2006;71:7521–7526. doi: 10.1021/jo0608570. [DOI] [PubMed] [Google Scholar]
- 21.Rivera D.G., Vercillo O.E., Wessjohann L.A. Combinatorial Synthesis of Macrocycles by Multiple Multicomponent Macrocyclization including Bifunctional Building Blocks (MiB) Synlett. 2007:308–312. [Google Scholar]
- 22.Bon R.S., van Vliet B., Sprenkels N.E., Schmitz R.F., de Kanter F.J.J., Stevens C., Swart M., Bickelhaupt F.M., Groen M.B., Orru R.V.A. Multicomponent Synthesis of 2-Imidazolines. J. Org. Chem. 2005;70:3542–3553. doi: 10.1021/jo050132g. [DOI] [PubMed] [Google Scholar]
- 23.Failli A., Immer H., Götz M. The synthesis of cyclic peptides by the four component condensation (4CC) Can. J. Chem. 1979;57:3257–3261. doi: 10.1139/v79-533. [DOI] [Google Scholar]
- 24.Davis A.P., Dresen S., Lawless L.J. Mitsunobu Reaction with Methanesulfoic Acid; The Replacement of Equatorial Hydroxyl Groups by Azide with Net Retention of Configuration. Tetrahedron Lett. 1997;38:4305–4308. doi: 10.1016/S0040-4039(97)00886-1. [DOI] [Google Scholar]
- 25.Davis A.P., Wareham R.S. Carbohydrate Recognition through Noncovalent Interactions: A Challenge for Biomimetic and Supramolecular Chemistry. Angew. Chem. Int. Ed. 1999;38:2978–2996. doi: 10.1002/(SICI)1521-3773(19991018)38:20<2978::AID-ANIE2978>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 26.Batta A.K., Aggarwal S.K., Salen G., Shefer S. Selective reduction of oxo bile acids: 3β-, 7β-, and 12β-hydroxy bile acids. J. Lipid Res. 1991;32:977–983. [PubMed] [Google Scholar]
- 27. Halgren T.A. MMFF VI. MMFF94s option for energy minimization studies. J. Comput. Chem. 1999;20:720–729. doi: 10.1002/(SICI)1096-987X(199905)20:7<720::AID-JCC7>3.0.CO;2-X. Halgren T.A. MMFF VII. Characterization of MMFF94, MMFF94s, and other widely available force fields for conformational energies and for intermolecular-interaction energies and geometries. J. Comput. Chem. 1999;20:730–748. doi: 10.1002/(SICI)1096-987X(199905)20:7<730::AID-JCC8>3.0.CO;2-T.