Abstract
Synthesis of ten 3-(arylideneamino)-2-phenylquinazoline-4(3H)-ones is reported. All the compounds contained a common phenyl group at the 2-position, while the substituents on the arylideneamino group were varied. The compounds were investigated for their antimicrobial activity against both Gram-positive (Staphylococcus aureus 6571 and Bacillus subtilis) and Gram-negative bacteria (Escherichia coli K12 and Shigella dysenteriae 6) using a turbidometric assay method. It was found that the incorporation of the 3-arylideneamino substituent enhanced the anti-bacterial activity of the quinazolone system. The preliminary QSAR studies were done using some computer derived property descriptors, calculated values of partition coefficients as well as usual Hammett’s sigma constants and the substituent’s molar refractivity.
Keywords: 2-Phenylquinazoline-4(3H)-one, antibacterial activities, QSAR, quinazolones
Introduction
Quinazolines have been frequently used in medicine because of their wide spectrum of biological activities [1]. Different quinazoline derivatives have been reported for their antibacterial, antifungal, anti-HIV [2,3], anthelmintic [4], CNS depressant [5] and antitubercular [6] activities. Antitumor activities are also reported for 2,3-dihydro-2-aryl-4-quinazolinones [7,8]. Some reports have suggested that 2-styrylquinazolin-4-ones (SQZ) [9,10] could be effective inhibitors of tubulin polymerization. The 2,3-disubstituted quinazolones have been predicted to possess antiviral and antihypertensive activities [11]. Synthesis of vascinone, a naturally occurring bioactive alkaloid having a quinazolone system, has been reported very recently [12]. In this paper, we would like to report the synthesis of ten 3-(arylidene-amino)-2-phenylquinazoline-4(3H)-ones and their antibacterial activity. Hydrazine-derived Schiff bases have potential antibacterial activity [13]. Expecting an enhancement of biological activity we have placed two potential bio-active sites, a quinazolone moiety as well as a Schiff base in our systems. The antibacterial activities of similar compounds have been reported very recently [14]. On the basis of antibacterial activities of the synthesized compounds a preliminary QSAR study was also attempted for future development of better drugs of similar structures.
Results and Discussion
Synthesis of 3-(arylideneamino)-2-phenylquinazolin-4(3H)-ones 3a-3j
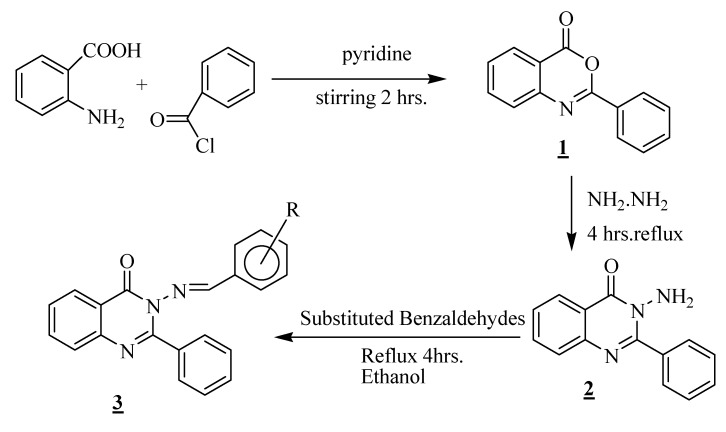
Synthesis of the ten novel 2-phenyl, 3-substituted benzalaminoquinazolinones involved three steps: benzoylation with simultaneous cyclization, addition of hydrazine and finally condensation to form a Schiff base. Thus, anthranilic acid was treated with benzoyl chloride in the presence of pyridine to undergo cyclization forming 2-phenyl-4H-benzo[d][1,3]oxazin-4-one (1), which on condensation with hydrazine hydrate yielded 3-amino-2-phenylquinazolin-4(3H)-one (2). The syntheses of the compounds 1 and 2 have been reported by earlier authors [15]. Compound 2 was then treated with different substituted benzaldehydes in the presence of ethanol to form the corresponding 3-(arylidene-amino)-2-phenylquinazolin-4(3H)-ones 3a-j. The synthetic route is summarized in Scheme 1. The synthesized compounds were identified from their elemental analyses, IR, NMR and mass spectral data. The carbon positions in 3 denoting the position/s of the substituent/s R in the 3-(arylideneamino)-group are shown in Scheme 1.
Scheme 1.
Interpretation of spectral data
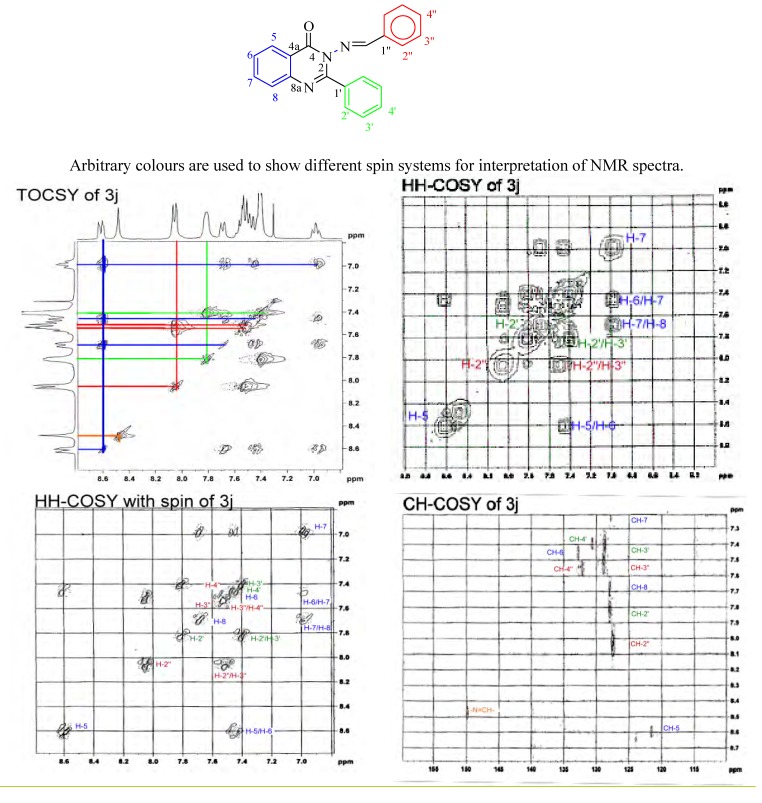
Structural elucidation of all the compounds 3a-j was accomplished by the procedures mentioned above. All these compounds have 3-(arylideneamino)-2-phenylquinazolin-4(3H)-one (3j) as the basic skeletal structure, with the other compounds of the series possessing different substitution patterns on the arylidene moiety phenyl ring. The structural assignment of 3j is illustrative. The molecular formula of the compound was established from mass and elemental analyses data. The IR data suggested the presence of the amidic bond in the characteristic region of the quinazolone system. The merging of signals from aldimine (N=C-H) proton along with all other aromatic C-H protons in a small range of δ–value (δ 6.98-8.62) made the interpretation of NMR spectra difficult. To overcome this difficulty 2D spectra were recorded. Since under the experimental condition of TOCSY the magnetization is dispersed over a complete spin system by successive scalar coupling, the protons located within a three bonds distance create a set of spin systems. Thus, for compound 3j we expected to observe three sets of correlations: the quinazolone protons marked as H5-8, the arylidene protons marked as H2”-4” (H2”-6” for other members of the series 3a-i) and the phenyl protons of the 2-phenyl group marked as H2’-4’ (Figure 1). In practice, the TOCSY spectrum showed distinct sets of four cross peaks, indicating four consecutive protons having δ values at 8.62, 7.67, 7.49 and 6.98 (Figure 1). The N=C-H peak was confirmed to be at δ 8.47. The off-diagonal peaks of other two sets of correlated protons were not clear. Since the cross peaks of COSY shows single step magnetization transfer thus inspection of the cross peaks of the protons in the set (H5-8) helped us to assign the nearest member of each proton. The sequential assignments, therefore were done as H5 (δ 8.62), H6 (δ 7.49), H7 (δ 6.98) and H8 (δ 7.67). The selection of the most deshielded proton was also important in the peak assignment. Since the proton located at the ortho-position of the amidic group was thought to be most deshielded it was assigned as H5. All other related proton peaks were then assigned with respect to the position of H5.
Figure 1.
Juxtaposed pictures of the 2D-NMR spectra of compound 3j.
As the protons at the meta and para positions of the two phenyl rings of the compound 3j showed proton shifts in a very close range of δ values, we had to record another COSY spectrum with spin. This spectrum enabled us to identify the H2” and H2’ proton peaks. When the off diagonal peaks in the three proton 2D spectra were examined together, the H2”-4” and H2’-4’ spin system was revealed. Better resolution on the 13C-axis of H3”/H4” and H3’/H4’ peaks was obtained in the C-H COSY spectrum. Thus, with the help of the correlation patterns the two sets of proton peaks, H2” (δ 8.05), H3” (δ 7.55) and H4” (δ 7.41) and H2’ (δ 7.81), H3’ (δ 7.40) and H4’ (δ 7.45) were assigned.
After proper assignment of the entire proton spectrum, the proton carbon correlation spectrum was further analyzed to assign the eleven 13C-H peaks. The quaternary carbon peaks were assigned intuitively with the help of 13C-NMR spectra. In order to validate our intuition for assigning the positions of the quaternary carbon atoms we have made a comparison of such assignments for all the compounds 3a to 3j, including 1 and 2.
Antibacterial activity
The compounds 3d, 3e and 3j showed similar antibacterial activity towards both Gram positive bacteria (Bacillus subtilis and Staphylococcus aureus 6571), while 3b and 3f were more active against B. subtilis and 3c on S. aureus 6571. The compounds 3d and 3h showed similar antibacterial activity towards both Gram negative bacteria, Escherichia coli and Shigella dysenteriae 6 while 3c, 3i and 3j were more active against S. dysenteriae 6. Other compounds exhibited moderate to weak anti-bacterial activity towards both species. The activity results are summarized in Table 1.
Table 1.
Antibacterial activity (MIC) of synthesized compounds 3a-j.
| Compound | R | M.F | Gram-Positive | Gram-Negative | ||
|---|---|---|---|---|---|---|
| B. subtilis* | S. aureus 6571* | E. coli K12* | S. dysenteriae 6* | |||
| 1 | - | C14H9NO2 | - | - | - | - |
| 2 | - | C14H11N3O | 400 | - | 400 | - |
| 3a | 2΄΄-OH | C21H15N3O2 | 300 | 200 | 200 | 300 |
| 3b | 4΄΄-OCH3 | C22H17N3O2 | 100 | 200 | 200 | 300 |
| 3c | 4΄΄-F | C21H14N3OF | 200 | 100 | 200 | 200 |
| 3d | 4΄΄-N(CH3)2 | C23H20N4O | 100 | 100 | 100 | 200 |
| 3e | 4΄΄-Cl | C21H14N3OCl | 100 | 100 | 200 | 300 |
| 3f | 3΄΄-OCH3 | C22H17N3O2 | 100 | 200 | 400 | 300 |
| 3g | 4’-OH | C21H15N3O2 | 300 | 200 | 400 | 400 |
| 3h | 3΄΄-OCH3, 4΄΄-OH |
C22H17N3O3 | 200 | 200 | 100 | 200 |
| 3i | 3΄΄-NO2 | C21H14N4O3 | 200 | 300 | 200 | 200 |
| 3j | H | C21H15N3O | 100 | 100 | 200 | 200 |
Compounds 3e, and 3i were also tested for their activity against two Multiple Antibiotic Resistant (MAR) enteric bacteria [MAR calculated on 12 antibiotics, namely amikacin, ampicillin, cefotaxim, cephalexin, chloramphenicol, gentamycin, kanamycin, netilmicin, nitrofurantoin, streptomycin, tetracycline, and tobramycin], Enterobacter sp. TR04 (resistance against 11 out of 12 antibiotics tested) and Citrobacter sp. TR06 (resistance against 08 out of 12 antibiotics tested) [16]. The MIC of 3i against TR04 and TR06 was determined to be 300 and 400 µg/mL, respectively. The MIC of 3e against TR04 and TR06 was determined to be 400 and 200 µg/mL, respectively. Antibiotic resistant pathogenic bacteria are potential threat to the disease management system, therefore compounds like 3i and 3e could be explored in details and further improvement of bactericidal property of this class of compounds is under development in our laboratory.
Preliminary QSAR study.
On the basis of the different substituents used in the arylideneamino-phenyl group, a preliminary quantitative structure activity (QSAR) study was done to optimize the influence of different functional groups in the said ring contributing to the bactericidal activity of the compounds 3a-j. The values of the chosen descriptors for each of the compounds are shown in Table 2.
Table 2.
Characterization data of 3a-j for the QSAR study.
| Compounds | 3a | 3b | 3c | 3d | 3e | 3f | 3g | 3h | 3i | 3j |
|---|---|---|---|---|---|---|---|---|---|---|
|
Log(P/C) for Gm –ve bacteria E. coli K12 |
7.9607 | 8.7209 | 8.6894 | 9.14782 | 9.4108 | 8.7204 | 7.9627 | 8.0490 | 3.7543* | 8.8084 |
|
Log(P/C) for Gm +ve bacteria B. subtilis |
8.1369 | 8.4199 | 8.6894 | 9.1478 | 9.1098 | 8.1184 | 7.8378 | 8.3500 | 3.7543* | 8.5074 |
| MR | 2.85 | 7.87 | 0.92 | 15.55 | 6.03 | 7.87 | 2.85 | 10.72 | 7.37 | 1.03 |
| σm | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 | 0.00 | 0.12 | 0.71 | 0.00 |
| σp | 0.00 | -0.27 | 0.06 | -0.83 | 0.23 | 0.00 | -0.37 | -0.37 | 0.00 | 0.00 |
| Ova | 1.5561 | 1.5970 | 1.5630 | 1.6166 | 1.5746 | 1.5937 | 1.5642 | 1.6012 | 1.5945 | 1.5506 |
| L | 2.85 | 3.98 | 2.65 | 3.53 | 3.52 | 3.98 | 2.85 | 6.83 | 3.44 | 2.06 |
| B1 | 2.74 | 1.35 | 1.35 | 1.5 | 1.8 | 1.35 | 2.74 | 4.09 | 1.7 | 1.00 |
| B4 | 1.35 | 2.87 | 1.35 | 2.8 | 1.8 | 2.87 | 1.35 | 4.22 | 2.44 | 1.00 |
Among the Verloop’s steric parameters, only the length parameter L and the breadth parameter B1 were considered, since the other breadth parameters B2, B3 and B4 were found to be highly correlated to B1. The correlation coefficients are shown in Table 3. Since the quantum chemical descriptors have tremendous applicability in QSAR studies [17] we preferred to use some computer derived property descriptors in our preliminary QSAR study, which may help to choose some quantum chemically derived descriptors in future QSAR studies. Four sets of non-correlated descriptors were chosen for regression analyses. In each of the sets hydrophobic, steric and electronic descriptors were kept in common. The regression analyses were done separately for the gram-positive and gram-negative bacteria. The results are shown in Table 4.
Table 3.
Correlation Matrix of the chosen QSAR descriptors.
| L | B1 | B2 | B3 | B4 | MR | OVA | σm | σp | |
|---|---|---|---|---|---|---|---|---|---|
| B1 | 0.119 | 1.000 | 0.171 | 0.220 | 0.024 | 0.071 | 0.116 | 0.163 | -0.203 |
| B2 | 0.881 | 0.171 | 1.000 | 0.854 | 0.854 | 0.834 | 0.815 | 0.326 | -0.355 |
| B3 | 0.738 | 0.220 | 0.854 | 1.000 | 0.805 | 0.786 | 0.861 | 0.521 | -0.355 |
| B4 | 0.929 | 0.024 | 0.854 | 0.805 | 1.000 | 0.786 | 0.768 | 0.456 | -0.304 |
| MR | 0.767 | 0.071 | 0.834 | 0.786 | 0.786 | 1.000 | 0.841 | 0.318 | -0.544 |
| OVA | 0.705 | 0.116 | 0.815 | 0.861 | 0.768 | 0.841 | 1.000 | 0.342 | -0.484 |
| σm | 0.382 | 0.163 | 0.326 | 0.521 | 0.456 | 0.318 | 0.342 | 1.000 | -0.034 |
| σp | -0.297 | -0.203 | -0.355 | -0.355 | -0.304 | -0.544 | -0.484 | -0.034 | 1.000 |
Table 4.
Regression equations.
| Equations | Eqn. Nos |
t | sig | ||
|---|---|---|---|---|---|
| Gram +ve | Log(P/C) = 8.240 + 0.397mr + 0.319 σp + (-1.06) σm N = 10 F = 52.72( sig 0.000) r = 0.853 Rsqr = 0.968 SEE = 0.377 D/W = 1.11 |
A | Intercept mr σp σm |
37.01 3.389 2.715 -12.37 |
0.000 0.015 0.035 0.000 |
| Gram -ve |
Log(P/C) = 8.131 + 0.356mr + 0.230 σp + (-1.05) σm N = 10 F = 75.46( sig 0.000) r = 0.559 Rsqr = 0.974 SEE = 0.3053 D/W = 1.762 |
B | Intercept mr σp σm |
45.09 3.620 2.333 -14.593 |
0.000 0.011 0.058 0.000 |
| Gram +ve |
Log(P/C) = -34.998 + 0.386Ova + 0.286 σp + (-1.109) σm N = 10 F = 51.72( sig 0.000) r = 0.870 Rsqr = 0.963 SEE = 0.380 D/W = 0.852 |
C | Intercept Ova σp σm |
-2.668 3.339 -11.910 2.562 |
0.037 0.016 0.000 0.043 |
| Gram -ve |
Log(P/C) = -26.253 + 0.319Ova + 0.183 σp + (-1.084) σm N = 10 F = 55.271( sig 0.000) r = 0.757 Rsqr = 0.965 SEE = 0.355 D/W = 1.818 |
D | Intercept Ova σp σm |
-2.146 2.850 1.686 -12.02 |
0.070 0.029 0.000 0.113 |
| Gram +ve |
Log(P/C) = 8.456 + 0.599L + (-0.345)B1 + 0.062 σp + (-1.003) σm N = 10 F = 41( sig 0.000) r = 0.62 Rsqr = 0.97 SEE = 0.369 D/W = 1.044 |
E | Intercept L B1 σp σm |
23.30 3.269 3.339 0.752 -12.6 |
0.000 0.022 0.021 0.486 0.150 |
| Gram -ve |
Log(P/C) = 8.061 + 0.304L + (-0.209)B1 + 0.019σp + (-1.002) σm N = 10 F = 34.287( sig 0.001) r = 0.762 Rsqr = 0.965 SEE = 0.390 D/W = 1.727 |
F | Intercept L B1 σp σm |
21.01 2.576 -1.844 0.214 -11.465 |
0.000 0.050 0.124 0.839 0.693 |
In equations A, B, E and F the right hand side descriptor values were derived for the substituents and the left hand side denoted the property of the whole molecule. In equations C and D the right hand side contained the ovality descriptor - a steric property of the whole molecule. The term ovality refers to the ratio of the Molecular Surface Area to the Minimum Surface Area. The significance of the ovality refers to a sphere having Minimum Surface Area equal to the Solvent-Excluded Volume of the molecule. Thus this shape descriptor illustrates the steric property of the molecule in which the contact surface as well as the volume within the contact surface is considered. The set solvent here is water.
The high positive value of the intercept added to low coefficients of the dependant variables in equations A, B, E and F suggested that hydrophobicity was the major constraint in achieving very low MIC value of the substrates. This observation did not correspond to what is reported for other Schiff bases [18] wherein the authors found a positive correlation between hydrophobicity and antibacterial activity. This difference in activity in relation to hydrophobicity in quinazolone derived Schiff base(s) and the earlier reported Schiff base(s) could be due to the differences in active sites.
Table 1.
Table 5a.
Compound-wise diagnostics values of regression analysis for Gram Negative bacteria.
| Equation Nos. | A | C | E | ||||
| 3a | 7.9607 | 8.6350 | -0.67432 | 8.4214 | -0.46072 | 8.0947 | -0.13400 |
| 3b | 8.7209 | 8.8798 | -0.15895 | 9.1579 | -0.43709 | 9.3161 | -0.59528 |
| 3c | 8.6894 | 8.4678 | 0.221566 | 8.7047 | -1.53E-02 | 8.8419 | -0.15254 |
| 3d | 9.1478 | 9.0093 | 0.138470 | 8.8645 | 0.283317 | 8.8503 | 0.29751 |
| 3e | 9.4108 | 9.4590 | -4.8E-02 | 9.2819 | 0.128812 | 9.0131 | 0.39762 |
| 3f | 8.7204 | 8.4049 | 0.315474 | 8.5034 | 0.216909 | 8.5288 | 0.19152 |
| 3g | 7.9627 | 8.0178 | -5.5E-02 | 8.0919 | -0.12929 | 7.9749 | -1.2E-02 |
| 3h | 8.0490 | 8.1824 | -0.13346 | 8.1587 | -0.10971 | 8.0473 | 1.664E-03 |
| 3i | 3.7543 | 3.7850 | -3.07E-02 | 3.7724 | -1.81E-02 | 3.7869 | -3.26E-02 |
| 3j | 8.8084 | 8.3829 | 0.42540 | 8.2671 | 0.54127 | 8.7700 | 3.83E-02 |
Table 5b.
Compound-wise diagnostics values of regression analysis for Gram Positive bacteria.
| Equation Nos. | B | D | F | ||||
|---|---|---|---|---|---|---|---|
|
Compound No. |
Observed value of log(P/C) |
Predicted value of log(P/C) |
Residual |
Predicted value of log(P/C) |
Residual |
Predicted value of log(P/C) |
Residual |
| 3a | 8.1369 | 8.4728 | -0.3359 | 8.3139 | -0.1771 | 8.1600 | -2.31E-02 |
| 3b | 8.4199 | 8.7608 | -0.3410 | 8.9742 | -0.5543 | 9.0213 | -0.601454 |
| 3c | 8.6894 | 8.3112 | 0.3781 | 8.5231 | 0.1662 | 8.5692 | 0.120200 |
| 3d | 9.1478 | 9.0311 | 0.1166 | 8.8937 | 0.2541 | 8.7516 | 0.396140 |
| 3e | 9.1098 | 9.1205 | -1.067E-02 | 8.9361 | 0.1736 | 8.7483 | 0.361502 |
| 3f | 8.1184 | 8.1903 | -7.194E-02 | 8.2388 | -0.1205 | 8.2062 | -8.78E-02 |
| 3g | 7.8378 | 8.0436 | -0.2058 | 8.1529 | -0.3151 | 8.1240 | -0.286304 |
| 3h | 8.3501 | 8.1024 | 0.2476 | 8.0655 | 0.2844 | 8.2693 | 8.077E-02 |
| 3i | 3.7543 | 3.7840 | -2.968E-02 | 3.7820 | -2.77E-02 | 3.7531 | 1.197E-03 |
| 3j | 8.5075 | 8.2548 | 0.2526 | 8.1911 | 0.31631 | 8.4684 | 3.898E-02 |
It is clear from the nature of descriptors and the associated coefficients that the substituents at arylideneamino group influence more the binding site than the site of action. The parent compound 2, not being a Schiff base, also has demonstrated antibacterial activity. Moreover, the high constant values found in all the regression equations suggested that the action site might reside in the quinazolone moiety and not in the imine moiety. However the substituents were predicted to have a definite role in binding to the drug receptor site and the electron withdrawing groups in the meta-position of the phenyl group resulted in an increment of activity. Moreover, it was noted that all the compounds, 3a-j, showed increased activity with respect to that of the parent compound 2. It was therefore concluded that the introduction of 3-(arylideneamino) chain in the quinazolone moiety increased the anti-bacterial activity and some degree of selectivity was also attained. The standardized coefficients were used to derive the equations. All the equations are more or less good fit as apparent from the regression data. The comparisons of observed and predicted values are shown in separate tables (Table 5a and Table 5b). Extension of the work is in progress.
Conclusions
The biological activity of the quinazolone system 2 was enhanced with the introduction of the Schiff base moiety; in addition some selectivity of antibacterial effect on Gram-Positive and Gram-Negative type of bacteria was also noted. The promising effect of such compounds against Multiple Antibiotic Resistant Gram-negative enteric bacteria could lead to the development of new drugs. However, at present the low water solubility of the compounds is the major impediment to achieving lower MIC values.
Experimental
General
NMR spectra were recorded in CDCl3 (unless noted otherwise) on a Bruker Avance 300 MHz FT-NMR spectrometer with a 5 mm BBO probe using tetramethylsilane (TMS) as internal standard. Mass spectra were recorded on a JEOL SX 102/DA-6000 instrument using argon as FAB gas. The acceleration voltage was 10 kV and the spectra were recorded at room temperature; m-nitrobenzyl alcohol was used as the matrix. The IR spectra were recorded on a Shimadzu FT-IR spectrometer in Mujol mulls mounted in potassium bromide discs. The TLC plates used were hand drawn plates with 0.1 mm coating of silicagel G. Reported Rf values correspond to elution with 3:1 benzene-ethyl acetate. The melting points are uncorrected.
General method for the preparation of 3a-j
A mixture of 2 (0.01 mole), the appropriate aromatic aldehyde (0.01 mole) and ethanol (20 mL) was refluxed for 4-6 hours. The resulting mixture was cooled and poured into ice water. The separated solid was filtered, washed with water and recrystallized from ethanol.
3-{[(2-Hydroxyphenyl)methylene]amino}-2-phenylquinazolin-4(3H)-one (3a). Yield 76%; mp 144 oC; Rf 0.77; IR (cm-1): 3200-3100, 1681.8, 1604.7, 1467.7; FAB MS (m/z): 342 (M+1); 1H-NMR: 6.69 (H-5”), 6.9 (H-3”), 7.35 (H-4”), 7.35 (H-6”), 7.4 (H-4’), 7.48 ( H-3’), 7.56 (H-6), 7.6 (H-8), 7.6 (H-7), 7.81 (H-2’), 8.36 (H-5), 9.19 (s, 1H, H-C=N), 9.99 (OH, H-bonded), 7.8 Hz (J56); 13C-NMR: 116.42 (C-1”), 117.50 (C-3’’), 119.69 (C-5”), 121.46 (C-4a), 127.32 (C-8), 127.39 (C-6), 128.00 (C-2’), 128.97 (C-5), 130.34 (C-3’), 132.5 (C-6”), 133.33 (C-4’), 134.19 (C-4”), 134.84 (C-7), 146.37 (C-8a), 153.63 (C-2), 159.14 (C-4), 159.74 ( =C(OH)-, C-2”), 164.75 (H-C=N), C-1’ quaternary peak not observed; Anal. Calcd. for C21H15N3O2: C, 73.89%; H, 4.45%; N, 12.31%; found: C, 74.10%; H, 4.45%; N, 12.28%.
3-{[(4-Methoxyphenyl)methylene]amino}-2-phenylquinazolin-4(3H)-one (3b). Yield 75%; m.p. 140°C; Rf 0.75; IR (cm-1): 1679.9, 1602.7, 1448.4, 1494.7, 1257.5, 1170.7; FAB MS (m/z): 356(M+1); 1H-NMR: 3.84 (s, 3H, methoxy protons), 7.37 (H-3’/ H-4’), 7.40 (H-3”), 7.46 (H-8), 7.53 (H-6), 7.78 (H-2”), 7.80 (H-7), 7.84 (H-2’), 8.36 (H-5), 8.87 (1H, s, H-C=N-N); 13C-NMR: 55.40 (-O-CH3), 114.34 (C-3”), 121.48 (C-4a), 125.89 (C-1”), 126.90 (C-8), 127.26 (C-2’), 127.70 (C-6), 127.88 (C-5), 129.89 (C-2”), 129.90 (C-4’), 130.75 (C-3’), 134.39 (C-7), 134.67 (C-1’), 146.50 (C-8a), 159.40 (C-4), 163.08 (=C(OCH3)-,C-4”), 164.0 (C-2), 166.55 (H-C=N); Anal. Calcd. for C22H17N3O2: C, 74.35%, H, 4.82%, N, 11.82%; found: C, 74.40%, H, 4.90%, N, 11.78%.
3-{[(4-Fluorophenyl)methylene]amino}-2-phenylquinazolin-4(3H)-one (3c). Yield 68%; m.p. 166°C; Rf 0.73; IR (cm-1): 1674.1, 1614.3, 1593.1, 1554.5, 1537.2, 1469.7, 1373.2, 1184.2; FAB MS (m/z): 344(M+1); 1H-NMR: 7.1 (H-4”), 7.41 (H-3’), 7.45 (H-4’), 7.49 (H-6), 7.53 (H-8), 7.54 (H-2”), 7.7 (H-2’), 7.8 (H-7), 8.3 (H-5), 9.04 (s, 1H, H-C=N); 7.8 Hz (J56), 0.9 Hz (J57), 0Hz (J58), 6.3 Hz (J67), 1.8 Hz (J68), 8.7 Hz (J2”3”); 13C-NMR: 166.30 (=C(F)-, C-4”), 164.84 (H-C=N), 153.97 (C-4), 153.90 (C-2), 146.60 (C-8a), 134.55 (C-1’), 134.15 (C-7), 131.00 (C-2”), 130.89 (C-4’), 130.50 (C-1”), 129.30 (C-2’), 128.98 (C-3’), 127.68 (C-6), 127.20 (C-5), 126.79 (C-8), 121.50 (C-4a), 116.33 (C-3”); Anal. Calcd. for C21H14N3OF: C, 73.46%, H, 4.11%, N, 12.24%; found: C, 73.50%, H, 4.10%, N, 12.18%.
3-{[4-Dimethylaminophenyl)methylene]amino}-2-phenylquinazolin-4(3H)-one (3d). Yield 70%; m.p. 178°C; Rf 0.8; IR (cm-1): 1681.8, 1589.2, 1556.4, 1508.2, 1456.2, 1375.2, 1328.9, 1313.4; FAB MS (m/z): 344(M+1); 1H-NMR: 3.04 (-N-CH3), 6.66 (H-3”), 7.40 (H-3’ and H-4’), 7.58 (H-6 and H-2”), 7.79 (H-8 and H-2’), 7.80 (H-7), 8.36 (H-5), 8.67 (s, 1H. H-C=N); 8.7 Hz (J56), 0.9 Hz (J57), 0 Hz (J58), 7.5 Hz (J67), 9.0 Hz (J2”3”); 13C-NMR: 187.65 (H-C=N), 159.80 (C-4), 154.07 (C-2), 153.06 (=C(N(CH3)2), C-4”), 146.66 (C-8a), 134.80 (C-1’), 134.15 (C-7), 130.72 (C-2”), 129.70 (C-4’), 129.30 (C-2’), 127.89 (C-3’), 127.68 (C-3), 127.20 (C-5), 126.79 (C-8), 121.58 (C-1”), 120.30 (C-4a), 111.52 (C-3”), 40.13 (-N-CH3); Anal. Calcd. for C23H20N4O: C, 74.98%, H, 5.47%, N, 15.2%; found: C, 74.99%, H, 5.50%, N, 15.13%.
3-{[4-Chloroyphenyl)methylene]amino}-2-phenylquinazolin-4(3H)-one (3e). Yield 72%; m.p. 162°C; Rf 0.81; IR (cm-1): 1679.9, 1591.2, 1554.5, 1377.1; FAB MS (m/z): 360(M+1); 1H-NMR: 7.37 (H-3’), 7.42 (H-4’), 7.49 (H-6), 7.53 (H-8), 7.54 (H-3”), 7.68 (H-2”), 7.80 (H-7), 7.82 (H-2’), 8.36 (H-5), 9.10 (s, 1H, H-C=N); 13C-NMR: 121.47 (C-4a), 127.16 (C-8), 127.33 (C-2’), 127.88 (C-6), 127.93 (C-5), 129.24 (C-3’), 129.77 (C-3”), 129.92 (C-4’), 130.00 (C-2”), 131.36 (C-1”), 134.36 (C-1”), 134.78 (C-7), 138.46 (=C(Cl)-, C-4”), 146.47 (C-8a), 154.00 (C-2), 159.22 (C-4), 164.40 (H-C=N); Anal. Calcd. for C21H14N3OCl: C, 70.10%; H, 3.92%; N, 11.68%; found: C, 70.20%; H, 4.10%; N, 11.62%.
3-{[3-Methoxyphenyl)methylene]amino}-2-phenylquinazolin-4(3H)-one (3f). Yield 75%; m.p. 134°C; Rf 0.67; IR (cm-1): 1679.9, 1575.7, 1465.8, 1367.4, 1317.3, 1276.8; FAB MS (m/z): 356(M+1);1H-NMR: 3.74 (s, 3H, methoxy protons), 7.30 (H-4”), 7.40 (H-2”), 7.43 (H-3’), 7.45 (H-4’), 7.55 ( H-5”/ H-6”), 7.62 (H-6), 7.69 (H-8), 7.72 (H-7), 7.82 (H-2’), 8.37 (H-5), 9.09 (s, 1H, H-C=N-N); 13C-NMR: 111.74 ( C-2”), 119.15 ( C-4”), 121.56 (C-4a), 121.76 ( C-6”), 122.33 (C-8), 127.08 (C-6), 127.31 (C-5), 127.87 (C-2’), 128.17 ( C-5”), 128.18 (C-3’), 129.84 (C-4’), 134.22 (C-1’), 134.51 (C-7), 134.88 (C-1”), 146.51 (C-8a), 154.13 (C-2), 159.27 (C-4), 159.86 (=C(OCH3), C-3”), 165.52 ( H-C=N); Anal. Calcd. for C22H17N3O2: C, 74.35%; H, 4.28%; N, 11.82%; found: C, 74.45%; H, 4.35%; N, 11.78%.
3-{[(4-Hydroxyphenyl)methylene]amino}-2-phenylquinazolin-4(3H)-one (3g). Yield 76%; m.p. 167°C; Rf 0.63; IR (cm-1): 3307.7, 3213.2, 1668.2, 1645.2, 1604.7, 1554.5, 1375.2, 1338.5; FAB MS ( m/z ): 342( M+1 ); 1H-NMR: 5.03 (-OH), 7.42 (H-3”/H-5”), 7.50 (H-3’/H-4’), 7.52 (H-6), 7.68 (H-2”/H-6”), 7.78 (H-7), 7.80 (H-8), 7.82 (H-2’), 8.29 ( m, H-5 ), 9.16 (s, 1H, H-C=N-N ); 13C-NMR: 110.00 (C-3”), 120.10 (C-4a), 126.61 ( C-8), 127.08 (C-6), 127.79 (C-5), 128.20 (C-2’), 129.28 (C-3’), 130.29 (C-4’), 133.94 (C-2”), 134.50 (C-7), 134.52 (C-1”), 143.12 (C-8a), 149.00 (C-2), 149.88 (-CH=N-), 155.00 (C-4), 161.54 (=C(OH)-, C-4”), C-1’ quaternary peak not observed; Anal. Calcd. for C21H15N3O2: C, 73.89%; H, 4.43%; N, 12.31%; found: C, 73.99%; H, 4.49%; N, 12.30%.
3-{[(4-Hydroxy-3-methoxyphenyl)methylene]amino}-2-phenylquinazolin-4(3H)-one (3h). Yield 74%; m.p. 155°C; Rf 0.62; IR (cm-1): 3305.8, 3215.1, 1749.3, 1712.7, 1664.5, 1575.7, 1467.7, 1377.1; FAB MS ( m/z) : 372 ( M+1 ); 1H-NMR: 3.81 (s, 3H, methoxy protons), 5.03 (-OH), 6.92 (H-5”), 7.20 (H-2”), 7.32 (H-6”), 7.50 (H-3’/H-4’), 7.52 (H-6), 7.76 (H-7), 7.78 (H-8), 7.83 (H-2’), 8.3 (m, H-5), 8.90 (s,1H, H-C=N-N); 13C-NMR: 55.95 (-O-CH3), 108.67 (C-2”), 114.46 (C-5”), 125.40 (C-6”), 126.40 (C-4a), 126.63 (C-8), 127.08 (C-1”), 127.27 (C-2’), 127.77 (C-6), 128.20 (C-5), 129.31 (C-1’), 129.93 (C-3’), 130.32 (C-4’), 134.50 (C-7), 143.23 (C-8a), 146.72 (=C(OH)-, C-4”), 147.28 (=C(OCH3)-, C-3”), 154.40 (C-4), 159.82 (-CH=N-); Anal. Calcd. for C22H17N3O3: C, 71.15%; H, 4.61%; N, 11.31%; found: C, 71.25%; H, 4.65%; N, 11.26%.
3-{[(3-Nitrophenyl)methylene]amino}-2-phenylquinazolin-4(3H)-one (3i). Yield 78%; m.p. 248°C; Rf 0.53; IR (cm-1): 1674.1, 1641.3, 1500, 1456.2, 1344.3; FAB MS ( m/z ): 371(M+1); 1H-NMR (CDCl3 + DMSO-d6): 7.22 (H-7), 7.30 (H-3’), 7.62 (H-6), 7.63 (H-4’/ H-4”), 7.9 (H-8), 8.02 (H-2’), 8.26 ( H-5”/ H-6”), 8.54 (H-5), 9.58 (H-2”), 8.59 (s, 1H, H-C=N); 13C-NMR (CDCl3 + DMSO-d6): 118.29 (C-4a), 120.65 (C-4”), 122.30 (C-8), 122.33 (C-2”), 126.90 (C-5”), 127.27 (C-5), 127.97 (C-6”), 128.17 (C-2’), 128.28 (C-6), 130.00 (C-7), 131.50 (C-3’), 132.40 (C-4’), 133.47 (C-1”), 134.09 (=C(NO3)-, C-3”), 139.82 (C-8a), 161.65 (-CH=N-), 164.88 (C-2), 165.50 (C-4), C-1’ quaternary peak not observed; Anal. Calcd. for C21H14N4O3: C, 68.10%; H, 3.84%; N, 15.15%; found: C, 68.15%; H, 3.81%; N, 15.13%.
3-{[(Pheny)lmethylene]amino}-2-phenylquinazolin-4(3H)-one (3j). Yield 80%; m.p. 196°C; Rf 0.68; IR (cm-1): 1662.52, 1647.1, 1645.2, 1556.4, 1454.2; FAB MS (m/z): 326(M+1); 1H-NMR (CDCl3 + DMSO-d6): 6.98 (H-7), 7.40 (H-3’), 7.41 (H-4’), 7.45 (H-6), 7.49 (H-3”), 7.55 (H-4”), 7.67 (H-8), 7.81 (H-2’), 8.05 (H-2”), 8.47 (s, 1H, H-C=N), 8.62 (H-5); 13C-NMR (CDCl3 + DMSO-d6): 119.83 (C-4a), 121.62 (C-5), 122.82 (C-2”), 127.47 (C-2’), 127.72 (C-6), 127.84 (C-8), 128.66 (C-3’), 128.82 (C-3”), 130.62 (C-4”), 132.10 (C-4’), 132.72 (C-7), 133.65 (C-1’), 134.31 (C-1”), 139.79 (C-8a), 149.98 (-CH=N-), 165.77 (C-4), 166.03 (C-2); Anal. Calcd. for C21H15N3O: C, 77.52%; H, 4.65%; N, 12.91%; found: C, 77.54%; H, 4.71%; N, 12.85%.
Antimicrobial activity
Minimum Inhibitory Concentration (MIC) values for all the synthesized compounds against two Gram-positive bacteria (S. aureus 6571 and B. subtilis) and two Gram-negative bacteria (E. coli K12 and S. dysenteriae 6) were obtained by a turbidometric method. The test compound was dissolved in DMF to prepare the stock solution and aseptically filtered through bacterial membrane. The required volume of filtrate was transferred to tubes containing a defined volume of nutrient broth to achieve a desired concentration of the compound. The concentrations of the tested compound were 800, 700, 600, 500, 400, 300, 200, 100, 50, and 25 μg/mL, in comparison with the standard drug ampicillin. The tubes containing nutrient broth were inoculated with 12 hrs old liquid culture (0.1 mL) in duplicate. The tubes were incubated at 370C for 18-24 hrs and the relative growths in the tubes were determined turbidometrically in a photoelectric colorimeter. The OD values, recorded at 530 nm were plotted against concentrations of the test compounds to get the standard curve and the MIC values of the compounds against the test organism were determined.
QSAR calculations
The QSAR study was done on the basis of Principal Component Analysis (PCA) and the best fit regression equations were derived with the use of SPSS-program package for statistical analysis. The descriptors chosen are logP where P is the partition coefficient in n-octanol/water system, MR (molar refractivity contribution of the substituents), Hammett’s sigma constants, Verloop’s steric parameters and ovality (Ova). For the QSAR calculations only the minimum inhibition concentration (MIC) values obtained form E. coli K12 as Gram negative bacteria B. subtilis as Gram positive bacteria were considered and the molar concentrations were used in the calculations. The ‘ovality’ values were generated using the software Chemoffice Ultra 2005 (CambridgeSoft) with the use of MOPAC and PM-3 methods of calculation. The logP values reflect the overall lipophilicity of a molecule, a property of major importance in Biochemical applications, and these values were also generated using the same software by fragmentation method using the command ‘best estimate’. The values of Hammett’s sigma parameters, the Verloop’s constants and molar refractivity were obtained from the standard tables [19,20].
Acknowledgments
The authors wish to thank RSIC, CDRI, Lucknow for recording the mass spectral data
Footnotes
Sample Availability: Samples of compounds 1, 2 and 3a-j are available from the authors.
References and Notes
- 1.Spirkova K., Stankovsky S., Mrvova A., Cipak L. Synthesis and Biological Activity of Some 2-substituted Quinazolin-4-ones. Chem. Pap. 1999;53:272–275. [Google Scholar]
- 2.Alagarsamy V., Giridhar R., Yadav H. R., Revathi R., Rukmani K., De Clercq E. Anti HIV, antibacterial and antifungal activities of some novel1,4-disubstituted-1,2,4-triazolo[4,3a] quinazolin-5(4h)-ones. Indian J. Pharm. Sci. 2006;68:532–535. doi: 10.4103/0250-474X.27840. [DOI] [Google Scholar]
- 3.Srivastava V. K., Singh A., Gucati A., Shankar K. Antiparkinsonian agent from qunazonyl thioazolidinones and azetidinones. Indian J. Chem. 1987;26:652–656. [Google Scholar]
- 4.Gupta D. P., Ahmed S., Kumar A., Shankar K. Newer Quinazolinone Derivatives as Anthelmintic Agents. Indian J. Chem. 1988;27:1060–1062. [Google Scholar]
- 5.Jatav V., Mishra P., Kaswa S., Stabes J. P. Synthesis and CNS depressant activity of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur. J. Med. Chem. 2007 doi: 10.1016/j.ejmech.2007.02.004. ? [DOI] [PubMed] [Google Scholar]
- 6.Joshi V., Chaurasia R. P. Synthesis of Some New 4-Quinazolinone-2-carboxylhydrazides and their Tosyl Derivatives Having Potential Biological Activity. Indian J. Chem. 1987;26:602–604. [Google Scholar]
- 7.Yale H. J., Kalkstein M. Substituted 2,3-dihydro-4(1H)-quinazolinones, a new class of inhibitors of cell multiplication. J. Med. Chem. 1967;10:334–336. doi: 10.1021/jm00315a010. [DOI] [PubMed] [Google Scholar]
- 8.Neil G. L., Li L. H., Buskirk H. H., Moxley T. E. Antitumor effects of the antispermatogenicagent, 2,3-dihydro-2-(1-naphthyl)-4(1H)-quinazolinone (NSC-145669) Cancer Chemother. Rep. 1972;56:163–173. [PubMed] [Google Scholar]
- 9.Jiang J. B, Hesson D. P., Dusak B. A., Dexter D. L., Kang G. J., Harmel E. Synthesis and Biological Evaluation of 2-Styrylquinazolin-4(3H)-ones, a New Class of Antimitotic Anticancer Agents which Inhibit Tubulin Polymerization. J. Med. Chem. 1990;33:1721–1728. doi: 10.1021/jm00168a029. [DOI] [PubMed] [Google Scholar]
- 10.Lin C. M., Kang G. J., Roach M. C., Jiang J. B., Hesson D. P., Luduena R. F., Hamel E. Investigation of the mechanism of the interaction of tubulin with derivatives of 2-styryl-quinazolin-4(3H)-one. Mol. Pharmacol. 1991;40:827–832. [PubMed] [Google Scholar]
- 11.Pandey V. K., Tusi S., Tusi Z., Raghubir R., Dixit M., Joshi M. N. Heterocyclic Compounds Thiazolyl quinazolones as Potential Antiviral and Antihypertensive Agents. Indian J. Chem. 2004;43:180–184. [Google Scholar]
- 12.Kamal A., Devaiah V., Sankaraiah N., Reddy K. L. A Polymer-assisted Solution-Phase Strategy for the Synthesis of Fused [2,1-b] quinazolones and the Preparation of Optically Active Vasicinone. Synlett. 2006:2609–2612. [Google Scholar]
- 13.Chohan Z. H., Supuran C.T., Scozzafava A, Farroq M.A. Antibacterial Schiff bases of oxalyl-hydrazine/diamide incorporating pyrrolyl and salicylyl moieties and their zinc(II) complexes. J. Enzym. Inhib. Med, Chem. 2002;17:1–7. doi: 10.1080/14756360290005598. [DOI] [PubMed] [Google Scholar]
- 14.Patel J. A., Mistry B. D., Desai K. R. Synthesis and Antibacterial Activity of Newer quinazolones. Molecules. 2006;3:97–102. [Google Scholar]
- 15.Alagarsamy V., Salomon V. R., Vanikavitha G., Paluchamy V., Chandran M. R., Sajin A. A., Thangathiruppathy A., Amuthalakshmi S., Revathi R. Synthesis, Analgesic, Anti-inflamatory and Antibacterial Activities of Some Novel 2-phenyl-3-substituted Quinazolin-4(3H)ones. Biol. Pharm. Bull. 2002;25:1432–1435. doi: 10.1248/bpb.25.1432. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee S., Chakraborty R. Incidence of class-1 integrons in multiple antibiotic-resistant Gram-negative copiotrophic bacteria from the River Torsa in India. Res. Microbiol. 2006;157:220–226. doi: 10.1016/j.resmic.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Karleson M., Lobanov V. S., Katrizky A. R. Quantum Chemical Descriptors in QSAR/QSPR studies. Chem. Rev. 1996;96:1027–1043. doi: 10.1021/cr950202r. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Li, Xiafiu Y., Cia L.-H., Hu Y.-J., Yin J., Hu P.-Z. Inhibitory Study of Some Novel Schiff base derivatives on Staphylococcus aureus by microcalorimetry. Thermochim. Acta. 2006;440:51–56. doi: 10.1016/j.tca.2005.10.012. [DOI] [Google Scholar]
- 19.Hantzsh C., Lee A. Electronic Parameters: Substituent Constants for Correlation Analysis in Chemistry and Biology. Jonh Wiley & Sons; New York: 1979. pp. 1–7. Chapter 1. [Google Scholar]
- 20.Verloop A., Hoogenstraaten W., Tipker J. In: Development and application of New Steric Substituent Parameters in Drug Design: Drug Design, Vol-VII. Ariens E. J., editor. Academic Press; New York: 1976. pp. 165–207. Chapter 4. [Google Scholar]