Abstract
Introduction
The objective of this study is to evaluate the survival and glucose-induced insulin secretion of rat-derived insulinoma cells (INS-1) from their aggregates incorporating different size of gelatin hydrogel microspheres comparing with microspheres-free cell aggregates.
Methods
The gelatin hydrogel microspheres were prepared by the conventional w/o emulsion method. The INS-1 cells were cultured in a V-bottomed well, combining with or without the gelatin hydrogel microspheres to form their aggregates with or without microspheres.
Results
When the cell viability, the live cell number, the reductase activity, and the insulin secretion of cell aggregates were evaluated 7 or 14 days after incubation, the cell aggregates incorporating gelatin hydrogel microspheres showed higher cell viability, reductase activity and a larger number of live cells. The cell aggregates incorporating larger size and number of gelatin hydrogel microspheres secreted a larger amount of insulin, compared with those incorporating smaller size and number of microspheres or without microspheres.
Conclusion
It is conceivable that the incorporation of gelatin hydrogel microspheres in cell aggregates is promising to improve their survival and insulin secretion function.
Keywords: Insulin secreting cells, Cell aggregates, Gelatin hydrogel microspheres, Glucose-induced insulin secretion
Abbreviations: INS-1, insulinoma; MSC, mesenchymal stem cell
Highlights
-
•
INS-1 cell aggregates incorporating gelatin hydrogel microspheres are prepared.
-
•
Gelatin hydrogel microspheres incorporation improves cell viability and glucose-induced insulin secretion of cell aggregates.
-
•
The size and number of gelatin hydrogel microspheres affected the cell condition and function.
1. Introduction
Islet transplantation has been investigated as a treatment of type 1 diabetes for patients with insufficient glucose control [1], [2], [3]. However, a big problem of islet transplantation therapy is the serious donor shortage [4], [5], [6]. To circumvent this issue, it has been reported to reconstitute islet-like aggregates of insulin secreting cells [7], [8]. However, for this approach, when the cell aggregates become larger than 200 μm in diameter, the cells in the center of cell aggregates tend to die because of a lack of oxygen and nutrients supply [9], [10]. It is well known that insulin secreting cells show a decreased function of insulin secretion under a hypoxic environment [11], [12]. Therefore, to achieve sufficient therapeutic effect with the insulin secreting cell aggregates, it is necessary to develop a method for the promotion of oxygen and nutrients supply.
Previous studies demonstrated that the incorporation of gelatin hydrogel microspheres in mesenchymal stem cells (MSC) aggregates enabled the cells to improve the viability, proliferation and osteogenic differentiation. This is because the microspheres improved the state of oxygen and nutrients supply for cells [13], [14]. In this study, the gelatin hydrogel microspheres technology was introduced to insulin secreting cell aggregates to assess the cell viability and insulin secretion function comparing with microspheres-free cell aggregates. Gelatin hydrogel microspheres with different sizes were prepared by the conventional w/o emulsion method previously reported [15]. Rat insulinoma cells (INS-1), the model of insulin secreting cells, were incubated with or without the gelatin hydrogel microspheres in a V-bottomed well to form the cell aggregates with or without the microspheres. We examined the effect of microspheres size and number on the cell viability, reductase activity, and insulin secretion ability in the aggregates.
2. Materials and methods
2.1. Preparation of gelatin hydrogel microspheres
Gelatin hydrogel microspheres were prepared by the chemical cross-linking of gelatin in a water-in-oil emulsion state according to the method previously reported [15]. Briefly, an aqueous solution (20 ml) of 10 wt% gelatin (isoionic point 5.0 (pI 5), weight-averaged molecular weight = 1,00,000, Nitta Gelatin Inc., Osaka, Japan) was preheated at 40 °C, and then added dropwise into 600 ml of olive oil (Wako Pure Chemical Industries Ltd., Osaka, Japan) at 40 °C, followed by stirring at 200 rpm for 10 min to prepare a water-in-oil emulsion. The emulsion temperature was decreased to 4 °C for the natural gelation of gelatin solution to obtain non-crosslinked microspheres. The resulting microspheres were washed three times with cold acetone in combination with centrifugation (5000 rpm., 4 °C, 5 min) to completely exclude the residual oil. Then, they were fractionated by size using sieves with apertures of 20, 32, and 53 μm (Iida Seisakusyo Ltd., Osaka, Japan) and air dried at 4 °C. The non-crosslinked and dried gelatin microspheres (200 mg) were treated in a vacuum oven at 140 °C and 0.1 Torr for 48 h for the dehydrothermal crosslinking of gelatin. Pictures of gelatin hydrogel microspheres in a dispersed state in RPMI medium 1640 containing l-glutamine (Invitrogen Ltd., Carlsbad, CA), were taken with a light microscope (BZ-X710, KEYENCE Corp., Osaka, Japan). The size of 100 microspheres for each sample was measured using the computer program of microscope (BZ-X710) to calculate the average diameter.
2.2. Preparation of INS-1 cell aggregates with or without gelatin hydrogel microspheres
A cell line 832/13, derived from INS-1 rat insulinoma cells, was obtained from Dr. Christopher B. Newgard (Duke University Medical Center, Durham, NC) [16]. Cells were grown in RPMI medium 1640 containing l-glutamine (Invitrogen Ltd.), 1 mM sodium pyruvate (Invitrogen Ltd.), 10 mM HEPES (Invitrogen Ltd.), 10 vol% heat-inactivated fetal bovine serum (Thermo Fisher Scientific Inc., Waltham, MA), 55 μM 2-mercaptoethanol (Invitrogen Ltd.), 100 IU/ml penicillin (Gibco, Grand Island, NY), and 100 μg/ml streptomycin (Gibco). Cells were cultured in a humidified atmosphere containing 5% CO2/95% air at 37 °C.
Gelatin hydrogel microspheres and INS-1 cells were separately suspended in the culture medium under different conditions (Table 1). Gelatin microsphere suspensions (100 μl) were added to each well of a 96-well culture plate with V-bottomed wells, followed by 50 μl of INS-1 cell suspensions at the initial density of 1.0 × 103 or 1.0 × 104 cells/well and 1.0 × 101, 0.5 × 102, 1.0 × 102 or 1.0 × 103 microspheres/well. The cells/microspheres number ratio is 10/1, 100/1, or 200/1. Pictures of INS-1 cell aggregates with or without gelatin hydrogel microspheres were taken with the microscope as described above.
Table 1.
Preparation conditions of INS-1 cells aggregate with or without gelatin microspheres incorporation
| Code | Number of INS-1 cells (cells/well) | Number of gelatin hydrogel microspheres (/well) | Size of gelatin hydrogel microspheres (mm) |
|---|---|---|---|
| a | 1.0 × 103 | 0 | --- |
| b | 1.0 × 103 | 1.0 × 101 | 46.0 ± 14.0 |
| c | 1.0 × 103 | 1.0 × 101 | 82.0 ± 18.0 |
| d | 1.0 × 104 | 0 | --- |
| e | 1.0 × 104 | 1.0 × 102 | 22.0 ± 9.0 |
| f | 1.0 × 104 | 1.0 × 103 | 22.0 ± 9.0 |
| g | 1.0 × 104 | 1.0 × 102 | 46.0 ± 14.0 |
| h | 1.0 × 104 | 0.5 × 102 | 82.0 ± 18.0 |
| i | 1.0 × 104 | 1.0 × 102 | 82.0 ± 18.0 |
2.3. Evaluation of cell viability, reductase activity, and live cell number
The number of live cells in INS-1 cell aggregates with or without gelatin hydrogel microspheres was determined by trypan-blue staining. After 7 or 14 days incubation, cell aggregates were collected into a 1.5 ml microtube and washed by 100 μl of phosphate-buffered saline solution (PBS, Gibco) once. After centrifugation and the supernatants removal, the aggregate pellets were incubated in 100 μl of trypsin–EDTA solution (Sigma–Aldrich Company Ltd., St. Louis, MO) for 15 min at 37 °C. Following the incubation, 100 μl of culture medium was added to stop the trypsin action. Live cells in the cell suspension were counted by a cell counter (Countess II FL, Thermo Fisher Scientific Inc.). Live/dead assays were conducted using a Live/Dead Viability/Cytotoxicity Assay (Invitrogen) according to the manufacturer's protocol. The cell aggregates were rinsed once with PBS 7 or 14 days after incubation, and then incubated with the mixed solution of 2 mM calcein AM and 4 mM ethidium homodimer-1 solution in PBS for 15 min at room temperature in the dark, followed by observation using a microscope (BX-X710).
Reductase activity of INS-1 cell aggregates with or without gelatin hydrogel microspheres was determined by colorimetric assay using water-soluble tetrazolium salt as one measure to assess a cell metabolic activity. After 7 or 14 days incubation, cell aggregates were collected into a well of 96 well flat-bottomed plate and the culture medium was adjusted 100 μl in each well. WST-8 reagent (10 μl, Dojindo Laboratories, Kumamoto, Japan) was added into each well, and then cell aggregates were incubated for 4 h at 37 °C. After the incubation, the absorbance at 450 nm was measured by SpectraMax M2/M2e Microplate Reader (Molecular Devices, LLC, CA). The reductase activity was normalized by the live cell number.
2.4. Evaluation of glucose-induced insulin secretion
After 7 or 14 days incubation, INS-1 cell aggregates with or without gelatin hydrogel microspheres were transferred to each well of a 12 mm Transwell (#3402, Coring Inc. Corning, NY) and washed once by PBS. Then, Krebs-Ringer-bicarbonate HEPES (KRB) buffer solution containing 10 mM glucose [16] was added to each well, and the cell aggregates were incubated for 1 h at 37 °C. The concentration of secreted insulin in the supernatants was measured by ELISA kit (Rat Insulin ELISA KIT, Shibayagi Co. Ltd., Gunma, Japan). The insulin secretion was normalized by the live cell number.
2.5. Statistical analysis
All the statistical data are expressed as mean ± standard error of the mean (SEM). The data were analyzed using the Tukey's test and the statistical significance was accepted at P < 0.05 or 0.01.
3. Result
3.1. Characterization of gelatin hydrogel microspheres
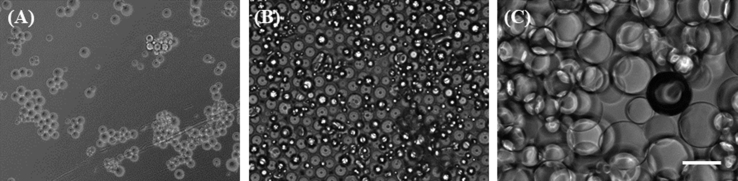
Fig. 1 shows the microscopic images of gelatin hydrogel microspheres. The microspheres had spherical shape and smooth surface. The size of microspheres in the swollen condition in the culture medium was 22.0 ± 9.0 (small), 46.0 ± 14.0 (middle) and 82.0 ± 18.0 μm (large).
Fig. 1.
Light microscopic pictures of gelatin hydrogel microspheres dispersed in the culture medium. The diameter of gelatin hydrogel microspheres is 22.0 ± 9.0 (small) (A), 46.0 ± 14.0 (middle) (B) or 82.0 ± 18.0 μm (large) (C). Scale bar is 100 μm.
3.2. Characterization of INS-1 aggregates with and without gelatin hydrogel microspheres
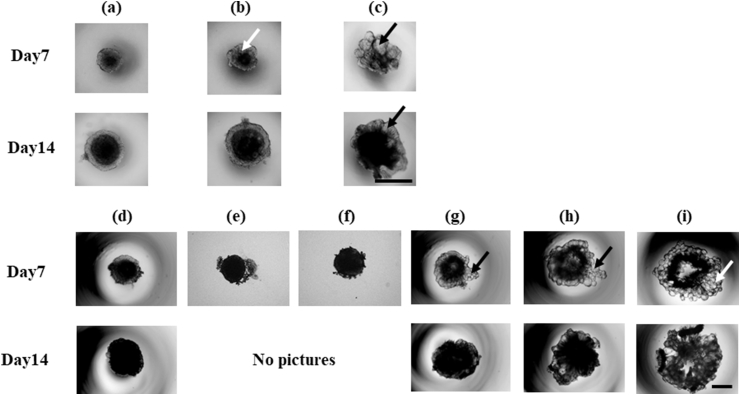
Table 1 shows the preparation condition of cell aggregate using middle or large microspheres. Small gelatin hydrogel microspheres with the diameter of 22.0 ± 9.0 μm were used for INS-1 cell aggregate preparation. However, the appearance of cell aggregate was similar to that of cell aggregate without microspheres (Fig. 2 (d)–(f)). Based on this, middle and large gelatin hydrogel microspheres were used for the formation of cell aggregates unless otherwise mentioned. Fig. 2 shows the microscopic pictures of INS-1 cell aggregates 7 or 14 days after incubation. Cell aggregates were successfully formed in V-bottomed wells. Cell aggregates had a fragile structure when they were prepared with larger size and number of microspheres at the initial seeding density of 1.0 × 104 cells/well even 14 days after incubation (Fig. 2 (i)). On the other hand, when the initial seeding density was 1.0 × 103 cells/well, cell aggregates were successfully obtained even in the case of larger size microspheres.
Fig. 2.
Light microscopic pictures of INS-1 cell aggregates 7 and 14 days after incubation with or without gelatin hydrogel microspheres. Symbols (a)–(i) correspond to those in Table 1. Arrows indicate gelatin hydrogel microspheres. Scale bar is 500 μm.
3.3. Effect of gelatin hydrogel microspheres on cell viability and reductase activity in cell aggregates
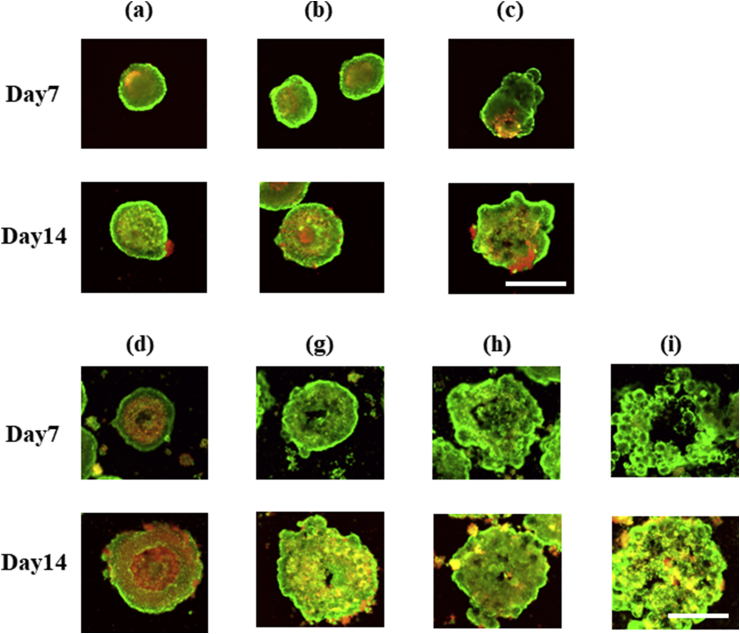
Fig. 3 shows the fluorescent images of cell aggregates after the live/dead assay. To assess the cell viability in INS-1 cell aggregates, the live/dead assay was conducted. At the initial seeding density of 1.0 × 104 cells/well, the cells in the center of microspheres-free cell aggregates died (Fig. 3 (d)). On the other hand, the microspheres suppressed the cell death (Fig. 3 (g)–(i)), irrespective of the size and number of microspheres incorporated. The microspheres incorporation did not affect the cell viability in cell aggregates at the initial seeding density of 1.0 × 103 cells/well (Fig. 3 (a)–(c)).
Fig. 3.
Live/dead assay pictures of INS-1 cell aggregates with or without gelatin hydrogel microspheres 7 and 14 days after incubation. Symbols (a)–(i) correspond to those in Table 1. Scale bar is 500 μm.
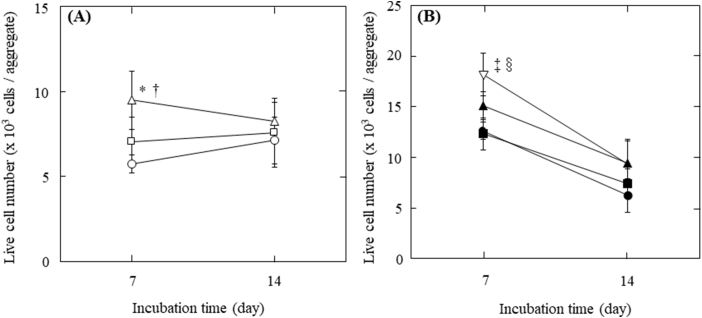
Trypan-blue exclusion test was performed to evaluate the number of live cells in cell aggregates. Fig. 4 shows the number of live cells 7 or 14 days after incubation. The number of live cells showed little change for cell aggregates prepared at the initial seeding density of 1.0 × 103 cells/well with incubation time. On the other hand, the number decreased for cell aggregates at the initial seeding density of 1.0 × 104 cells/well. For cell aggregates at the initial seeding density both of 1.0 × 103 and 1.0 × 104 cells/well, the number of live cells in cell aggregates incorporating large size and number of gelatin hydrogel microspheres was significantly larger than that of cell aggregates incorporating middle microspheres and without microspheres 7 days after incubation.
Fig. 4.
Live INS-1 cell number per aggregate with or without gelatin hydrogel microspheres 7 and 14 days after incubation. (A) Cell aggregates with 1.0 × 101 of middle size (□) and large size gelatin hydrogel microspheres (△) or without gelatin hydrogel microspheres (〇) at the initial seeding density of 1.0 × 103 cells/well. *p < 0.01, significant against the number of live INS-1 cells in cell aggregate without gelatin hydrogel microspheres at the corresponding time; †p < 0.05, significant against the number of live INS-1 cells with middle size of gelatin hydrogel microspheres at the corresponding time. (B) Cell aggregates with 1.0 × 102 of middle size (■), 0.5 × 102 of large size (▲) and 1.0 × 102 of large size of gelatin hydrogel microspheres (▽) or without gelatin hydrogel microspheres (●) at the initial seeding density of 1.0 × 104 cells/well. ‡p < 0.01, significant against the number of live INS-1 cells in cell aggregate without gelatin hydrogel microspheres at the corresponding time; §p < 0.01, significant against the number of live INS-1 cells with middle size of gelatin hydrogel microspheres at the corresponding time.
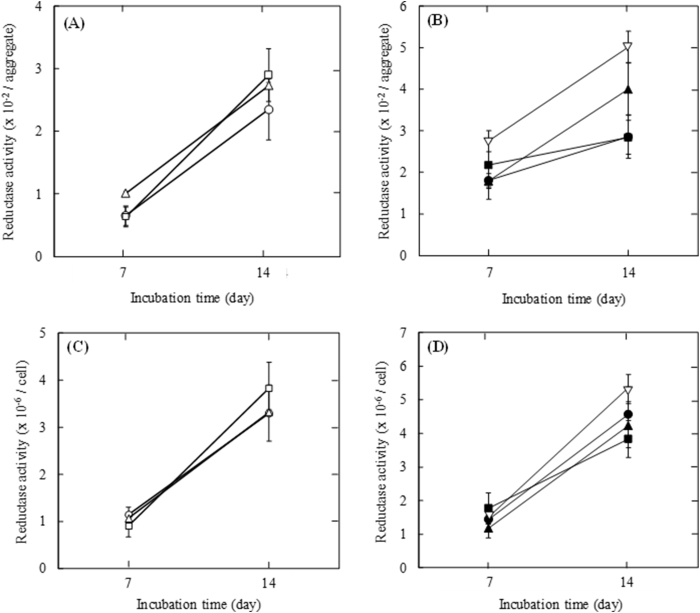
Fig. 5 shows the reductase activity per INS-1 cell aggregate or INS-1 cell 7 and 14 days after incubation with or without gelatin hydrogel microspheres. The reductase activity per aggregate increased with incubation time. For cell aggregates at the initial seeding density of 1.0 × 103 and 1.0 × 104 cells/well, the activity per aggregate incorporating large size and number of gelatin hydrogel microspheres tended to be larger than that of cell aggregate incorporating middle microspheres and without microspheres (Fig. 5A and B). The profile of the reductase activity per cell was the similar to that per aggregate (Fig. 5C and D).
Fig. 5.
Reductase activity 7 and 14 days after incubation. (A) Reductase activity of INS-1 cell aggregate with 1.0 × 101 of middle size (□) and large size gelatin hydrogel microspheres (△) or without gelatin hydrogel microspheres (〇) at the initial seeding density of 1.0 × 103 cells/well. (B) Reductase activity of INS-1 cell aggregate with 1.0 × 102 of middle size (■), 0.5 × 102 of large size (▲) and 1.0 × 102 of large size of gelatin hydrogel microspheres (▽) or without gelatin hydrogel microspheres (●) at the initial seeding density of 1.0 × 104 cells/well. (C) Reductase activity of INS-1 cell in aggregates with 1.0 × 101 of middle size (□) and large size gelatin hydrogel microspheres (△) or without gelatin hydrogel microspheres (〇) at the initial seeding density of 1.0 × 103 cells/well. (D) Reductase activity of INS-1 cell in aggregates with 1.0 × 102 of middle size (■), 0.5 × 102 of large size (▲) and 1.0 × 102 of large size of gelatin hydrogel microspheres (▽) or without gelatin hydrogel microspheres (●) at the initial seeding density of 1.0 × 104 cells/well.
3.4. Effect of gelatin hydrogel microspheres on glucose-induced insulin secretion of cell aggregates
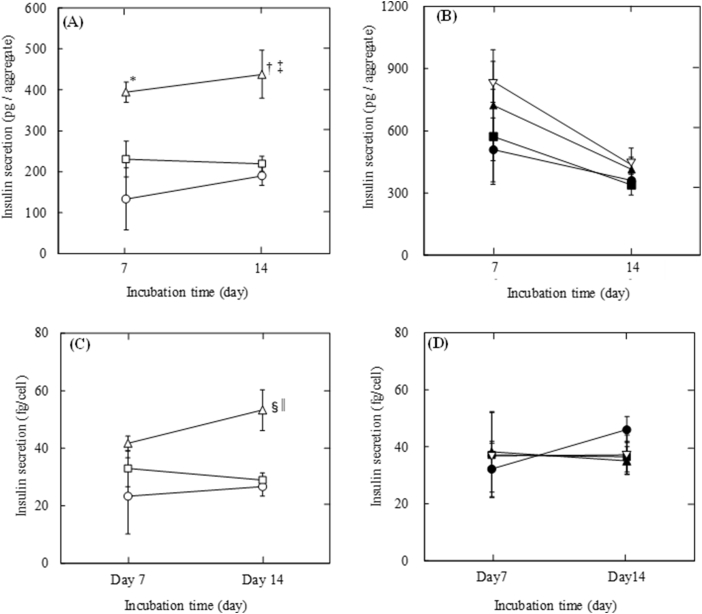
Fig. 6 shows the insulin secretion per INS-1 cell aggregate or INS-1 cell 7 and 14 days after incubation with or without gelatin hydrogel microspheres. Cell aggregates with large microspheres secreted insulin at significantly larger amount than those with middle microspheres and without microspheres for 14 days at the initial seeding density of 1.0 × 103 cells/well. On the other hand, at the initial seeding density of 1.0 × 104 cells/well, cell aggregates with large microspheres tended to secrete insulin at larger amount than those with middle microspheres and without microspheres, and the decreasing tendency of insulin secretion was observed (Fig. 6A and B). The insulin secretion per cell in aggregate incorporating large microspheres was also significantly higher than that in aggregate with middle microspheres and without microspheres at 14 days after incubation at the initial seeding density of 1.0 × 103 cells/well. However, at the initial seeding density of 1.0 × 104 cells/well, there was no difference among cells in each cell aggregate (Fig. 6C and D).
Fig. 6.
Insulin secretion 7 and 14 days after incubation. (A) Insulin secretion per INS-1 cell aggregate with 1.0 × 101 of middle size (□) and large size gelatin hydrogel microspheres (△) or without gelatin hydrogel microspheres (〇) at the initial seeding density of 1.0 × 103 cells/well. *p < 0.05, †p < 0.01, significant against insulin secretion per aggregate without gelatin hydrogel microspheres at the corresponding time. ‡p < 0.05, significant against insulin secretion per aggregate with middle size of gelatin hydrogel microspheres at the corresponding time. (B) Insulin secretion per INS-1 cell aggregate with 1.0 × 102 of middle size (■), 0.5 × 102 of large size (▲) and 1.0 × 102 of large size of gelatin hydrogel microspheres (▽) or without gelatin hydrogel microspheres (●) at the initial seeding density of 1.0 × 104 cells/well. (C) Insulin secretion per INS-1 cell in aggregates with 1.0 × 101 of middle size (□) and large size gelatin hydrogel microspheres (△) or without gelatin hydrogel microspheres (〇) at the initial seeding density of 1.0 × 103 cells/well. §p < 0.05, significant against insulin secretion per aggregate without gelatin hydrogel microspheres at the corresponding time. ||p < 0.05, significant against insulin secretion per aggregate with middle size of gelatin hydrogel microspheres at the corresponding time. (D) Insulin secretion per INS-1 cell in aggregates with 1.0 × 102 of middle size (■), 0.5 × 102 of large size (▲) and 1.0 × 102 of large size of gelatin hydrogel microspheres (▽) or without gelatin hydrogel microspheres (●) at the initial seeding density of 1.0 × 104 cells/well.
4. Discussion
Generally, U and V-bottomed multi-well plates or a hanging-drop method are used to prepare cell aggregates in lab-scale [17], [18], [19], [20]. However, the aggregates formation depended on the type of cells. For example, INS-1 cell aggregates did not form when 96-well U-bottomed or Flat-bottomed plate was used. This is because cells adhered to the bottom surface or wall of well unless the well was coated for anti-cell adhesion (data not shown) [13], [14]. On the other hand, cell aggregates were obtained in the V-bottomed well even without the anti-cell adhesion coating. It is reported that cells did not remain on the wall of wells because the V-bottomed well had too steep wall to induce cell adhesion [20]. The formation of cell aggregate depended on the size and number of microspheres (Fig. 2). Cell aggregates were not completely formed upon using larger size and number of microspheres even 14 days after incubation at the initial seeding density of 1.0 × 104 cells/well. It is likely that microspheres tended to sink down to the well bottom faster than cells because of their heavy weight, and larger number of microspheres (0.5 or 1 × 102 microspheres/well) tended to occupy the larger volume of the well bottom. Consequently, the microspheres were firstly gathered on the bottom surface of wells and then cells surrounded them (Supplemental Fig. 1), because cells could not sufficiently go through the spaces inbetween larger number of microspheres. It is highly conceivable that heterogeneous distribution of cells and microspheres structurally weakened cell aggregates. This result suggests that there is an optimal number ratio of cells to microspheres.
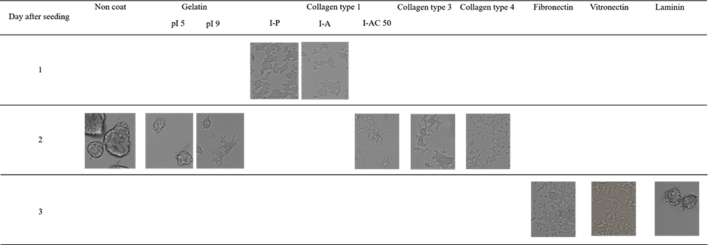
In this study, gelatin was used for the material of hydrogel microspheres because of its high chemical susceptibility and biocompatibility. Gelatin is biodegradable material and has been extensively used for food, pharmaceutical, and medical purposes and its biosafety has been proven through their long practical applications [21], [22], [23], [24], [25]. However, for the formation of more rigid cell aggregates, it is required to modify or optimize the hydrogel microspheres to promote INS-1 cell attachment to the surface of microspheres. The coating of cell adhesion proteins, such as fibronectin, laminin, vitronectin, and collagen, is known to improve cell adhesion to the surface of microspheres [26], [27], [28]. Isoionic point 9.0 (pI 9) gelatin has a positive charge at the physiological pH and might promote cell adhesion because cell surfaces have a negative charge [29], [30]. We evaluated INS-1 cell attachment to the surface of low-attachment 24 well plate (#3473, Corning Inc.) which had been coated by gelatin of pI5 or 9 (weight-averaged molecular weight≃1,00,000, Nitta Gelatin Inc.), collagens, and cell adhesion proteins. The concentration of coating solutions and coating methods were decided according to the manufacturer's protocol. The protocol for the coating of gelatin (pI 5 or 9) referred to that of collagen. The gelatin of pI9, collagen type 1 (Type I-A, I-P; Nitta Gelatin Inc./I-AC, Koken Co., Ltd., Tokyo), type 3 (PSC-3-100-05; Nippi Inc., Tokyo, Japan), or type 4 (ASC-4-104-01; Nippi), fibronectin (Wako Pure Chemical Industries Ltd.), and vitronectin (Sigma–Aldrich Company Ltd.) improved the INS-1 cells attachment compared with gelatin of pI 5 (Supplemental Fig. 2). On the other hand, laminin (Wako Pure Chemical Industries Ltd.) did not promote the cell attachment. Taken together, the surface coating of gelatin hydrogel microsphere by collagen, fibronectin or vitronectin might be promising to improve cell attachment and form more rigid aggregates, although the coating onto the surface of tissue culture plates does not represent directly that of gelatin hydrogel microspheres.
The viability of cells in the center of cell aggregates without microspheres was obviously bad when the initial seeding density was 1.0 × 104 cells/well even 7 days after incubation (Fig. 3). On the other hand, it is apparent that the microspheres incorporation suppressed the cell death, although a little decrease in the cell viability was detected, because the aggregates showed yellow color which were mixed with green live cells and red dead cells. The number of live cells in cell aggregates was improved by the incorporation of gelatin hydrogel microspheres, especially for larger size and number of microspheres (Fig. 4). It is demonstrated that oxygen and nutrients did not supply sufficiently to cells present in the center of aggregates without microspheres [9], [10]. We previously demonstrated that the incorporation of gelatin hydrogel microspheres enabled MSC in their aggregates to improve the cell viability and proliferation, and osteogenic differentiation [13], [14]. We can say with certainty that the gelatin hydrogel microspheres gave a pathway of oxygen to the cell aggregates. Especially, larger size and number of microspheres would give more efficient pathway of oxygen to cells inside the cell aggregates than smaller size and number of microspheres. Therefore, a large number of live cells would remain in the cell aggregates incorporating larger size and number of microspheres. As seen in Fig. 4B, the live cell number was notably decreased with the incubation time when the initial seeding density was 1.0 × 104 cells/well. In addition, the cell number only slightly increased compared with the cell number seeded initially, whereas the cell number increased 5–10 folds compared with the initial cell number at the initial seeding density of 1.0 × 103 cells/well. The initial seeding density of 1.0 × 104 cells/well would be too high to allow cell aggregates to grow in the V-bottomed well. It is conceivable that the insufficient space physically hindered the cell proliferation. Taken together, this result suggests that there must be an optimal seeding density for each culture substrate not to physically damage the cell aggregates.
It is apparent from Fig. 2 that cell number increased with incubation time for cell aggregates at the initial seeding density of 1.0 × 103 cells/well (a-c), aggregates without microspheres (d) and with middle microspheres (e) at the initial seeding density of 1.0 × 104 cells/well, because the size of aggregate increased with incubation time. The aggregates with larger microspheres at the initial seeding density of 1.0 × 104 cells/well (h, i) did not increase the size, but the structural density seems to become higher with the incubation time. We can think that this is because the increasing cell number resulted in filling the spaces between aggregates. However, trypan-blue staining showed that live cell number did not increase at the initial seeding density of 1.0 × 103 cells/well, and live cell number decreased at that of 1.0 × 104 cells/well (Fig. 4). This implies that the lack of oxygen and nutrients caused the necrosis of cells in the center of aggregate, and the narrow bottom space of well damaged cells in aggregate. At the initial seeding density of 1.0 × 103 cells/well, cell aggregates with large microspheres secreted significantly larger amount if insulin than aggregates with middle microspheres or without microspheres (Fig. 6A). We got the equivalent result on the insulin secretion per cell (Fig. 6C). On the other hand, at the initial seeding density of 1.0 × 104 cells/well, insulin secretion amount per aggregate decreased with the decrease of live cell number, and the insulin secretion amount per cell did not change with incubation time (Fig. 6B and D). It is well known that insulin secreting cells are susceptible to a hypoxic environment and need large amount of oxygen to physiologically produce insulin [11], [12]. In this connection, this result experimentally confirms that the presence of gelatin hydrogel microspheres could give INS-1 cells a larger amount of oxygen to secrete insulin. As mentioned above, it is possible that the V-bottomed well physically damaged the cell aggregates because the well bottom area is narrow compared with that of U-bottomed or flat-bottomed well. This may be explained in terms of the physical stress which greatly affects the profile of insulin secretion. The result of reductase activity was different from that of live cell number at both initial seeding densities of 1.0 × 103 and 1.0 × 104 cells/well, namely the activity per cell and aggregate increased with incubation time (Fig. 5). It is considered to be difficult to definitely determine the live or dead of cells by trypan blue staining method because the method can show the cell viability only by the damage degree of cell membrane. Cells detected as dead cells maintained the reductase activity and then the activity increased with incubation time. This is because the cell number increased as mentioned above. This result implies that the insulin secretion ability was sensitive to cell viability compared with reductase activity.
In this study, glucose-induced insulin secretion was evaluated as the function of insulin secreting cell aggregates because the function is the most important for the diabetes treatment. It is known that this function is damaged by the hypoxic environment most seriously. The improvement of insulin secretion ability by the microspheres is important for treatment of type 1 diabetes.
Particularly, in the present study, INS-1 cell aggregate with large gelatin hydrogel microspheres with the initial seeding density of 1.0 × 103 cells/well would be cell aggregates prepared with the best condition for treatment of type 1 diabetes, because the aggregates secrete significantly higher amount of insulin after the same incubation time as aggregates with middle microspheres and without microspheres.
Pseudo-islets artificially formed from insulin secreting cells undergo necrosis because the cell aggregates are generally cultured under a static condition. Lock et al. or Matta et al. used the bioreactor for suspension culture to improve the oxygen and nutrients transport [31], [32]. The supplementation of all-trans retinoic acid or nicotinamide to the culture medium also maintained or enhanced the functions of pseudo-islets [33], [34], [35], [36]. Primarily isolated islets show a tendency of self-aggregation and the islet clumping often causes the serious necrosis because of the lack of oxygen and nutrients after the islet transplantation [37], [38], [39]. To tackle this issue, Liao et al. made islets separate to each other by their embedding into a hydrogel [37]. Qi et al. or Aung et al. encapsulated islets into a PVA sheet or tube not to form the clumping [38], [39], [40]. Similarly, Onoe et al. encapsulated islets into alginate fibers [41]. The gelatin hydrogel microspheres technology might be promising to avoid the necrosis under the conventional static culture condition without any devices to suppress the clumping because the microspheres function as the pathway of oxygen and nutrients in insulin secreting cell aggregates or islets. When the cell aggregate become larger, the simple supplementation to the culture medium would not reach cells in the center of aggregates sufficiently because of the inherent barrier of cell aggregates. We previously reported that gelatin hydrogel microspheres function as not only the pathway of oxygen and nutrients, but also biological factors release carrier [24], [42], [43], [44]. If the supplementation of all-trans retinoic acid or nicotinamide is performed from gelatin hydrogel microspheres in cell aggregates, cells in aggregates might uniformly interact with these factors. Furthermore, it is known that primary islets contain cells secreting vascular endothelial growth factor-A (VEGF-A) to regulate islet differentiation, form highly vascularized islets, and maintain islet functions [45], [46], [47]. It is easily predicted that the release of VEGF from gelatin hydrogel microspheres in cell aggregates would realize the artificially formed insulin secreting cell aggregates more similar to natural islets.
The present study demonstrates that the incorporation of gelatin hydrogel microspheres at the certain size and number could improve the cell viability and insulin secretion function. This technology is promising to give insulin secreting cell aggregates a better condition for their functional improvement, although more optimization of microspheres is needed in the future.
5. Conclusions
INS-1 cell aggregates with or without gelatin hydrogel microspheres were formed. Cell viability and glucose-induced insulin secretion of cell aggregates were improved by the incorporation of microspheres especially at the larger size and number.
Acknowledgement
We thank Hiroo Iwata, Tetsuo Hoshino, Shigeo Yanai, Kenichi Anai, Kenichiro Kiyoshima, and Aya Nitta for valuable comments, proof-reading, and experimental supports. This work has been done during the first author's tenure at Takeda under Takeda sponsorship.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.reth.2017.12.002.
Conflict of interest
There is no conflict of interest to disclose.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplemental materials & methods
-
1.
Materials
Gelatin (isoionic point 5.0 (pI 5) and 9.0 (pI 9), weight-averaged molecular weight = 1,00,000, were kindly provided from Nitta Gelatin Inc., Osaka, Japan. Other chemicals were purchased from Nitta Gelatin Inc., Wako Pure Chemical Industries Ltd., Osaka, Japan, Koken Co., Ltd., Tokyo, Japan or Sigma–Aldrich Company Ltd., St. Louis, MO and used without further purification. The low-attachment plate (#3473, Coring Inc. Corning, NY) was used for this evaluation.
-
2.
Evaluation of INS-1 cell attachment
Gelatins, collagens, and other cell adhesion proteins were coated according to the manufacturer's protocol. Briefly, gelatins and collagens were dissolved in water at 0.3 mg/ml. Each solution (200 μl) was added into each well and naturally dried in the clean-bench. Fibronectin or laminin was dissolved in PBS at 10 μg/ml. Each solution (300 μl) was added into each well and the well plate was incubated for 2 h at 37 °C, following the wash with PBS. Vitronectin was dissolved in water at 0.7 μg/ml. The following process was the same as that for fibronectin and laminin. INS-1 cells of 5.0 × 104 cells were seeded to the well with or without coating.
Supplemental Fig. 1.
Light microscopic pictures of INS-1 cell aggregates 1 day after incubation with gelatin hydrogel microspheres. Symbols correspond to those in Table 1. Scale bar is 500 μm.
Supplemental Fig. 2.
Light microscopic images of INS-1 cells 1, 2, or 3 days after seeding to the well coated by gelatins, collagens, or other cell adhesion proteins.
References
- 1.Ryan E.A., Lakey J.R., Rajotte R.V., Korbutt G.S., Kin T., Imes S. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50(4):710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 2.Ricordi C., Strom T.B. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4(4):259–268. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 3.Barton F.B., Rickels M.R., Alejandro R., Hering B.J., Wease S., Naziruddin B. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35(7):1436–1445. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro A.M., Lakey J.R., Ryan E.A., Korbutt G.S., Toth E., Warnock G.L. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 5.Sakuma Y., Ricordi C., Miki A., Yamamoto T., Pileggi A., Khan A. Factors that affect human islet isolation. Transplant Proc. 2008;40(2):343–345. doi: 10.1016/j.transproceed.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto S., Okitsu T., Iwanaga Y., Noguchi H., Nagata H., Yonekawa Y. Insulin independence of unstable diabetic patient after single living donor islet transplantation. Transplant Proc. 2005;37(8):3427–3429. doi: 10.1016/j.transproceed.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita N., Echigo Y., Shinohara S., Gu Y., Miyazaki J., Inoue K. Regulation of cell proliferation using tissue engineering in MIN6 cells. Cell Transplant. 2001;10(4–5):473–477. [PubMed] [Google Scholar]
- 8.Brereton H., Carvell M.J., Persaud S.J., Jones P.M. Islet alpha-cells do not influence insulin secretion from beta-cells through cell-cell contact. Endocrine. 2007;31(1):61–65. doi: 10.1007/s12020-007-0004-0. [DOI] [PubMed] [Google Scholar]
- 9.Kellner K., Liebsch G., Klimant I., Wolfbeis O.S., Blunk T., Schulz M.B. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol Bioeng. 2002;80(1):73–83. doi: 10.1002/bit.10352. [DOI] [PubMed] [Google Scholar]
- 10.Malda J., Klein T.J., Upton Z. The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng. 2007;13(9):2153–2162. doi: 10.1089/ten.2006.0417. [DOI] [PubMed] [Google Scholar]
- 11.Sato Y., Endo H., Okuyama H., Takeda T., Iwahashi H., Imagawa A. Cellular hypoxia of pancreatic beta-cells due to high levels of oxygen consumption for insulin secretion in vitro. J Biol Chem. 2011;286(14):12524–12532. doi: 10.1074/jbc.M110.194738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porterfield D.M., Corkey R.F., Sanger R.H., Tornheim K., Smith P.J., Corkey B.E. Oxygen consumption oscillates in single clonal pancreatic beta-cells (HIT) Diabetes. 2000;49(9):1511–1516. doi: 10.2337/diabetes.49.9.1511. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi K., Tabata Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011;7(7):2797–2803. doi: 10.1016/j.actbio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Tajima S., Tabata Y. Preparation and functional evaluation of cell aggregates incorporating gelatin microspheres with different degradabilities. J Tissue Eng Regen Med. 2013;7(10):801–811. doi: 10.1002/term.1469. [DOI] [PubMed] [Google Scholar]
- 15.Tabata Y., Ikada Y., Morimoto K., Katsumata H., Yabuta T., Iwanaga K. Surfactant-free preparation of biodegradable hydrogel microspheres for protein release. J Bioact Compat Polym. 1999;14:371–384. [Google Scholar]
- 16.Tsujihata Y., Ito R., Suzuki M., Harada A., Negoro N., Yasuma T. TAK-875, an orally available G protein-coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J Pharmacol Exp Therapeut. 2011;339(1):228–237. doi: 10.1124/jpet.111.183772. [DOI] [PubMed] [Google Scholar]
- 17.Ivascu A., Kubbies M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol Screen. 2006;11(8):922–932. doi: 10.1177/1087057106292763. [DOI] [PubMed] [Google Scholar]
- 18.Ng E.S., Davis R.P., Azzola L., Stanley E.G., Elefanty A.G. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106(5):1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 19.Tung Y.C., Hsiao A.Y., Allen S.G., Torisawa Y.S., Ho M., Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;136(3):473–478. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ungrin M.D., Joshi C., Nica A., Bauwens C., Zandstra P.W. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One. 2008 Feb 13;3(2):e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J., Yoo J.J., Atala A., Lee S.J. The effect of controlled release of PDGF-BB from heparin-conjugated electrospun PCL/gelatin scaffolds on cellular bioactivity and infiltration. Biomaterials. 2012 Oct;33(28):6709–6720. doi: 10.1016/j.biomaterials.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. [DOI] [PubMed]
- 23.Vardakou M., Mercuri A., Naylor T.A., Rizzo D., Butler J.M., Connolly P.C. Predicting the human in vivo performance of different oral capsule shell types using a novel in vitro dynamic gastric model. Int J Pharm. 2011 Oct 31;419(1–2):192–199. doi: 10.1016/j.ijpharm.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 24.Sakakibara Y., Nishimura K., Tambara K., Yamamoto M., Lu F., Tabata Y. Prevascularization with gelatin microspheres containing basic fibroblast growth factor enhances the benefits of cardiomyocyte transplantation. J Thorac Cardiovasc Surg. 2002 Jul;124(1):50–56. doi: 10.1067/mtc.2002.121293. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y., Yamamoto M., Tabata Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials. 2005 Aug;26(23):4856–4865. doi: 10.1016/j.biomaterials.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe R., Hayashi R., Kimura Y., Tanaka Y., Kageyama T., Hara S. A novel gelatin hydrogel carrier sheet for corneal endothelial transplantation. Tissue Eng Part A. 2011 Sep;17(17–18):2213–2219. doi: 10.1089/ten.TEA.2010.0568. [DOI] [PubMed] [Google Scholar]
- 27.Masters K.S., Shah D.N., Walker G., Leinwand L.A., Anseth K.S. Designing scaffolds for valvular interstitial cells: cell adhesion and function on naturally derived materials. J Biomed Mater Res A. 2004 Oct 1;71(1):172–180. doi: 10.1002/jbm.a.30149. [DOI] [PubMed] [Google Scholar]
- 28.Lu H.H., Cooper J.A., Jr., Manuel S., Freeman J.W., Attawia M.A., Ko F.K. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005 Aug;26(23):4805–4816. doi: 10.1016/j.biomaterials.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 29.Saito T., Tabata Y. Preparation of gelatin hydrogels incorporating low-molecular-weight heparin for anti-fibrotic therapy. Acta Biomater. 2012 Feb;8(2):646–652. doi: 10.1016/j.actbio.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Solorio L., Zwolinski C., Lund A.W., Farrell M.J., Stegemann J.P. Gelatin microspheres crosslinked with genipin for local delivery of growth factors. J Tissue Eng Regen Med. 2010 Oct;4(7):514–523. doi: 10.1002/term.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matta S.G., Wobken J.D., Williams F.G., Bauer G.E. Pancreatic islet cell reaggregation systems: efficiency of cell reassociation and endocrine cell topography of rat islet-like aggregates. Pancreas. 1994 Jul;9(4):439–449. [PubMed] [Google Scholar]
- 32.Lock L.T., Laychock S.G., Tzanakakis E.S. Pseudoislets in stirred-suspension culture exhibit enhanced cell survival, propagation and insulin secretion. J Biotechnol. 2011 Feb 10;151(3):278–286. doi: 10.1016/j.jbiotec.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Lee D.Y., Park S.J., Nam J.H., Byun Y. Optimal aggregation of dissociated islet cells for functional islet- like cluster. J Biomater Sci Polym Ed. 2008;19(4):441–452. doi: 10.1163/156856208783719527. [DOI] [PubMed] [Google Scholar]
- 34.Ohgawara H., Mochizuki N., Karibe S., Omori Y. Survival and B-cell function of neonatal pig pancreatic islet-like cell clusters in an extracellular matrix. Pancreas. 1991 Nov;6(6):625–630. doi: 10.1097/00006676-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Otonkoski T., Beattie G.M., Mally M.I., Ricordi C., Hayek A. Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J Clin Invest. 1993 Sep;92(3):1459–1466. doi: 10.1172/JCI116723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beattie G.M., Rubin J.S., Mally M.I., Otonkoski T., Hayek A. Regulation of proliferation and differentiation of human fetal pancreatic islet cells by extracellular matrix, hepatocyte growth factor, and cell-cell contact. Diabetes. 1996 Sep;45(9):1223–1228. doi: 10.2337/diab.45.9.1223. [DOI] [PubMed] [Google Scholar]
- 37.Liao S.W., Rawson J., Omori K., Ishiyama K., Mozhdehi D., Oancea A.R. Maintaining functional islets through encapsulation in an injectable saccharide-peptide hydrogel. Biomaterials. 2013 May;34(16):3984–3991. doi: 10.1016/j.biomaterials.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aung T., Kogire M., Inoue K., Fujisato T., Gu Y., Burczak K. Insulin release from a bioartificial pancreas using a mesh reinforced polyvinyl alcohol hydrogel tube. An in vitro study. ASAIO J. 1993 Apr–Jun;39(2):93–96. [PubMed] [Google Scholar]
- 39.Aung T., Inoue K., Kogire M., Doi R., Kaji H., Tun T. Comparison of various gels for immobilization of islets in bioartificial pancreas using a mesh-reinforced polyvinyl alcohol hydrogel tube. Transplant Proc. 1995 Feb;27(1):619–621. [PubMed] [Google Scholar]
- 40.Qi M., Gu Y., Sakata N., Kim D., Shirouzu Y., Yamamoto C. PVA hydrogel sheet macroencapsulation for the bioartificial pancreas. Biomaterials. 2004 Dec;25(27):5885–5892. doi: 10.1016/j.biomaterials.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 41.Onoe H., Okitsu T., Itou A., Kato-Negishi M., Gojo R., Kiriya D. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat Mater. 2013 Jun;12(6):584–590. doi: 10.1038/nmat3606. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto M., Ikada Y., Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed. 2001;12(1):77–88. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- 43.Patel Z.S., Ueda H., Yamamoto M., Tabata Y., Mikos A.G. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm Res. 2008 Oct;25(10):2370–2378. doi: 10.1007/s11095-008-9685-1. [DOI] [PubMed] [Google Scholar]
- 44.Ichinohe N., Kuboki Y., Tabata Y. Bone regeneration using titanium nonwoven fabrics combined with fgf-2 release from gelatin hydrogel microspheres in rabbit skull defects. Tissue Eng Part A. 2008 Oct;14(10):1663–1671. doi: 10.1089/ten.tea.2006.0350. [DOI] [PubMed] [Google Scholar]
- 45.Brissova M., Aamodt K., Brahmachary P., Prasad N., Hong J.Y., Dai C. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration. Cell Metab. 2014 Mar 4;19(3):498–511. doi: 10.1016/j.cmet.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinert R.B., Cai Q., Hong J.Y., Plank J.L., Aamodt K., Prasad N. Vascular endothelial growth factor Coordinates islet innervation via vascular scaffolding. Development. 2014 Apr;141(7):1480–1491. doi: 10.1242/dev.098657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brissova M., Shostak A., Shiota M., Wiebe P.O., Poffenberger G., Kantz J. Pancreatic islet production of vascular endothelial growth factor-A is essential for islet vascularization, revascularization, and function. Diabetes. 2006 Nov;55(11):2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]