Abstract
Mesenchymal stem cells (MSCs) are multipotent stem cells and capable of differentiating into multiple cell types including osteoblastic, chondrogenic and adipogenic lineages. We previously identified BMP9 as one of the most potent BMPs that induce osteoblastic differentiation of MSCs although exact molecular mechanism through which BMP9 regulates osteogenic differentiation remains to be fully understood. Here, we seek to develop a recombinant adenovirus system to optimally silence mouse BMP9 and then characterize the important role of BMP9 in osteogenic differentiation of MSCs. Using two different siRNA bioinformatic prediction programs, we design five siRNAs targeting mouse BMP9 (or simB9), which are expressed under the control of the converging H1 and U6 promoters in recombinant adenovirus vectors. We demonstrate that two of the five siRNAs, simB9-4 and simB9-7, exhibit the highest efficiency on silencing exogenous mouse BMP9 in MSCs. Furthermore, simB9-4 and simB9-7 act synergistically in inhibiting BMP9-induced expression of osteogenic markers, matrix mineralization and ectopic bone formation from MSCs. Thus, our findings demonstrate the important role of BMP9 in osteogenic differentiation of MSCs. The characterized simB9 siRNAs may be used as an important tool to investigate the molecular mechanism behind BMP9 osteogenic signaling. Our results also indicate that recombinant adenovirus-mediated expression of siRNAs is efficient and sustained, and thus may be used as an effective delivery vehicle of siRNA therapeutics.
Keywords: BMP9, Bone formation, Mesenchymal stem cells, Osteogenic differentiation, RNA interference, Recombinant adenovirus, siRNA
Introduction
Mesenchymal stem cells (MSCs) are multipotent stem cells with self-renewal and capability of differentiating into multiple cell types, such as osteoblastic, chondrogenic, and adipogenic lineages.1, 2, 3, 4, 5, 6, 7, 8 MSCs have attracted profound attentions in the arena of stem cell biology and regenerative medicine.4, 7, 8, 9, 10, 11, 12 Osteogenic differentiation from MSCs is a tightly regulated process of sequential events that recapitulate most of the molecular processes occurring during bone and skeletal development.7, 8, 13 While osteogenic differentiation is regulated by numerous pathways, such as Wnt, Insulin-like growth factors (IGFs), Fibroblast growth factor (FGFs) and Notch,3, 7, 8, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 bone morphogenetic proteins (BMPs) are considered as the only group of osteoinductive factors that can induce de novo bone formation from MSCs.24, 25
BMPs are members of the TGF-β superfamily and play critical roles in skeletal development, bone formation and stem cell differentiation.3, 24, 25, 26 At least 14 types of BMPs have been identified in humans and rodents.24, 25, 27 We conducted a comprehensive analysis of the osteogenic activities of the 14 types of BMPs, and found that BMP9 (also known as growth differentiation factor 2, or GDF2) is one of the most potent BMPs that induce osteoblastic differentiation of MSCs.24, 28, 29, 30, 31 However, BMP9 is one of the least studied BMPs, and the exact molecular mechanism through which BMP9 regulates osteogenic differentiation remains to be fully understood.
Originally discovered in C. elegans and subsequently demonstrated in diverse eukaryotes, such as insects, plants, fungi and vertebrates, RNA interference (RNAi) is a cellular process of sequence-specific, post-transcriptional gene silencing initiated by short double-stranded RNAs (dsRNA), or short interfering RNAs (siRNAs), which are homologous to the gene being suppressed through the RNA-induced silencing complex (RISC).32, 33, 34, 35, 36, 37, 38, 39 Since its discovery, RNAi has become a valuable and powerful tool to analyze loss-of-function phenotypes,33, 34, 35, 36, 37, 38 as well as offers unprecedented opportunities for developing novel and effective therapeutics for human diseases.40, 41, 42, 43, 44, 45 Nonetheless, the silencing efficiency of siRNAs is highly empirical and varies drastically, depending on many parameters, such as mRNA secondary structures, target availability, status of matching and intrinsic characteristics of mRNA and siRNA.33, 46, 47, 48, 49
In this study, we developed a recombinant adenovirus system to express the optimal siRNAs targeting mouse BMP9 and characterized the important role of BMP9 in osteogenic differentiation of MSCs. Using different siRNA bioinformatic prediction programs, we designed five siRNAs targeting mouse BMP9 (or simB9 siRNAs), which were expressed under the control of converging H1 and U6 promoters in recombinant adenovirus vectors. We demonstrated that two of the five siRNAs, simB9-4 and simB9-7, exhibited the highest efficiency on silencing exogenous mouse BMP9 in MSCs. Furthermore, we demonstrated that simB9-4 and simB9-7 acted synergistically in impairing BMP9-induced expression of osteogenic markers, matrix mineralization and ectopic bone formation from MSCs. Thus, our findings demonstrate the important role of BMP9 in osteogenic differentiation of MSCs. The characterized simB9 siRNAs may be used as an important tool to investigate the molecular mechanism behind BMP9 osteogenic signaling. Lastly, our results indicate that recombinant adenovirus-mediated expression of siRNAs is efficient and sustained, and thus may be used as a potential delivery vehicle of siRNA therapeutics.
Materials and methods
Cell culture chemicals
HEK-293 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). HEK-293 derivatives 293pTP and RAPA were previously characterized.50, 51 The immortalized mouse adipose-derived multipotent (iMAD) cells are mouse mesenchymal stem cells as previously characterized.52 All cell lines were maintained in the completed Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum, 100 units of penicillin and 100 μg streptomycin, cultured at 37 °C in 5% CO2 incubator as described.53, 54, 55, 56 Unless indicated otherwise, all reagents were purchased from Sigma–Aldrich (St. Louis, MO, USA) or Thermos Fisher Scientific (Waltham, MA, USA).
Construction and generation of recombinant adenoviruses expressing mouse BMP9, RPF, and simB9s
Recombinant adenoviruses were generated using the AdEasy system as previously described.57, 58, 59 The coding region of mouse BMP9 was amplified and subcloned into adenoviral shuttle vector pAdTrace-TOX. The siRNAs targeting mouse Bmp9 coding region (or simB9s) were designed by using the Dharmacon's siDESIGN and IDT's DsiRNA design tools as described previously.39, 49 The expression of the siRNAs was driven by converging U6 and H1 promoters as described (Fig. 1).39, 49 The siRNA oligo cassettes (Fig. 1A) were subcloned into the homemade adenoviral shuttle vector pSES1 or pAdTrace-OK.39, 49 All adenoviral shuttle vectors were subjected to homologous recombination with adenoviral backbone in BJ5183/AdEasy1 cells. The resultant recombinants were used to package recombinant adenoviruses in 293pTP and/or RAPA cell lines,50, 51 yielding AdBMP9, AdsimB9-1, 2, 4, 7 and 8, respectively, all of which co-express monomeric RFP (mRFP). AdRFP was used as a mock virus control as reported.53, 60, 61, 62, 63 For all adenovirus infection, polybrene (4–8 μg/ml) was added to improve infection efficiency as previously reported.64
Figure 1.
Selection and construction of recombinant adenoviruses expressing optimal siRNAs targeting the mouse BMP9 coding region (simB9s). (A) Schematic representation of mouse Bmp9 coding region and the location and sequences of the five siRNA targeting sites designed by the Dharmacon's siDESIGN and IDT's DsiRNA design tools. (B) Schematic depiction of the expression of siRNAs driven by the convergent H1 and U6 promoters in recombinant adenoviral vectors through the homemade shuttle vector pSES1 or pAdTrace-61, which co-expresses mRFP as a tracking marker. The resultant siRNA-expressing simB9 siRNA adenoviruses are designated as AdsimB9-1, 2, 4, 7 and 8, respectively. AdBMP9 expresses mouse BMP9 and mRFP, while AdRFP was used as a mock control virus.
RNA isolation and touchdown quantitative real-time PCR (TqPCR)
Subconfluent iMAD cells were infected with the indicated adenoviruses. At the indicated time points, total RNA was isolated by using NucleoZOL Reagent (Takara Bio USA, Mountain View, CA) according to the manufacturer's introduction and subjected to reverse transcription with a hexamer and M-MuLV Reverse Transcriptase (New England Biolabs, Ipswich, MA). The resultant cDNA products were diluted 50-folds and used as templates for TqPCR. PCR primers were designed by Primer3 Plus program (Supplemental Table 1). The touchdown-quantitative PCR analysis was carried out by our optimized TqPCR protocol65 using the 2 × SYBR Green qPCR master mix (Bimake, Houston, TX). All sample values were normalized to Gapdh expression by using the 2–ΔΔCt method as described.7, 8
Alkaline phosphatase (ALP) assays
Subconfluent iMAD cells were infected with the indicated adenoviruses and subjected to ALP assays at the indicated time points. ALP activity was quantitatively assessed by using the modified Great Escape SEAP chemiluminescence assay (using p-nitrophenyl phosphate as a substrate) and/or histochemical staining assay (using a mixture of 0.1 mg/ml napthol AS-MX phosphate and 0.6 mg/ml Fast Blue BB salt) as previously described.66, 67, 68, 69, 70
Matrix mineralization assay (Alizarin Red S staining)
Subconfluent iMAD cells were infected with the indicated adenoviruses and cultured in the presence of ascorbic acid (50 mg/ml) and β-glycerophosphate (10 mM). At day 7 and day 11 after infection, mineralized matrix nodules were stained for calcium precipitation by means of Alizarin Red S staining as described.71, 72, 73, 74, 75 The staining of calcium mineral deposits was documented under bright field microscopy.
Subcutaneous stem cell implantation and ectopic bone formation
The use and care of animals in this study was approved by the Institutional Animal Care and Use Committee. All experimental procedures were carried out in accordance with the approved guidelines. The subcutaneous stem cell implantation was performed as previously described.29, 76, 77, 78, 79, 80 Briefly, subconfluent iMAD cells were infected with various adenoviruses for 36 h and collected for subcutaneous injection into the flanks of athymic nude mice (Envigo/Harlan Research Laboratories; n = 5/group, female, 5–6 week old; 5 × 106 cells per injection). The animals were maintained ad lib in the biosafety barrier facility. At 4 weeks after injection, animals were sacrificed for ectopic mass harvest.
Hematoxylin and eosin (H & E) staining
The retrieved specimens were fixed with 10% formalin, decalcified and embedded in paraffin. Serial sections at 5 μm of the embedded specimens were carried out, and mounted onto treated slides. Then the sections were deparaffinized and then rehydrated in a graduated fashion. H & E staining was done as described.66, 72, 75
Statistical analysis
All quantitative experiments were performed in triplicate and/or repeated three times. Data were expressed as mean ± standard deviation (SD). The one-way analysis of variance was used to analyze statistical significance.81 A value of p < 0.05 was considered statistically significant.
Results and discussion
Selection of optimal siRNAs that effectively silence the expression of mouse BMP9 in mesenchymal stem cells (MSCs)
We previously developed a fluorescence-based optimal siRNA selection system and demonstrated its utility for evaluating the siRNA silencing efficiency.49 We and others have since used this siRNA expression system and demonstrated effective gene silencing in numerous studies.7, 8, 39, 71, 72, 82, 83 In order to effectively knock down mouse BMP9 expression in mesenchymal stem cells (MSCs), we designed five siRNAs targeting the mouse BMP9 coding region, namely simB9-1, simB9-2, simB9-4, simB9-7 and simB9-8 (Fig. 1A). The expression of these siRNAs was driven by the converging H1 and U6 promoters as described,39, 49 followed by generation of recombinant adenoviruses to ensure high transduction efficiency (Fig. 1B).
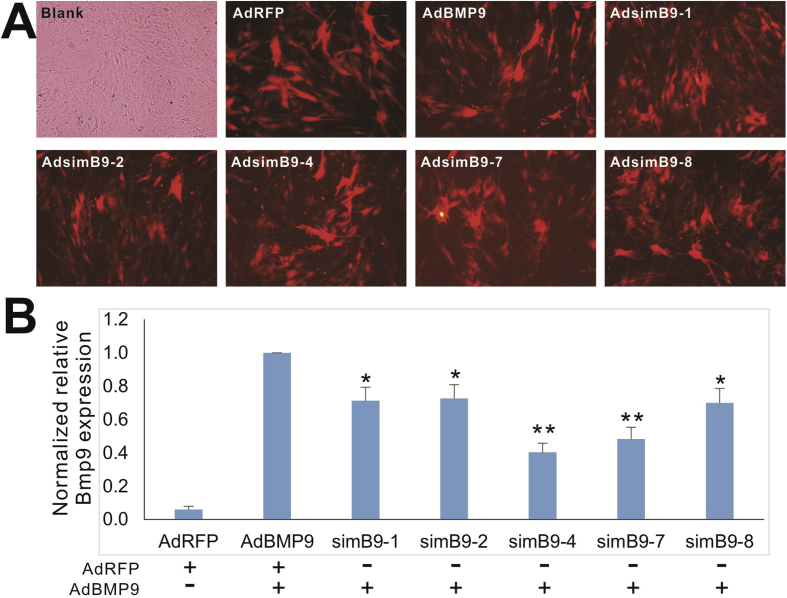
We first examined whether exogenously expressed mouse BMP9 could be effectively silenced by adenovirus-mediated transduction of simB9 siRNAs in MSCs. As expected, the recombinant adenoviruses transduced the iMAD cells with high efficiency (Fig. 2A). Through qPCR analysis, we found that the endogenous BMP9 expression was fairly low in iMADs cells (Fig. 2B). However, the exogenously expressed mouse BMP9 was significantly silenced by the five simB9 siRNAs to various degrees, with simB9-4 and simB9-7 being the most effective (Fig. 2B). It is noteworthy that we constructed two scrambled siRNAs as negative controls and obtained similar results to that of the GFP or RFP control group (data not shown). To simplify the experimental design we thus used Ad-GFP or Ad-RFP as a negative control.
Figure 2.
Characterization of the silencing efficiency of the selected five simB9 siRNAs targeting exogenously expressed mouse BMP9. (A) Efficient transduction of subconfluent iMAD MSCs by recombinant adenoviruses expressing siRNAs targeting mouse Bmp9. RFP signal was recorded at 48 h after infection. Representative results are shown. (B) siRNA-mediated knockdown of exogenously expressed mouse BMP9 in iMADs cells. Subconfluent iMADs were infected with the equal titer of the indicated adenoviruses. At 48 h after infection, total RNA was isolated and subjected to reverse transcription and qPCR analysis of the indicated mouse genes. All samples were normalized with Gapdh. Each assay condition was done in triplicate. “*” p < 0.05, “**” p < 0.01 compared with the AdBMP9-infected group.
Exogenous mouse BMP9-induced osteogenic differentiation can be attenuated by simB9 siRNAs in MSCs
We previously demonstrated that BMP9 is one of the most potent osteogenic factors and yet least studied.24, 28, 29, 30, 76 Using these simB9 siRNA adenoviral vectors, we examined whether BMP9-induced osteogenic differentiation would be effectively hampered by BMP9-specific siRNAs in MSCs. We found that co-expression of mouse BMP9 and its simB9 siRNAs resulted in decreases in early osteogenic marker ALP activity to various degrees both qualitatively (Fig. 3A) and quantitatively (Fig. 3B). Consistent with qPCR findings from Fig. 2B, simB9-4 and simB9-7 were shown to exhibit the highest inhibitory effects on BMP9-induced ALP activity, at as early as day 3 and day 5 (Fig. 3A–a vs 3A-b; and Fig. 3B). Interestingly, simB9-4 and simB9-7 target sites are located more proximal to the 5′-end of the mouse BMP9 coding region than that of the other three siRNA target sites. Nonetheless, the above results suggest that the adenovirus-mediated expression of simBP9 siRNAs, especially simB9-4 and simB9-7, can effectively silence BMP9 expression and hence BMP9-induced osteogenic differentiation in MSCs.
Figure 3.
Adenovirus-mediated expression of simB9 siRNAs significantly diminishes exogenous BMP9-induced ALP activity in MSCs. (A) Qualitative ALP assay. Subconfluent iMADs were infected with the indicated adenoviruses. At day 5 (a) and day 7 (b), the infected cells were subjected to histochemical staining of ALP activity. Each assay condition was done in duplicate. Representative results are shown. (B) Quantitative ALP assay. Subconfluent iMADs were infected with the indicated adenoviruses. At days 3, 5, and 7, the infected cells were subjected to bioluminescence assay of ALP activity. Each assay was done in triplicate. “*” p < 0.05, “**” p < 0.01 compared with the AdBMP9-infected iMADs group.
Synergistic silencing effect between simB9-4 and simB9-7 leads to an effective inhibition of exogenous BMP9-induced osteogenic differentiation of MSCs in vitro
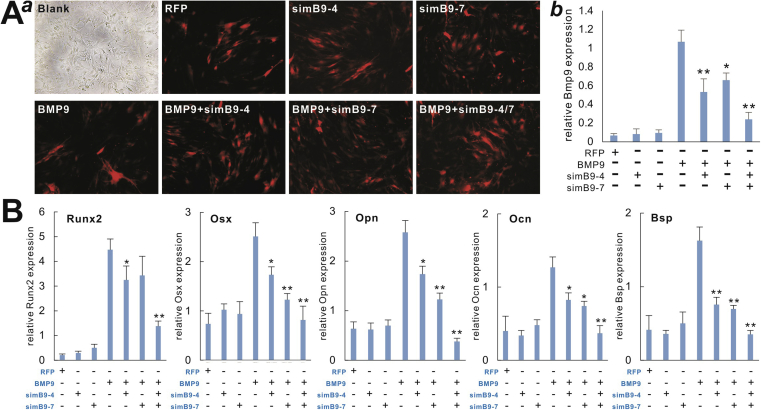
The above results indicate that simB9-4 and simB9-7 may represent two most potent siRNAs targeting mouse BMP9. We further examined whether these two siRNAs would exhibit a synergistic effect on inhibiting BMP9-induced osteogenic differentiation in MSCs. We showed that when the iMADs cells were effectively co-infected with AdBMP9, AdsimB9-4 and/or AdsimB9-7 (Fig. 4A–a) the co-expression of simB9-4 and simB9-7 led to greater reduction of exogenous BMP9 expression than either simB9-4 or simB9-7 siRNA alone (Fig. 4A–b).
Figure 4.
BMP9-induced expression of osteogenic regulators and biomarkers is effectively inhibited by the co-expression of simB9-4 and simB9-7 siRNAs in MSCs. (A) Efficient knockdown of exogenous BMP9 by co-expression of simB9-4 and simB9-7. Subconfluent iMADs were infected with the indicated adenoviruses for 48 h (a) and total RNA was isolated from the cells and subjected to qPCR analysis of mouse Bmp9 expression (b). All samples were normalized with Gapdh. Each assay condition was done in triplicate. “*” p < 0.05, “**” p < 0.01 compared with the AdBMP9-infected group. (B) BMP9-induced expression of osteogenic regulators and biomarkers is effectively inhibited by simB9-4 and simB9-7 siRNAs. Subconfluent iMADs were infected with the indicated adenoviruses. Total RNA was isolated from the cells at day 3 (for Runx2 and Osx) and day 5 (for Opn, Ocn and Bsp), and subjected to qPCR analysis of mouse gene expression. All samples were normalized with Gapdh. Each assay condition was done in triplicate. “*” p < 0.05, “**” p < 0.01 compared with the AdBMP9-infected group.
We next analyzed the effect of co-targeting of BMP9 by simB9-4 and simB9-7 on BMP9-induced expression of osteogenic regulators Runx2 and Osterix (Osx), and found that exogenous BMP9-induced expression of Runx2 and Osx was most significantly inhibited by the co-expression of simB9-4 and simB9-7 siRNAs while either simB9-4 or simB9-7 exerted certain inhibitory effects (Fig. 4B). Furthermore, the three late stage osteogenic markers induced by exogenous BMP9, such as Opn, Ocn and Bsp, exhibited the lowest level of expression in the presence of both simB9-4 and simB9-7, compared with that of either simB9-4 or simB9-7 alone (Fig. 4B).
We also assessed the effect of co-targeting of BMP9 by simB9-4 and simB9-7 on BMP9-induced ALP activity and matrix mineralization in MSCs. While adenovirus-mediated expression of either simB9-4 or simB9-7 alone exhibited inhibitory effects on BMP9-induced ALP activities to varied degrees, a co-expression of simB9-4 and simB9-7 exerted highest inhibitory effects on BMP9-induced ALP activities, as assessed by either histochemical staining (Fig. 5A) or quantitative bioluminescence assay (Fig. 5B). Accordingly, the co-expression of simB9-4 and simB9-7 exerted highest inhibitory effects on BMP9-induced matrix mineralization in iMADs, as assessed by Alizarin Red S staining (Fig. 6AB). Collectively, these results provide strong evidence to demonstrate that simB9-4 and simB9-7 may effectively silence BMP9 expression in MSCs, and subsequently inhibit BMP9-induced osteogenic signaling.
Figure 5.
BMP9-induced ALP activity is effectively inhibited by the co-expression of simB9-4 and simB9-7 siRNAs in MSCs. Subconfluent iMADs were infected with the indicated adenoviruses. At days 3, 5, and 7, the infected cells were subjected to ALP activity assays by either histochemical staining (A), or quantitative bioluminescence assay (B). Each assay was done in triplicate. Representative staining results are shown. “*” p < 0.05, “**” p < 0.01 compared with the AdBMP9-infected iMADs group.
Figure 6.
BMP9-induced matrix mineralization is effectively inhibited by the co-expression of simB9-4 and simB9-7 siRNAs in MSCs. Subconfluent iMADs were infected with the indicated adenoviruses. At days 7 and 11, the infected cells were subjected to Alizarin Red S staining. Staining results were recorded macrographically (A) or under a low power microscope (B). Each assay condition was done in duplicate. Representative staining results are shown.
Synergistic silencing of BMP9 with simB9-4 and simB9-7 effectively diminishes BMP9-induced ectopic bone formation in vivo
To further confirm the knockdown efficiency of BMP9 through the adenovirus-mediated transient co-expression of simB9-4 and simB9-7 in vivo, we also conducted ectopic bone formation assays in athymic nude mice. When the iMADs cells were transduced with AdBMP9 or AdRFP and AdsimB9-4 and/or AdsimB9-7 and injected into the flanks of athymic nude mice, at four weeks after injection apparent bony masses were formed in the BMP9-treated group. The animals injected with iMADs co-infected with AdBMP9, AdsimB9-4 and AdsimB9-7 formed smallest masses, while the masses formed in the animals injected with iMADs co-infected with AdBMP9 and AdsimB9-4 or AdsimB9-7 were smaller than that of AdBMP9 only group (data not shown). Consistent with our earlier studies,52, 63, 69 the iMADs cells transduced with AdRFP, AdsimB9-4 or AdsimB9-7 alone failed to form any detectable masses under the same condition (data not shown).
Histologic evaluation indicated that masses retrieved from BMP9 treatment group exhibited active and robust bone formation, consisting of ample trabecular bone structure and well-mineralized matrices (Fig. 7A). Overexpression of AdsimB9-4 or AdsimB9-7 inhibited BMP9-induced ectopic bone formation and matrix mineralization in iMADs cells (Fig. 7BC). Accordingly, co-expression of AdsimB9-4 and AdsimB9-7 significantly impaired BMP9-induced bone formation and matrix mineralization (Fig. 7D). These in vivo results further demonstrate that co-expression of simB9-4 and simB9-7 may effectively silence BMP9 expression and drastically inhibit exogenous BMP9-induced osteogenic signaling in MSCs.
Figure 7.
BMP9-induced ectopic bone formation is significantly inhibited by the co-expression of simB9-4 and simB9-7 siRNAs in MSCs. Subconfluent iMADs were infected with the indicated adenoviruses for 36 h, and then collected for subcutaneous injection into the flanks of athymic nude mice (n = 5/group). Ectopic bony masses were harvested at 4 weeks after injection and subjected to H & E staining. No masses were retrieved from the groups injected with AdRFP, AdsimB9-4 and AdsimB9-7 infected iMADs. Representative histologic images are shown.
Discussion
As a transforming growth factor β member, BMP9 was originally identified in developing mouse liver.30, 84 In addition to its role in inducing osteogenic differentiation, BMP9 plays important roles in inducing and maintaining embryonic basal forebrain cholinergic neurons, inhibiting hepatic glucose production and inducing the expression of key enzymes of lipid metabolism, stimulating hepcidin 1 expression, and regulating angiogenesis.30 We also found that BMP9 is resistant to the naturally occurring antagonist noggin,85 and demonstrated that TGFβ/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in MSCs.78 As BMP9 is one of the least studied BMP, we performed mechanism-based studies and identified several early downstream targets,30, 31, 70, 82, 86, 87, 88, 89 and demonstrated that BMP9 signaling has extensive cross-talks with other signaling pathways, especially Wnt and Notch signaling.7, 8, 31, 71, 72, 73, 77, 79, 80, 90, 91 Nonetheless, many mechanistic aspects of BMP9 in regulating lineage-specific differentiation of BMP9 remain to be fully understood. In this study, we have developed a highly effective tool to silence mouse Bmp9 expression, which should be useful for delineating the molecular mechanisms underlying BMP9 osteogenic signaling.
RNAi-mediated gene silencing has become a valuable tool for functional studies, reverse genomics, and drug discoveries.33, 34, 35, 36, 37, 38, 40, 41, 42, 43, 44, 45 One major challenge of using RNAi is to identify the most effective short interfering RNAs (siRNAs) sites of a given gene. Despite several published bioinformatic prediction models, the process to select and validate optimal siRNA sites for a given gene is empirical and laborious.39, 49 In this study, we analyzed five siRNAs targeting mouse Bmp9 and found that simB9-4 and simB9-7 exhibited highest silencing efficiency, while other three simB9 siRNAs also were able to silence Bmp9 expression to varied extents. Interestingly, both simB9-4 and simB9-7 are located at the 5′-end of Bmp9 coding region, compared with other three simB9 siRNAs, suggesting siRNAs located at 5′-end of coding regions may silence target genes more efficiently. Furthermore, we have demonstrated that simB9-4 and simB9-7 can act synergistically in terms of silencing Bmp9 expression and hence its biological activities, indicating that it is highly beneficial to combine multiple siRNAs in order to accomplish high knockdown efficiency.
Efficient introduction and sustained expression of siRNAs in mammalian cells are important perquisites for effective target gene silencing.40, 41, 42, 43, 44, 45, 92 One common-used approach is to transfect mammalian cells with synthetic siRNAs, However, this approach is limited by transfection efficiency and short-term transient knockdown, and can be only used for cultured cells in vitro. Thus, a more commonly-used approach is to express short hairpin RNAs (shRNAs) or siRNAs in cells.43, 44, 92 In this case, the shRNA or siRNA expression of siRNAs is driven by Pol III promoters such U6 and H192. In our studies, the siRNAs are driven by converging U6 and H1 promoters, yielding functional siRNAs without the requirement of additional processing by RISC machinery, which is significantly advantageous over shRNA processing.39, 49 Another formidable technical challenge is to effectively deliver RNAi expression vectors into mammalian cells in order to knock down target genes with high efficiency. Among many exogenous gene delivery methods, recombinant adenovirus-based delivery system remains as one of the most favorable strategies because of its high efficiency in vitro and in vivo.59, 62, 93 In this study, we have demonstrated that adenovirus-mediated expression of siRNAs can achieve effective gene silencing both in vitro and in vivo. Thus, adenoviral vectors should be useful for effective delivery of siRNAs into mammalian cells in vitro and/or animal tissues in vivo.
It should point out that the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated 9 (Cas9), or CRISPR/Cas9-based genome editing technology has recently become a popular tool for gene manipulations.54, 94, 95, 96, 97, 98, 99, 100, 101, 102 CRISPR/Cas9 is a recently developed and naturally occurred genome editing tool, which is used by bacteria for immune defense.94, 95, 96 CRISPR RNA-guided Cas9 nucleases have been shown to work in a wide range of model organisms and mediate genome editing by introducing a double-stranded break (DSB) in a target DNA sequence, which in turn can lead to the efficient generation of insertions or deletion mutations (indels) through nonhomologous end-joining repair, or in the presence of an appropriately designed homologous donor DNA template, precise DNA alterations can be made through homology-directed repair of the DSB.54, 94, 95, 96, 97, 98, 99, 100, 101, 102 While CRISPR/Cas9 and related Cas nuclease systems will continue to evolve and undoubtedly change the landscape of genome editing and gene manipulations, the gene editing efficiency in cultured cells is limited by the technical challenges in expressing multiple components including Cas9 nuclease and small guide-RNAs, and/or delivering homologous donor DNA templates. While it is conceivable that the BMP9 gene can be deleted at genome level, the technical challenges will prevent accomplishing such feat effectively in cultured MSCs. Thus, siRNA-mediated knockdown of Bmp9 expression in MSCs provides a viable alternative to investigate the loss of function effect on BMP9-induced osteogenic differentiation of mesenchymal stem cells.
In summary, using different siRNA bioinformatic prediction programs, we designed five simB9 siRNAs, which were expressed under the control of converging H1 and U6 promoters in recombinant adenovirus vectors. We demonstrated that simB9-4 and simB9-7 exhibited the highest efficiency on silencing exogenous mouse BMP9 in MSCs individually. Furthermore, simB9-4 and simB9-7 acted synergistically in inhibiting exogenous BMP9-induced expression of osteogenic markers, matrix mineralization and ectopic bone formation from MSCs. Thus, the characterized simB9 siRNAs may be used as an important tool to investigate the molecular mechanism behind BMP9 osteogenic signaling. Furthermore, our results indicate that recombinant adenovirus-mediated expression of siRNAs is efficient and sustained, and thus may be used as a potential delivery vehicle of siRNA therapeutics.
Conflicts of interests
The authors declare no competing interests.
Acknowledgements
The reported work was supported in part by research grants from the National Institutes of Health (CA226303, DE020140 to TCH and RRR), the U.S. Department of Defense (OR130096 to JMW), the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust (RRR, TCH), the Scoliosis Research Society (TCH and MJL), and the National Key Research and Development Program of China (2016YFC1000803 and 2011CB707906 to TCH). Funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.gendis.2018.04.006.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Caplan A.I., Bruder S.P. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7(6):259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 3.Deng Z.L., Sharff K.A., Tang N. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 4.Rastegar F., Shenaq D., Huang J. Mesenchymal stem cells: molecular characteristics and clinical applications. World J Stem Cell. 2010;2(4):67–80. doi: 10.4252/wjsc.v2.i4.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shenaq D.S., Rastegar F., Petkovic D. Mesenchymal progenitor cells and their orthopedic applications: forging a path towards clinical trials. Stem Cell Int. 2010;2010:519028. doi: 10.4061/2010/519028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teven C.M., Liu X., Hu N. Epigenetic regulation of mesenchymal stem cells: a focus on osteogenic and adipogenic differentiation. Stem Cell Int. 2011;2011:201371. doi: 10.4061/2011/201371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao J., Wei Q., Zou Y. Notch signaling augments BMP9-induced bone formation by promoting the osteogenesis-angiogenesis coupling process in mesenchymal stem cells (MSCs) Cell Physiol Biochem. 2017;41(5):1905–1923. doi: 10.1159/000471945. [DOI] [PubMed] [Google Scholar]
- 8.Liao J., Yu X., Hu X. lncRNA H19 mediates BMP9-induced osteogenic differentiation of mesenchymal stem cells (MSCs) through Notch signaling. Oncotarget. 2017;8(32):53581–53601. doi: 10.18632/oncotarget.18655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noel D., Djouad F., Jorgense C. Regenerative medicine through mesenchymal stem cells for bone and cartilage repair. Curr Opin Investig Drugs. 2002;3(7):1000–1004. [PubMed] [Google Scholar]
- 10.Chan J.L., Tang K.C., Patel A.P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006;107(12):4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corcione A., Benvenuto F., Ferretti E. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 12.Djouad F., Charbonnier L.M., Bouffi C. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25(8):2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 13.Olsen B.R., Reginato A.M., Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 14.Raucci A., Bellosta P., Grassi R., Basilico C., Mansukhani A. Osteoblast proliferation or differentiation is regulated by relative strengths of opposing signaling pathways. J Cell Physiol. 2008;215(2):442–451. doi: 10.1002/jcp.21323. [DOI] [PubMed] [Google Scholar]
- 15.Kim J.H., Liu X., Wang J. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther Adv Musculoskelet Dis. 2013;5(1):13–31. doi: 10.1177/1759720X12466608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang K., Wang X., Zhang H. The evolving roles of canonical WNT signaling in stem cells and tumorigenesis: implications in targeted cancer therapies. Lab Invest. 2016;96(2):116–136. doi: 10.1038/labinvest.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denduluri S.K., Idowu O., Wang Z. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015;2(1):13–25. doi: 10.1016/j.gendis.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teven C.M., Farina E.M., Rivas J., Reid R.R. Fibroblast growth factor (FGF) signaling in development and skeletal diseases. Genes Dis. 2014;1(2):199–213. doi: 10.1016/j.gendis.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo A., Denduluri S.K., Zhang B. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014;1(2):149–161. doi: 10.1016/j.gendis.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louvi A., Artavanis-Tsakonas S. Notch and disease: a growing field. Semin Cell Dev Biol. 2012;23(4):473–480. doi: 10.1016/j.semcdb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanotti S., Canalis E. Notch and the skeleton. Mol Cell Biol. 2010;30(4):886–896. doi: 10.1128/MCB.01285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guruharsha K.G., Kankel M.W., Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13(9):654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F., Song J., Zhang H. Wnt and BMP signaling crosstalk in regulating dental stem cells: implications in dental tissue engineering. Genes Dis. 2016;3(4):263–276. doi: 10.1016/j.gendis.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luu H.H., Song W.X., Luo X. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25(5):665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 25.Wang R.N., Green J., Wang Z. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddi A.H. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16(3):247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 27.Zou H., Choe K.M., Lu Y., Massague J., Niswander L. BMP signaling and vertebrate limb development. Cold Spring Harbor Symp Quant Biol. 1997;62:269–272. [PubMed] [Google Scholar]
- 28.Cheng H., Jiang W., Phillips F.M. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Kang Q., Sun M.H., Cheng H. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11(17):1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 30.Luther G., Wagner E.R., Zhu G. BMP-9 induced osteogenic differentiation of mesenchymal stem cells: molecular mechanism and therapeutic potential. Curr Gene Ther. 2011;11(3):229–240. doi: 10.2174/156652311795684777. [DOI] [PubMed] [Google Scholar]
- 31.Lamplot J.D., Qin J., Nan G. BMP9 signaling in stem cell differentiation and osteogenesis. Am J Stem Cells. 2013;2(1):1–21. [PMC free article] [PubMed] [Google Scholar]
- 32.Hammond S.M., Bernstein E., Beach D., Hannon G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404(6775):293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 33.Castel S.E., Martienssen R.A. RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. Nat Rev Genet. 2013;14(2):100–112. doi: 10.1038/nrg3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dykxhoorn D.M., Novina C.D., Sharp P.A. Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol. 2003;4(6):457–467. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- 35.Ghildiyal M., Zamore P.D. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10(2):94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammond S.M., Caudy A.A., Hannon G.J. Post-transcriptional gene silencing by double-stranded RNA. Nat Rev Genet. 2001;2(2):110–119. doi: 10.1038/35052556. [DOI] [PubMed] [Google Scholar]
- 37.Okamura K., Lai E.C. Endogenous small interfering RNAs in animals. Nat Rev Mol Cell Biol. 2008;9(9):673–678. doi: 10.1038/nrm2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkies P., Miska E.A. Small RNAs break out: the molecular cell biology of mobile small RNAs. Nat Rev Mol Cell Biol. 2014;15(8):525–535. doi: 10.1038/nrm3840. [DOI] [PubMed] [Google Scholar]
- 39.Deng F., Chen X., Liao Z. A simplified and versatile system for the simultaneous expression of multiple siRNAs in mammalian cells using Gibson DNA Assembly. PLoS One. 2014;9(11):e113064. doi: 10.1371/journal.pone.0113064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bumcrot D., Manoharan M., Koteliansky V., Sah D.W. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2(12):711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czech M.P., Aouadi M., Tesz G.J. RNAi-based therapeutic strategies for metabolic disease. Nat Rev Endocrinol. 2011;7(8):473–484. doi: 10.1038/nrendo.2011.57. [DOI] [PubMed] [Google Scholar]
- 42.de Fougerolles A., Vornlocher H.P., Maraganore J., Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6(6):443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iorns E., Lord C.J., Turner N., Ashworth A. Utilizing RNA interference to enhance cancer drug discovery. Nat Rev Drug Discov. 2007;6(7):556–568. doi: 10.1038/nrd2355. [DOI] [PubMed] [Google Scholar]
- 44.Kim D.H., Rossi J.J. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8(3):173–184. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 45.Pecot C.V., Calin G.A., Coleman R.L., Lopez-Berestein G., Sood A.K. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11(1):59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshinari K., Miyagishi M., Taira K. Effects on RNAi of the tight structure, sequence and position of the targeted region. Nucleic Acids Res. 2004;32(2):691–699. doi: 10.1093/nar/gkh221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudek P., Picard D.T.R.O.D. T7 RNAi oligo designer. Nucleic Acids Res. 2004;32(Web Server issue):W121–W123. doi: 10.1093/nar/gkh360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins R.E., Cheng X. Structural domains in RNAi. FEBS Lett. 2005;579(26):5841–5849. doi: 10.1016/j.febslet.2005.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo Q., Kang Q., Song W.X. Selection and validation of optimal siRNA target sites for RNAi-mediated gene silencing. Gene. 2007;395(1–2):160–169. doi: 10.1016/j.gene.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 50.Wu N., Zhang H., Deng F. Overexpression of Ad5 precursor terminal protein accelerates recombinant adenovirus packaging and amplification in HEK-293 packaging cells. Gene Ther. 2014;21(7):629–637. doi: 10.1038/gt.2014.40. [DOI] [PubMed] [Google Scholar]
- 51.Wei Q., Fan J., Liao J. Engineering the rapid adenovirus production and amplification (RAPA) cell line to expedite the generation of recombinant adenoviruses. Cell Physiol Biochem. 2017;41(6):2383–2398. doi: 10.1159/000475909. [DOI] [PubMed] [Google Scholar]
- 52.Lu S., Wang J., Ye J. Bone morphogenetic protein 9 (BMP9) induces effective bone formation from reversibly immortalized multipotent adipose-derived (iMAD) mesenchymal stem cells. Am J Transl Res. 2016;8(9):3710–3730. [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao C., Jiang W., Zhou N. Sox9 augments BMP2-induced chondrogenic differentiation by downregulating Smad7 in mesenchymal stem cells (MSCs) Genes Dis. 2017;4(4):229–239. doi: 10.1016/j.gendis.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu X., Li L., Yu X. CRISPR/Cas9-mediated reversibly immortalized mouse bone marrow stromal stem cells (BMSCs) retain multipotent features of mesenchymal stem cells (MSCs) Oncotarget. 2017;8(67):111847–111865. doi: 10.18632/oncotarget.22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao J., Wei Q., Fan J. Characterization of retroviral infectivity and superinfection resistance during retrovirus-mediated transduction of mammalian cells. Gene Ther. 2017;24(6):333–341. doi: 10.1038/gt.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang J., Bi Y., Zhu G.H. Retinoic acid signalling induces the differentiation of mouse fetal liver-derived hepatic progenitor cells. Liver Int. 2009;29(10):1569–1581. doi: 10.1111/j.1478-3231.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 57.He T.C., Zhou S., da Costa L.T., Yu J., Kinzler K.W., Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95(5):2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo J., Deng Z.L., Luo X. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2(5):1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 59.Lee C.S., Bishop E.S., Zhang R. Adenovirus-mediated gene delivery: potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017;4(2):43–63. doi: 10.1016/j.gendis.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan J., Wei Q., Liao J. Noncanonical Wnt signaling plays an important role in modulating canonical Wnt-regulated stemness, proliferation and terminal differentiation of hepatic progenitors. Oncotarget. 2017;8(16):27105–27119. doi: 10.18632/oncotarget.15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song D., Zhang F., Reid R.R. BMP9 induces osteogenesis and adipogenesis in the immortalized human cranial suture progenitors from the patent sutures of craniosynostosis patients. J Cell Mol Med. 2017;21(11):2782–2795. doi: 10.1111/jcmm.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang N., Zhang H., Zhang B.Q. Adenovirus-mediated efficient gene transfer into cultured three-dimensional organoids. PLoS One. 2014;9(4):e93608. doi: 10.1371/journal.pone.0093608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang N., Zhang W., Cui J. The piggyBac transposon-mediated expression of SV40 T antigen efficiently immortalizes mouse embryonic fibroblasts (MEFs) PLoS One. 2014;9(5):e97316. doi: 10.1371/journal.pone.0097316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao C., Wu N., Deng F. Adenovirus-mediated gene transfer in mesenchymal stem cells can be significantly enhanced by the cationic polymer polybrene. PLoS One. 2014;9(3):e92908. doi: 10.1371/journal.pone.0092908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Q., Wang J., Deng F. TqPCR: a touchdown qPCR assay with significantly improved detection sensitivity and amplification efficiency of SYBR Green qPCR. PLoS One. 2015;10(7):e0132666. doi: 10.1371/journal.pone.0132666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye J., Wang J., Zhu Y. A thermoresponsive polydiolcitrate-gelatin scaffold and delivery system mediates effective bone formation from BMP9-transduced mesenchymal stem cells. Biomed Mater. 2016;11(2):025021. doi: 10.1088/1748-6041/11/2/025021. [DOI] [PubMed] [Google Scholar]
- 67.Li Y., Wagner E.R., Yan Z. The calcium-binding protein S100A6 accelerates human osteosarcoma growth by promoting cell proliferation and inhibiting osteogenic differentiation. Cell Physiol Biochem. 2015;37(6):2375–2392. doi: 10.1159/000438591. [DOI] [PubMed] [Google Scholar]
- 68.Li R., Yan Z., Ye J. The prodomain-containing BMP9 produced from a stable line effectively regulates the differentiation of mesenchymal stem cells. Int J Med Sci. 2016;13(1):8–18. doi: 10.7150/ijms.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang E., Bi Y., Jiang W. Conditionally immortalized mouse embryonic fibroblasts retain proliferative activity without compromising multipotent differentiation potential. PLoS One. 2012;7(2):e32428. doi: 10.1371/journal.pone.0032428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang E., Zhu G., Jiang W. Growth hormone synergizes with BMP9 in osteogenic differentiation by activating the JAK/STAT/IGF1 pathway in murine multilineage cells. J Bone Miner Res. 2012;27(7):1566–1575. doi: 10.1002/jbmr.1622. [DOI] [PubMed] [Google Scholar]
- 71.Tang N., Song W.X., Luo J. BMP9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signaling. J Cell Mol Med. 2009;13(8B):2448–2464. doi: 10.1111/j.1582-4934.2008.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H., Wang J., Deng F. Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs) Biomaterials. 2015;39:145–154. doi: 10.1016/j.biomaterials.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu X., Qin J., Luo Q. Cross-talk between EGF and BMP9 signalling pathways regulates the osteogenic differentiation of mesenchymal stem cells. J Cell Mol Med. 2013;17(9):1160–1172. doi: 10.1111/jcmm.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J., Liao J., Zhang F. Nel-like Molecule-1 (Nell1) is regulated by bone morphogenetic protein 9 (BMP9) and potentiates BMP9-induced osteogenic differentiation at the expense of adipogenesis in mesenchymal stem cells. Cell Physiol Biochem. 2017;41(2):484–500. doi: 10.1159/000456885. [DOI] [PubMed] [Google Scholar]
- 75.Wang J., Zhang H., Zhang W. Bone morphogenetic Protein-9 (BMP9) effectively induces osteo/odontoblastic differentiation of the reversibly immortalized stem cells of dental apical papilla. Stem Cell Dev. 2014;23(12):1405–1416. doi: 10.1089/scd.2013.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang Q., Song W.X., Luo Q. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cell Dev. 2009;18(4):545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li R., Zhang W., Cui J. Targeting BMP9-promoted human osteosarcoma growth by inactivation of notch signaling. Curr Cancer Drug Targets. 2014;14(3):274–285. doi: 10.2174/1568009614666140305105805. [DOI] [PubMed] [Google Scholar]
- 78.Luo J., Tang M., Huang J. TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J Biol Chem. 2010;285(38):29588–29598. doi: 10.1074/jbc.M110.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu N., Jiang D., Huang E. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J Cell Sci. 2013;126(Pt 2):532–541. doi: 10.1242/jcs.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L., Jiang W., Huang J. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res. 2010;25(11):2447–2459. doi: 10.1002/jbmr.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang F., Li Y., Zhang H. Anthelmintic mebendazole enhances cisplatin's effect on suppressing cell proliferation and promotes differentiation of head and neck squamous cell carcinoma (HNSCC) Oncotarget. 2017;8(8):12968–12982. doi: 10.18632/oncotarget.14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J., Weng Y., Liu X. Endoplasmic reticulum (ER) stress inducible factor cysteine-rich with EGF-like domains 2 (Creld2) is an important mediator of BMP9-regulated osteogenic differentiation of mesenchymal stem cells. PLoS One. 2013;8(9):e73086. doi: 10.1371/journal.pone.0073086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao Y., Huang E., Zhang H. Crosstalk between Wnt/beta-catenin and estrogen receptor signaling synergistically promotes osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2013;8(12):e82436. doi: 10.1371/journal.pone.0082436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song J.J., Celeste A.J., Kong F.M., Jirtle R.L., Rosen V., Thies R.S. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology. 1995;136(10):4293–4297. doi: 10.1210/endo.136.10.7664647. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y., Hong S., Li M. Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J Orthop Res. 2013;31(11):1796–1803. doi: 10.1002/jor.22427. [DOI] [PubMed] [Google Scholar]
- 86.Peng Y., Kang Q., Cheng H. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J Cell Biochem. 2003;90(6):1149–1165. doi: 10.1002/jcb.10744. [DOI] [PubMed] [Google Scholar]
- 87.Peng Y., Kang Q., Luo Q. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279(31):32941–32949. doi: 10.1074/jbc.M403344200. [DOI] [PubMed] [Google Scholar]
- 88.Luo Q., Kang Q., Si W. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279(53):55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 89.Sharff K.A., Song W.X., Luo X. Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. J Biol Chem. 2009;284(1):649–659. doi: 10.1074/jbc.M806389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang W., Deng Z.L., Chen L. Retinoic acids potentiate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2010;5(7):e11917. doi: 10.1371/journal.pone.0011917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang H., Li L., Dong Q. Activation of PKA/CREB signaling is involved in BMP9-induced osteogenic differentiation of mesenchymal stem cells. Cell Physiol Biochem. 2015;37(2):548–562. doi: 10.1159/000430376. [DOI] [PubMed] [Google Scholar]
- 92.Fellmann C., Lowe S.W. Stable RNA interference rules for silencing. Nat Cell Biol. 2014;16(1):10–18. doi: 10.1038/ncb2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Breyer B., Jiang W., Cheng H. Adenoviral vector-mediated gene transfer for human gene therapy. Curr Gene Ther. 2001;1(2):149–162. doi: 10.2174/1566523013348689. [DOI] [PubMed] [Google Scholar]
- 94.Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Doudna J.A., Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 96.Dominguez A.A., Lim W.A., Qi L.S. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat Rev Mol Cell Biol. 2016;17(1):5–15. doi: 10.1038/nrm.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim H., Kim J.S. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15(5):321–334. doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- 98.Zhang H., Yan Z., Li M., Peabody M., He T.C. CRISPR clear? Dimeric Cas9-Fok1 nucleases improve genome-editing specificity. Genes Dis. 2014;1(1):6–7. doi: 10.1016/j.gendis.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mali P., Esvelt K.M., Church G.M. Cas9 as a versatile tool for engineering biology. Nat Methods. 2013;10(10):957–963. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sander J.D., Joung J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2015;32(4):347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang H., La Russa M., Qi L.S. CRISPR/Cas9 in genome editing and beyond. Annu Rev Biochem. 2016;85:227–264. doi: 10.1146/annurev-biochem-060815-014607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.