Abstract
The hurdles in realizing successful cancer immunotherapy stem from the fact that cancer patients are either refractory to immune response and/or develop resistance. Here, we propose that the sephenomena are due,inpart,to the deployment/secretion of a “decoy flare,” for example, anomalous cancer-associated antigens by the tumor cells. The cancer secretome, which resembles the parent cell make-up, is composed of soluble macromolecules (proteins, glycans, lipids, DNAs, RNAs, etc.) and insoluble vesicles (exosomes),thus hindering cancerdetection/recognition by immuno the rapeutic agents, resulting in a “cancer-stealth” effect. Immunotherapy, or any treatment that relies on antigens’ expression/ function,couldbe improved by the understanding of the properties of the cancer secretome, as its clinical evaluation may change the therapeutic landscape.
Introduction
Cancers have developed various stratagems to propagate within an immunocompetent host. Immune regulation and surveillance play an imperative role in early attempts of cancer prevention by the host. This is evident by the presence of endogenous anticancer-associated antibodies, that is, autoantibodies (AAb), which occur as a result of immunosurveillance by which the immune system recognizes and destroys normal cells that have transitioned to malignancy (1). Because of their specificity and stability in the sera, AAbs are regarded as early and sensitive serologic biomarkers for the diagnosis and prognosis of cancer and may serve as a potential means of identifying and tracking (1, 2). However, despite their presence, cancers do develop and progress, and the possible beneficial role of AABs to the patients’ clinical outcome is still unsettled and seems to differ among cancer types and AAb specificity. To explore the presence and functionality of AAbs, in a recent clinical study, we identified the presence of two forms of cancer AAbs: (i) noninducible/nonfunctional (galectin-3) and (ii) inducible/functional [prostate-specific antigen (PSA)]. Both antigens induce malignancy and are biomarkers of prostate cancer progression (3–7). The study highlighted that PSA AAbs alter PSA levels, elucidating that the antigen– AAb interactions are a part of cancer–host immune defense (8). It was also noted that AAbs’ response may be due to tumor-associated antigens’ cross-presentation by tumor-derived exosomes, as they resemble the parental cell make-up and thus may also interfere with antitumor immunotherapies (9). In addition, it was reported that cancer patient-derived AAbs recognize tumor antigens on exosomes (10). Taken together, these studies suggest that soluble and membrane-bound cancer-associated antigens both elicit immune response and interfere with host immunity, leading to cancer cells’ escape from immunosurveillance.
Here, we propose that cancers resort to a “self-defense” mechanism that shields them from auto- and exo-Abs by deploying “decoy flares” (soluble and insoluble antigens) that may shield them and thus hinder therapeutic intervention (Fig. 1).
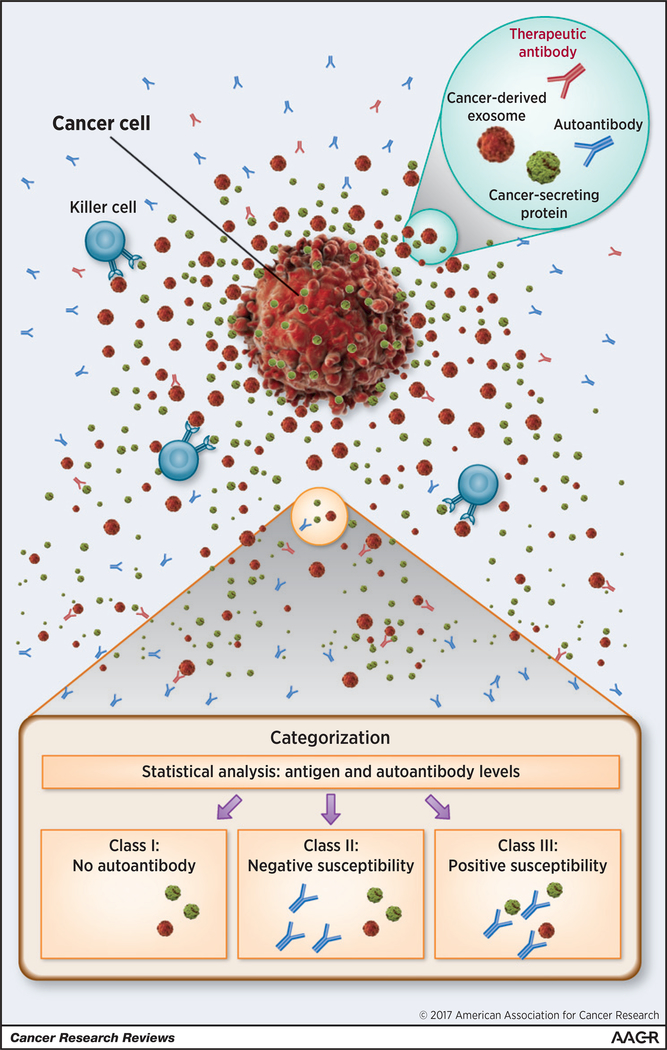
Figure 1.
The complexity of cancer secretory elements and immune interventions is illustrated. Cancer cell releases secretory factors composed of cancer-derived soluble antigens (green) and exosomes (red). Endogenous/exogenous antibodies, that is, patient-derived AAbs (blue) and therapeutic AAbs (red) are blocked due to the cancer secretory stealth effect. Killer cells, for example, natural killer cells, T lymphocytes, tumor-associated macrophages, neutrophils, and dendritic cells (light blue) may also join the reactions. On the basis of correlation analyses between AAbs and tumor-derived factors, the tumor-derived factors may be classified into three groups: class I, not inducible; class II, not susceptible; class III, positive susceptibility. The cancer cell image was purchased from Shutterstock.
Cancer-Secretory Immunity: Cause and Effect
Cancer-secretory autoimmunity is a collection of complex networks of cancer-derived molecules and patient-derived AAbs that coordinate the self-defense needed to protect the host from the developing neoplasm. It should be noted that the AAbs’ recognition site of the functional domain of cancer-secretory antigen needs to be evaluated in precise detail. For example, cancer cells have been known to secrete mutated proteins and/or produce posttranslational modifications, such as altered glycosylation (11, 12). The recognition of these molecular alterations is crucial in understanding the composition of the cancer secretome. The AAbs’ response may be specific for the altered target molecules, as they were shown to recognize and interact with proteins harboring a point mutation and single codon deletion (13), as well as detect aberrant protein glycosylation associated with oncogenic transformation (14). Thus, the secretion of mutated protein/ aberrant glycosylation may be a molecular mimicry (a decoy flare) to immune systems, masking the presence of cancer cells.
It has been estimated that the circulatory system of patients with cancer contains approximately 2 ×1012 more exosomes compared with that of healthy volunteers (15, 16). The robust exosome release leads to exosome-mediated shielding of target cells as a critical determinant of tumor cell susceptibility to antibody therapy (17). The cancer-derived exosomes efficiently bind and sequester anticancer antibodies and dramatically reduce their binding to cancer cells and escape immunosurveillance (18). The above-mentioned diverse cancer-masking phenomena are used by cancers to disguise and shield them from AAbs and should be preclinically considered.
Mouse models of spontaneous cancer showed that despite the presence of cancer AAbs, cancers developed (19), implying that AAbs are either nonfunctional or the “cancer stealth” effect neutralizes host autoimmunity. In fact, the proteomic analysis using a spontaneous cancer model showed that only a portion of AAbs passed through the shielding and interacted with cancer cells (13, 14). Considering that >2,000 AAbs have been identified in cancer patient sera, most may be eliminated by “cancer stealth” effect in systemic circulation or tumor interstitial fluid.
Thus, a possible cause for failure of immune therapy may be a “cancer stealth” effect, depicting the interactions between a cancer secretome acting as a “decoy flare” and patient AAbs/therapeutic AAb and/or cancer-attacking immune cells (Fig. 1). The immune conflicts shown above can be assessed in a clinical setting and are outlined below.
Cancer Autoantibody Influence: A Clinical Categorization of Cancer-Secretory Factors
Various methodologies have been utilized for the detection of multiple AAbs, as summarized by Zaenker and colleagues and Rich and colleagues (1, 2). Most of these methods are labor intensive, with sensitivities varying from 12% to 95%. A recent clinical study offers insight into elements that potentially affect the functionality of cancer AAbs (8). The levels of circulating cancer-secretory antigens and AAbs were determined, followed by mathematical calculations using correlation tests and regression modeling, yielding a correlation coefficient and fitted slope values. The values were used to predict a possible association between the two variables, allowing the influential level to be estimated (8). Subsequently, the values have classified the cancer-derived macromolecules into three categories: class 1, not inducible; class 2, not susceptible; class 3, statistically significant positive susceptibility. AAbs in the case of either class 1 or 2 were near zero or nonsignificant (Fig. 1). This categorization may be used to further the understanding of the complexity of the cancer-associated AAbs and the secretome, where tumor type and/or molecular structural categorization have been the primary method to classify the secretory factors (15). The above criteria may allow a refined clinical grading, “Patient Autoantibody-based Grading.” Subsequently, for class I, additional therapeutic antibodies essentially need to be administered, as host immunity does not produce AAbs against the cancer-derived antigens, for example, rituximab targeting CD20 and cetuximab targeting EGFR, as seen in current clinical settings. For class II, an alternative therapeutic method could be considered based on the anticipated potential factors affecting AAbs’ function as described, for example, targeting cancer-specific exosome(s) (16). For class 3, where tumor-derived antigens may possibly react to host AAbs, immunotherapy, such as tumor vaccines, may be considered as a therapeutic priority to induce further production of intrinsic antibodies.
As examples in clinical practice, there is increasing evidence that therapeutic targeting of secretory factors in the tumor microenvironment improves patients’ clinical outcomes, for example, VEGF-A, an angiogenic factor activated in various cancers, and RANKL, an osteoclastogenic factor of bone metastasis. Exogenous antibodies targeting these molecules, bevacizumab and denosumab, respectively, have been administered with no consideration of patient’s endogenous antibody, that is, AAb status. Similarly, clinical trials of therapeutic antibodies targeting tumor surface proteins have not been too encouraging. Indeed, considering that the primary location of expression is the cell membrane, it may need additional approaches or methodologies to quantitate and estimate the immune interactions, although portions of the antigens, such as CD20, EGFR, and HER2, are released to extracellular space (17, 18). Given that the AAbs can be either functional or nonfunctional, it is reasonable to consider the AAb status and the stealth effect in clinical trials, which may enhance the efficacy of immunotherapy.
Conclusion and Future Perspective
In the search for personalized immune medicine, cancer patient AAbs and the secretome make-up status need to be guiding elements prior to implementation of immunotherapies, as overlooking them may derail clinical interventions and lead to unsuccessful results (4). Further development of new therapeutic modalities based on the clinical categorization of the cancer secretome is necessary to interrupt the cancer self-defense that leads to a stealth effect. Furthermore, this could be tested by proteomic analysis of the sera following cancer development in transgenic mice or use of mice with “knock-in” and conditional specific gene-targeting strategies. These models that are reflective of the human cancers could provide an opportunity to study the immunotherapy during cancer progression.
Acknowledgments
Grant Support
This work was supported by an internal grant from Karmanos Cancer Institute (to A. Raz), the Paul Zuckerman Endowment (to A. Raz) and by the NCI at the NIH, Cancer Center Support Grant (CA-22453 to G. Bepler).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zaenker P, Gray ES, Ziman MR. Autoantibody production in cancer–the humoral immune response toward autologous antigens in cancer patients. Autoimmun Rev 2016;15:477–83. [DOI] [PubMed] [Google Scholar]
- 2.Rich BS, Honeyman JN, Darcy DG, Smith PT, Williams AR, Lim II, et al. Endogenous antibodies for tumor detection. Sci Rep 2014;4:5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balan V, Nangia-Makker P, Kho DH, Wang Y, Raz A. Tyrosine-phosphorylated galectin-3 protein is resistant to prostate-specific antigen (PSA) cleavage. J Biol Chem 2012;287:5192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakajima K, Heilbrun LK, Hogan V, Smith D, Heath E, Raz A. Positive associations between galectin-3 and PSA levels in prostate cancer patients: a prospective clinical study-I. Oncotarget 2016;7: 82266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Balan V, Gao X, Reddy PG, Kho D, Tait L, et al. The significance of galectin-3 as a new basal cell marker in prostate cancer. Cell Death Dis 2013;4:e753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Nangia-Makker P, Tait L, Balan V, Hogan V, Pienta KJ, et al. Regulation of prostate cancer progression by galectin-3. Am J Pathol 2009;174:1515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams SA, Jelinek CA, Litvinov I, Cotter RJ, Isaacs JT, Denmeade SR. Enzymatically active prostate-specific antigen promotes growth of human prostate cancers. Prostate 2011;71: 1595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima K, Heilbrun LK, Smith D, Hogan V, Raz A,Heath E.The influence of PSA autoantibodies in prostate cancer patients: a prospective clinical study-II. Oncotarget 2017;8:17643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiteside TL. Tumor-derived exosomes and their role in tumor-inducedimmune suppression. Vaccines 2016;4:pii:E35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberson CD, Atay S, Gercel-Taylor C, Taylor DD. Tumor-derived exosomesas mediators ofdisease and potential diagnostic biomarkers. Cancer Biomark 2010;8:281–91. [DOI] [PubMed] [Google Scholar]
- 11.Wandall HH, Blixt O, Tarp MA, Pedersen JW, Bennett EP, Mandel U,et al. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res 2010;70:1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathivanan S, Ji H, Tauro BJ, Chen YS, Simpson RJ. Identifying mutatedproteins secreted by colon cancer cell lines using mass spectrometry. J Proteomics 2012;76:141–9. [DOI] [PubMed] [Google Scholar]
- 13.Rich BS, Honeyman JN, Darcy DG, Smith PT, Williams AR, Lim II, et al. Endogenous antibodies for tumor detection. Sci Rep 2014;4:5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladd JJ, Chao T, Johnson MM, Qiu J, Chin A, Israel R, et al. Autoantibody signatures involving glycolysis and splicesome proteins precede a diagnosis of breast cancer among postmenopausal women. Cancer Res 2013;73:1502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feizi A, Banaei-Esfahani A, Nielsen J. HCSD: the human cancer secretome database. Database (Oxford) 2015;2015:bav051. [DOI] [PMC free article] [PubMed]
- 16.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015;523:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A,et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 2008;10: 619–24. [DOI] [PubMed] [Google Scholar]
- 18.Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M,et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol 2012;227:658–67. [DOI] [PubMed] [Google Scholar]
- 19.Barderas R, Villar-Vazquez R, Fernandez-Acenero MJ, Babel I, PelaezGarcia A, Torres S, et al. Sporadic colon cancer murine models demonstrate the value of autoantibody detection for preclinical cancer diagnosis. Sci Rep 2013;3:2938. [DOI] [PMC free article] [PubMed] [Google Scholar]