Abstract
Polymeric unimolecular nanoparticles (NPs) exhibiting a core-shell structure and formed by a single multi-arm molecule containing only covalent bonds have attracted increasing attention for numerous biomedical applications. This unique single-molecular architecture provides the unimolecular NP with superior stability both in vitro and in vivo, a high drug loading capacity, as well as versatile surface chemistry, thereby making it a desirable nanoplatform for therapeutic and diagnostic applications. In this review, we surveyed the architecture of various types of polymeric unimolecular NPs, including water-dispersible unimolecular micelles and water-soluble unimolecular NPs used for the delivery of hydrophobic and hydrophilic agents, respectively, as well as their diverse biomedical applications. Future opportunities and challenges of unimolecular NPs were also briefly discussed.
Keywords: Unimolecular nanoparticles, unimolecular micelles, drug delivery, nanomedicine
Graphical Abstract
1. Introduction
Polymeric nanoparticle (NP)-based delivery systems have been extensively investigated to improve the diagnostic and treatment efficacy of a wide range of diseases, ranging from cancer [1–16] and cardiovascular diseases [17–26], to bacterial and viral infections [27–33]. Polymeric NPs are attractive for drug delivery applications because many polymers are biocompatible and biodegradable. Polymer chemistry is also very versatile, thereby making it possible to precisely control the molecular structure, NP morphology, and surface characteristics (e.g., zeta potential and ligand conjugation) of polymeric NPs. The design of polymeric NPs can drastically impact the safety, pharmacokinetics, pharmacodynamics, and ultimate in vivo fate of their payloads [6–9, 34–42].
Certain types of polymers can form NPs with a core–shell structure in aqueous media owing to the various types of inter/intra-molecular interactions, including electrostatic interactions, hydrophobic interactions, and hydrogen bonding [43–45]. A broad spectrum of payloads for therapeutic and diagnostic purposes have been delivered by polymeric NPs. The stability of the NPs, or the ability to control NP stability, is of great importance for in vivo/human applications. However, conventional polymeric NP systems, which mostly rely on relatively weak interactions as previously mentioned, often exhibit insufficient in vivo stability in terms of nanostructures [46–49]. Specifically, it is well-documented that dilution in the bloodstream, flow stress, environmental factors (e.g., pH and ionic strength), and interactions with serum proteins can lead to the disruption of the polymeric NPs before functioning [50–55]. For instance, a recent report attributed poor micelle stability to the failure of NK-911, a self-assembled polymeric micelle, at the early clinical stage as it bursts too rapidly after i.v. injection [55] Moreover, a recent study also found that more than 80% of the self-assembled PEG-polyester micelles dissociated within 1 h after intravenous administration [54].
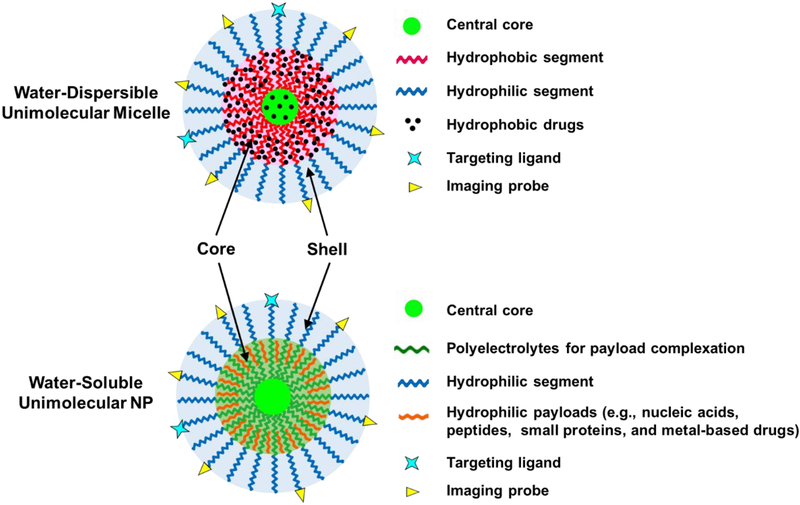
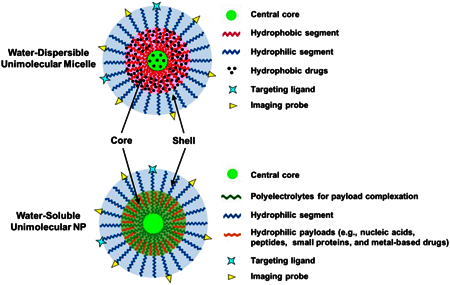
Among all of the strategies that can be applied to address this instability issue, unimolecular NPs have received increasing attention because they are stable regardless of their concentration or the microenvironment [56]. The concept of unimolecular NPs was introduced in the 1990s [57–59]. Thereafter, development of the polymeric unimolecular NPs has been accelerated owing to their desirable characteristics as drug nanocarriers as well as versatile polymer chemistry [60–66]. In particular, polymeric unimolecular NPs formed by a single multi-arm polymer molecule containing only covalent bonds and exhibiting a core–shell structure are especially valuable for biomedical applications. Polymeric unimolecular NPs can be made from a variety of polymers. One way to classify unimolecular NPs is based on their chemical composition, which can be divided into two main categories: water-dispersible unimolecular micelles and water-soluble unimolecular NPs (Figure 1). Water-dispersible unimolecular micelles are typically formed by single/individual dendritic multi-arm amphiphilic block copolymers, conferring excellent in vitro and in vivo stabilities [67, 68]. Their unique hydrophobic core is of particular interest in delivering hydrophobic therapeutics or imaging probes. Hydrophobic agents can be loaded into the hydrophobic core of the unimolecular micelles through hydrophobic interactions, hydrogen bonding, or covalent conjugation [67–69]. Water-soluble unimolecular NPs are typically formed by single/individual dendritic multi-arm water-soluble block copolymers [70, 71]. The cores of the water-soluble unimolecular NPs are usually polyelectrolytes (e.g., cationic or anionic polymers), which can be used to encapsulate hydrophilic payloads (e.g., nucleic acids, peptides, small proteins, metal-based drugs, etc.) via electrostatic interaction, hydrogen bonding, chelation, and/or ion–dipole interactions [70–72]. For biomedical applications, the shells of the unimolecular NPs are commonly formed by poly(ethylene glycol) (PEG) or other types of hydrophilic polymers (e.g., polyzwitterions) to provide good water dispersity, reduce opsonization during circulation in the bloodstream, and improve biocompatibility [68]. Both nanoplatforms have diverse applications.
Figure 1:
An illustration of a representative multifunctional water-dispersible unimolecular micelle and a water-soluble unimolecular NP. The functional core of the water-dispersible unimolecular micelle is formed by hydrophobic segments and is used to encapsulate hydrophobic drugs. The functional core of the water-soluble unimolecular NP is typically formed by polyelectrolytes and is usually used to encapsulate hydrophilic payloads. The hydrophilic shell for both types of unimolecular NPs is typically formed by hydrophilic segments, such as PEG and polyzwitterions. The hydrophilic shell provides the NP with good water dispersity and better biocompatibility. It also helps to reduce opsonization during circulation in the bloodstream.
This review focuses on the recent progress of the design and application of core–shell structured polymeric unimolecular NPs for various biomedical applications. Different types of polymeric unimolecular NPs and their diverse applications are highlighted. Future opportunities and challenges related to unimolecular NPs are also discussed briefly.
2. Types of Core–Shell Structured Unimolecular Nanoparticles
2.1. Water-Dispersible Unimolecular Micelles
Unimolecular micelles can be engineered using various types of organic NPs including dendritic polymers (e.g., hyperbranched polymers (HBPs) and dendrimers) and brush polymers as the initiating central core for the multi-arm amphiphilic block copolymers (Figure 1). Although inorganic NPs (e.g., Au NP, quantum dots, CuS NPs, and upconversion NPs) have also been employed as the central core of unimolecular micelles [73–79], those are beyond the scope of this review.
2.1.1. Unimolecular Micelles Based on Dendritic Polymers
Both HBPs and dendrimers are highly branched, three-dimensional dendritic macromolecules [80]. Their globular and dendritic architectures endow them with unique properties including abundant functional groups, intramolecular cavities, non/low entanglement, and low viscosity. HBPs and dendrimers differ in that dendrimers have regular structures, while HBPs have irregular structures [81, 82]. Dendrimers are synthesized step-by-step in an iterative fashion, while HBPs are typically synthesized via a one-step process [83]. Both HBPs and dendrimers have been widely used to fabricate unimolecular micelles.
2.1.1.1. Unimolecular Micelles Based on Hyperbranched Polymers
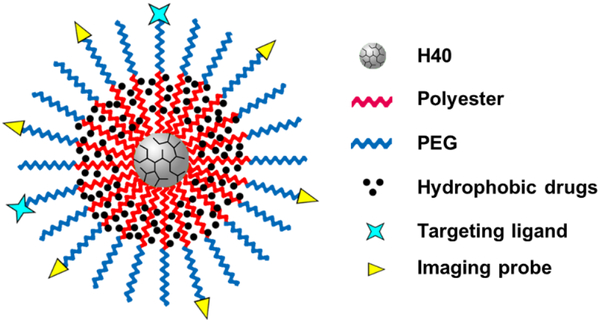
Hyperbranched polyesters are an attractive class of HBPs because they are biodegradable and biocompatible, which is extremely important if these molecules are to be used for drug delivery or other biological applications [84, 85]. Boltorn™ (e.g., H30 and H40) is one of the most studied hyperbranched polyesters, and its monomer unit is 2,2-bis(methylol) propionic acid [86, 87]. Because of its biodegradability, biocompatibility, globular architecture, and abundant functional groups, Boltorn hyperbranched polyesters have received a lot of attention in the design of nanoplatforms, including unimolecular NPs. Since H40 itself is hydrophobic, when conjugated directly with PEG, it can also be used to encapsulate hydrophobic drugs [88]. However, due to the extremely small size (~3 nm) of H40, the drug (e.g., paclitaxel) loading level of H40-PEG unimolecular micelles is limited to less than 0.3 wt.% [88]. To significantly enhance the drug loading content, we and others reported H40-based unimolecular micelles formed by multi-arm amphiphilic block copolymers H40-polyester (e.g., poly (l-lactide) (PLA) and polycaprolactone (PCL))-poly(ethylene glycol) in aqueous media (Figure 2) [61, 89–96]. For example, H40 with –OH terminal groups was used as the macroinitiator to synthesize H40-PLA via ring-opening polymerization (ROP), followed by PEG conjugation via esterification [89, 90]. The H40-PLA formed a hydrophobic functional core for hydrophobic drug encapsulation, while the hydrophilic PEG shell provided the unimolecular micelles with good water dispersity. These core–shell structured unimolecular micelles can be a stable drug nanocarrier with a much higher drug loading level (for instance, ~12 wt.% for doxorubicin (Dox) [89] and ~33 wt.% for 5-fluorouracil (5-FU) [90]).
Figure 2:
A representative illustration of an H40-based unimolecular micelle formed by a single multi-arm star amphiphilic block copolymer H40-polyester (e.g., (PLA) and (PCL))-PEG.
Zhang et al. also synthesized a temperature and pH dual-responsive unimolecular micelle based on H40 via reversible addition-fragmentation chain transfer (RAFT) polymerization [97]. The inner block was thermo-responsive poly(N, N-diethylacrylamide) (PDEAAM) and the outer shell was pH-sensitive cationic poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA). The size of the unimolecular micelles changed in response to both pH and temperature, which can be used for drug and gene delivery with a controlled release manner.
Hyperbranched polyglycerol (HPG) [98] is a type of water-soluble HBP that exhibits good biocompatibility and a low viscosity, prompting its use in drug nanocarrier systems. For example, a unimolecular micelle composed of an HPG core conjugated with diblock copolymer polycaprolactone-PEG (HPG-PCL-PEG) via amidization was reported [99]. These unimolecular micelles showed superior skin permeation of the entrapped payloads compared to the conventional cream formulation. Moreover, their good stability, low cytotoxicity, and ease of scaled-up production make them a promising nanoplatform for topical drug delivery.
2.1.1.2. Unimolecular Micelles Based on Dendrimers
Dendrimers are nano-sized, radially symmetric molecules with a well-defined, homogeneous, and monodispersed structure consisting of tree-like arms or branches [100]. It was first developed by Vögtle et al. in 1978 under the name of cascade polymers [101, 102]. Dendrimers can be prepared by the convergent or divergent synthesis approach [103, 104]. For the convergent strategy, it begins from the synthesis of dendrons that will eventually become the dendrimer shell. A dendrimer structure is then formed by coupling several dendrons to a multifunctional core [103, 104]. In contrast, the divergent strategy initiates growth at the core of the dendrimer and continues outward by the repetition of coupling and activation steps [103, 104]. So far, over 100 different dendrimers have been realized. Several of the most commonly referenced dendrimers include polyamidoamine (PAMAM) [105], poly(L-lysine) (PLL) [106], polyamide [107], polyester (PGLSA-OH) [108], and polypropylenimine (PPI) dendrimers [109]. The high level of control over the synthesis process gives dendrimers a perfect branching structure with a compact molecular structure, a high number of functional groups, and predictable properties. Due to these unique characteristics, tremendous progress on the development of dendrimers for a wide range of biomedical applications has been witnessed in recent years [110–113]. Unimolecular micelles formed by dendrimer-based multi-arm star amphiphilic block copolymers have also been reported.
PAMAM dendrimers are the most common class of dendrimers suitable for many applications [114]. The original primary amino groups (–NH2) on the surface of PAMAM have been modified into other functional terminals, such as –OH and –COOH, thereby allowing for versatile chemistry to form unimolecular micelles [115–121]. For example, we utilized an –OH functionalized generation 4 (G4) PAMAM (PAMAM-OH) dendrimer to grow amphiphilic block copolymer poly(L-lactide)-PEG (PAMAM-PLA-PEG) [115]. The resulting dendritic multi-arm amphiphilic block copolymer can form stable unimolecular micelles as a drug nanocarrier with a typical drug loading content around 20 wt.% [122]. Moreover, because of the ease of surface modification, the unimolecular micelle can be tailored with targeting ligands and imaging probes for multifunctionalities. Other types of polyesters (e.g., PCL and polyvalerolactone (PVL)) were also used as the hydrophobic segments in PAMAM-based unimolecular micelles [119–121].
2.1.2. Unimolecular Micelles Based on Brush-Shaped Polymers
Brush-shaped polymers are a special category of synthetic macromolecules with multiple side chains/polymers stemming from a polymer backbone [123–125]. There are three main strategies to synthesize brush-shaped polymers: “grafting through”, “grafting onto”, and “grafting from” [126–128]. In recent years, the investigation of brush-shaped core–shell unimolecular NPs has gained increasing attention. For example, a wormlike unimolecular micelle based on a densely grafted brush polymer polymethacrylate-g-(poly(ε-caprolactone)-b-poly(ethylene oxide)) (PGA-g-(PCL-b-PEO), prepared by “grafting onto” chemistry, was reported [129]. This polymer brush in an aqueous solution exhibited a uniform, wormlike conformation both before and after anticancer drug (DOX) encapsulation, as demonstrated by atomic force microscopy (AFM) images.
We also fabricated a unimolecular micelle system based on a brush-shaped amphiphilic block copolymer. The backbone of the brush-shaped polymer was poly(2-hydroxyethyl methacrylate) (PHEMA) and the side chains were poly(L-lactide)-poly(ethylene glycol) (PHEMA-PEG) [130]. The brush-shaped polymer was prepared via the “graft from” method and was able to form a stable unimolecular micelle for targeted drug delivery. Ito et al. also engineered a unimolecular NP formed by a branched graft copolymer poly(styrene-graft-PEO) that was synthesized via a “graft through” approach [60]. The polystyrene backbone of the brushed polymer served as a hydrophobic domain of the unimolecular micelle.
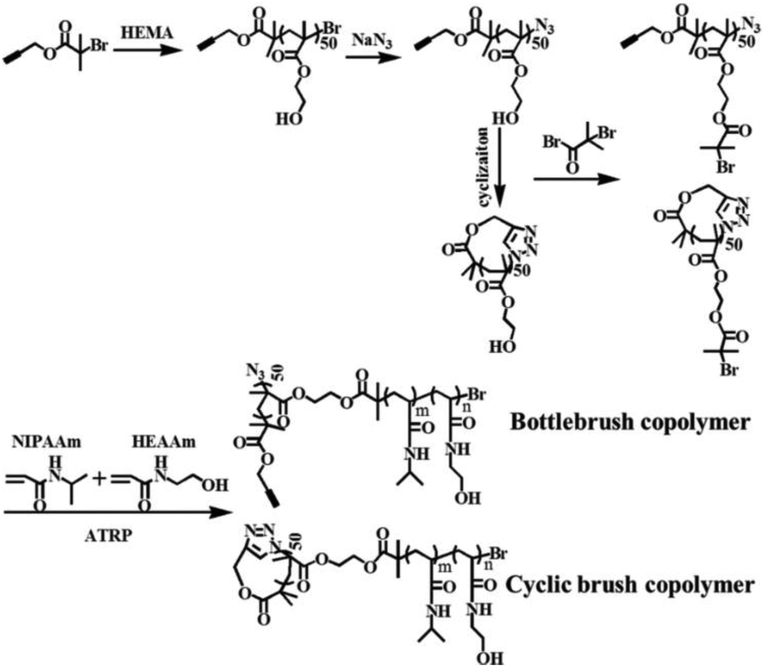
More recently, Tu et al. reported a unimolecular NP formed by a cyclic brush copolymer named poly(2-hydroxyethyl methacrylate-g-poly(N-isopropylacrylamide-st-N-hydroxyethylacrylamide)) (P(HEMA-g-P(NIPAAm-st-HEAAm)) (Figure 3) [131]. They found that the unimolecular micelles formed by these cyclic brush copolymers possessed better stability and a higher anti-proliferation effect compared to the ones formed by the bottlebrush analogues.
Figure 3:
Synthesis of cyclic brush and bottlebrush copolymers. Reproduced with permission from Ref. [131].
2.1.3. Other Types of Unimolecular Micelles
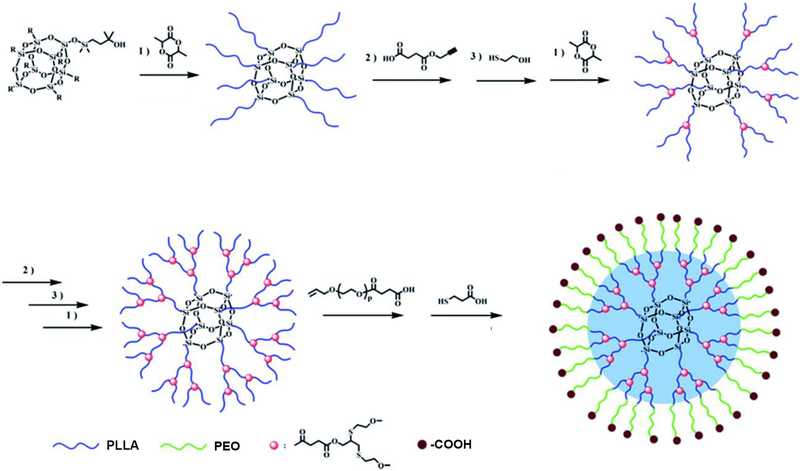
Unimolecular micelles formed by multi-arm amphiphilic polymers based on other types of central cores have also been reported. For example, polyhedral oligomeric silsesquioxane (POSS) with eight functional groups on the vertex of the silica cage has been used in the design of drug delivery systems due to its good biocompatible and non-toxic properties [132]. The eight corner functional groups can be conveniently used to grow multifunctional polymers. For instance, Fan et al. [133] reported a unimolecular micelle composed of POSS as the central core, PLA as the hydrophobic segment, and PEG as the hydrophilic block for drug delivery (Figure 4). In an aqueous solution, a stable unimolecular micelle with a unique core–shell structure was obtained as evidenced by DLS and TEM. The hydrophobic domain formed by POSS-PLA was loaded with the anticancer drug, DOX, with a high drug-loading content (18.5%) capable of sustained drug release.
Figure 4:
Schematic illustration of the core–shell structured unimolecular micelle formed by the amphiphilic block copolymer POSS-(G3-PLLA-b-PEO-COOH)8. Reproduced with permission from Ref. [133].
β-CD has also been used to fabricate unimolecular NPs. β-CD is a cyclic oligosaccharide consisting of seven glucose molecules linked by α(1,4)-glucosidic bonds [134]. The 21 available hydroxyl groups on the surface of β-CD make it a suitable central core for unimolecular NPs. Lin et al. reported a β-CD-polystyrene-block-poly(3-hexylthiophene) unimolecular micelle as a potential drug nanocarrier [135].
2.2. Water-Soluble Unimolecular NPs
Water-soluble unimolecular NPs are generally designed for the delivery of hydrophilic payloads, such as metal-based compounds (e.g., platinum-based drugs), nucleic acids (e.g., siRNA and microRNA), peptides, and small proteins. These payloads are typically loaded into the unimolecular NPs, mainly through electrostatic interactions, hydrogen bonding, ion–dipole interactions, and chelation [70–72, 136–138]. Hence, polyelectrolytes are often incorporated into the design of such unimolecular NPs (Figure 1).
We reported a water-soluble unimolecular NP made of a hydrophilic multi-arm star block copolymer poly(amidoamine)-b-poly(aspartic acid)-b-poly(ethylene glycol) (PAMAM-PAsp-PEG) [136]. Carboplatin, a hydrophilic platinum-based drug, was complexed into the carboxyl-bearing PAsp segments through an ion–dipole interaction.
Jiang et al. reported a polyelectrolyte brush (PFNBr), which was a brush-like polymer consisting of a polyfluorene backbone and multiple positively charged poly(2-(dimethyl-amino)ethyl methacrylate) (PDMAEMA) side chains [72]. This unique cationic brush polymer can complex siRNA through electrostatic interactions and form an siRNA-complexed unimolecular NP. Its intrinsic fluorescence property also makes it a promising candidate for precise bioimaging.
We also reported a pH and redox dual-responsive unimolecular NP for the delivery of siRNA [137]. The unimolecular NP was formed by an H40-poly(aspartic acid-(2-aminoethyl disulfide)-(4-imidazolecarboxylic acid))-poly(ethylene glycol) (H40-P(Asp-AED-ICA)-PEG)) multi-arm star block copolymer. The cationic segments (P(Asp-AED-ICA)-PEG)) used for siRNA complexation contained disulfide bonds and were linked to the H40 core through pH-sensitive bonds. These pH and redox dual-response properties facilitate the release of the siRNA once they are taken up by the cells.
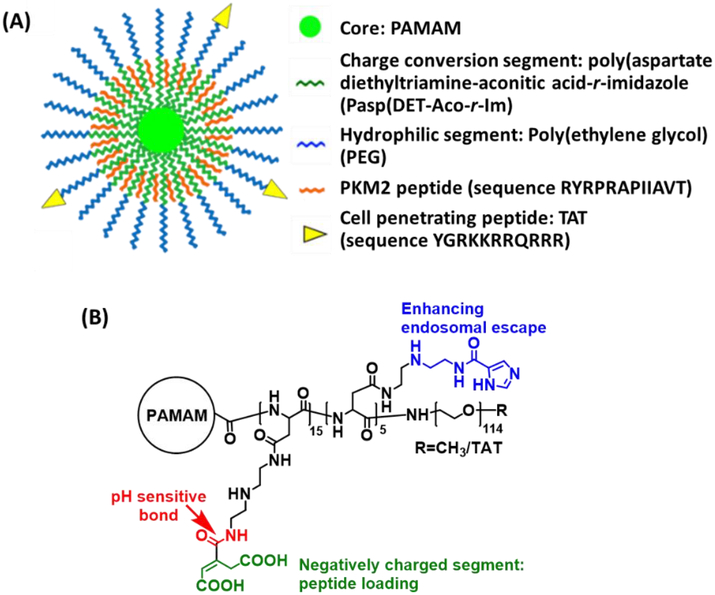
We recently reported a unique charge-conversional unimolecular NP made of multi-arm star block copolymer poly(amidoamine)–poly(aspartate diethylenetriamine-aconitic acid-r-imidazole)-poly(ethylene glycol) (PAMAM-PAsp(DET-Aco-r-Im)-PEG) for the delivery of positively charged peptides (Figure 5) [70]. In acidic conditions, the anionic PAsp(DET-Aco) segment can turn to positively charged PAsp(DET), thereby facilitating the release of peptides to function.
Figure 5.
(A) Illustration of the unimolecular NP for PKM2 peptide delivery. (B) Chemical structure of PAMAM-PAsp(DET-Aco-r-Im)-PEG. Reproduced with permission from Ref. [70].
3. Unimolecular NPs for In Vivo Applications
Unimolecular NPs have been extensively investigated as drug nanocarriers for various therapeutic and diagnostic applications. In this section, we will summarize the state-of-the-art usage of unimolecular NPs for the delivery of therapeutic and diagnostic agents. Table 1 summarizes the representative core-shell structured polymeric unimolecular for biomedical applications.
Table 1:
Representative polymeric unimolecular NPs for biomedical applications.
| Chemical composition | Therapeutic payloads | Imaging Probes | Reference | |
|---|---|---|---|---|
| Water-dispersible unimolecular micelles | H40-PLA-PEG | DOX | - | 62 |
| H40-PCL-PEG | Resveratrol | - | 151 | |
| PAMAM-PLA-PEG | DOX | 64Cu (PET) | 115 | |
| PAMAM-PVL-PEG | TDP-A | Cy5 (optical imaging) | 120 | |
| PGA-g-(PCL-b-PEG) | DOX | - | 129 | |
| POSS-(G3-PLLA-b-PEO-COOH)8 | DOX | - | 133 | |
| β-CD-P(DPA-co-OEGMA) | DOX + BBT-2FT | - | 156 | |
| H40-BPLP-PEG | DOX | BPLP (self-fluorescent) | 183 | |
| H-P(CPTM-co-DOTA(Gd))-P(OEGMA-co-GPMA) | CPT | DOTA/Gd (MRI) | 196 | |
| β-CD-(PCL-PAEMA-PPEGMA)21/AuNPs | DOX | AuNPs (CT imaging) | 197 | |
| Water-soluble unimolecular nanoparticles | PAMAM-PAsp-PEG | Carboplatin | Cy5 (optical imaging) | 136 |
| PAMAM-PGA-PEG | DACHPt | - | 71 | |
| PFNBr-PDMAEMA | siRNA | - | 72 | |
| H40-P(Asp-AED-ICA)-PEG | siRNA | Cy5 (optical imaging) | 137 | |
| PAMAM-PAsp(DET-Aco-r-Im)-PEG | Peptide | - | 70 |
3.1. Unimolecular NPs for Drug Delivery
The success of the therapeutic applications of unimolecular NPs critically depends on a rational design of the unimolecular NPs. Different therapeutic payloads may require distinctively different designs of unimolecular NPs. For instance, unimolecular micelles are used to deliver hydrophobic drugs, while water-soluble unimolecular NPs containing polyelectrolytes are needed to deliver charged payloads such as nucleic acids and peptides.
3.1.1. Unimolecular Micelles for Drug Delivery
Since Duncan and Kopecek introduced the concept of using polymers to deliver drugs back in the 1980s [139], the application of polymers in the drug delivery field has been explosively investigated. Polymeric micelles, due to their unique core–shell structure, have been extensively studied for delivering hydrophobic drugs [41, 42, 140–143]. It has been estimated that about 40% of the drugs in the market present poor water solubility [144, 145]. The inner hydrophobic core of the micelles can solubilize drugs through hydrophobic interactions and hydrogen bonding, while the hydrophilic shell can provide the micelles with good water dispersity and reduced opsonization. However, as aforementioned, the instability issue associated with traditional polymer micelles severely hinders their in vivo application [46–49]. Premature disruption of the micelles could lead to a burst release of the entrapped drugs, causing serious systemic toxicity and undermining their multifunctionality, including loss of specific tissue/cell targeting ability [50–52]. In contrast, judiciously designed unimolecular micelles can offer excellent in vitro and in vivo stabilities due to their covalent nature. It has been previously demonstrated that unimolecular NPs can transport hydrophobic guests into living cells under biologically relevant high-dilution conditions, which cannot be achieved by an analogous structure prepared by self-assembly [146].
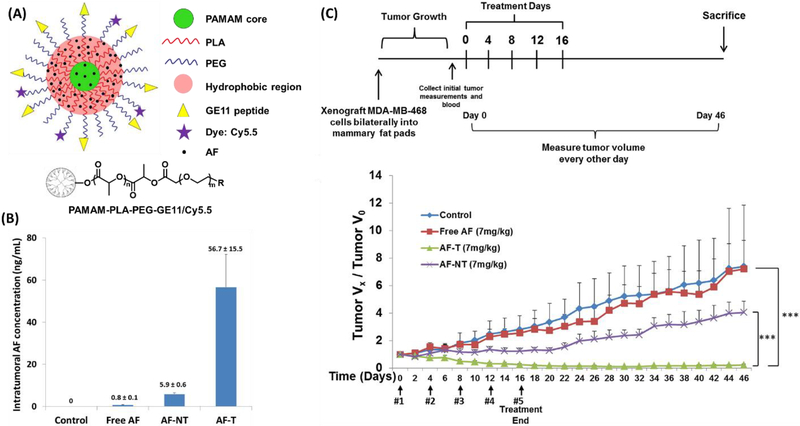
Drug molecules can be encapsulated into unimolecular micelles via either physical encapsulation or chemical conjugation. All hydrophobic drugs can be easily loaded into unimolecular micelles via physical encapsulation through hydrophobic interactions and/or hydrogen bonding [61, 62, 91–93, 99, 120, 147, 148]. Thus, physical encapsulation is a more general and easier approach than chemical conjugation. However, chemical conjugation can offer stimuli-responsive drug release profiles [69, 149, 150]. We have reported a series of drug-loaded unimolecular NPs for cancer treatment [62, 115, 116, 119, 130, 151, 152]. In order to improve delivery efficiency, various targeting ligands—such as folate (for folate receptor) [62], GE11 peptide (for epidermal growth factor receptor (EGFR)) [116], octreotide (for somatostatin receptor) [151], aptamer (e.g., A10 aptamer for prostate-specific membrane antigen) [152], and antibody (e.g., anti-CD105 monoclonal antibody (TRC105)) [115, 130]—were tagged to the surface of the unimolecular micelles for targeted cancer therapy. The resulting drug-loaded unimolecular micelles exhibited superior stability and tumor-targeting ability, as well as enhanced therapeutic efficacy. For example, aminoflavone (AF; an anticancer drug)-loaded and GE11-conjugated unimolecular micelles were tested in an orthotropic triple-negative breast cancer (TNBC) xenograft mouse model (Figure 6) [116]. GE11 peptide can bind specifically to EGFR, which is frequently amplified in TNBC tumors. The AF concentration in the tumor treated with AF-loaded, GE11-conjugated unimolecular micelles was about 72-fold and 10-fold higher than the AF concentration in the tumor treated with free AF and AF-loaded unimolecular micelles without GE11 targeting ligand, respectively (Figure 6 (B)). Remarkably, the AF-loaded, GE11-conjugated unimolecular micelles induced complete tumor regression at a significantly lower dosage (7 mg/kg) than the common dosage required for free drug (70–120 mg/kg) (Figure 6 (C)) [153–155], and this treatment did not result in any detectable systemic toxicity. A unimolecular micelle designed for the co-delivery of an anticancer drug (DOX) and an NIR-photothermal agent (benzo [1,2-c;4,5-c’]bis[1,2,5]thiadiazole-4,7-bis(9,9-dioctyl-9H-fluoren-2-yl)thiophene (BBT-2FT)) for combination chemotherapy and photothermal therapy (PTT) was also reported [156]. The unimolecular micelle was formed by β-CD-poly(2-(diisopropylamino) ethyl methacrylate-oligo (ethylene glycol)-methyl ether-methacrylate) (β-CD-P(DPA-OEGMA)). The PDAP segment, with a pKa value around 6.4, is hydrophobic at neutral pH, and becomes hydrophilic in an acidic environment (pH < 6.4). The unimolecular micelles loaded with DOX and BBT-2FT showed pH-responsive drug release kinetics that enhanced the therapeutic effect against cancer cells by chemo-photothermal combination therapy. The combination therapy induced approximately 23-fold and 3-fold more cell death than chemotherapy and PTT alone, respectively.
Figure 6:
(A) A schematic illustration of multifunctional unimolecular micelles formed by multi-arm star amphiphilic block copolymer PAMAM–PLA–PEG–OCH3/Cy5.5/GE11 for targeted triple-negative breast cancer therapy. (B) Graphical representation of AF concentration in the tumors of nude mice treated with saline control, free AF, and AF encapsulated in GE11-conjugated targeted (AF-T) or non-targeted (AF-NT) micelles as determined by LC/MS/MS. (C) Anticancer study of AF-loaded, GE11-conjugated unimolecular micelles in an MDA-MB-468 TNBC xenograft mouse model. Reproduced with permission from Ref. [116].
Besides cancer therapy, drug-loaded unimolecular micelles have also been investigated for the treatment of other diseases. For instance, glaucoma is a common eye disease characterized by loss of retinal ganglion cells (RGCs), resulting in irreversible blindness. An RGC-targeted intraocular drug delivery system employing unimolecular micelles was engineered to deliver a neuroprotective drug, dehydroepiandrosterone (DHEA), to prevent RGC loss [157]. The unimolecular micelles were formed by multi-arm star amphiphilic block copolymers poly(amidoamine)–polyvalerolactone–poly(ethylene glycol) (PAMAM-PVL-PEG) conjugated with the cholera toxin B (CTB) domain that can bind specifically to GM1 ganglioside on the RGC surface. Following intravitreal administration, the CTB-conjugated (targeted) unimolecular micelles exhibited a much higher accumulation in the RGC layer than the non-targeted ones. Targeted unimolecular micelles can be detected at 7 days post-injection, while non-targeted micelles can only be detected one day post-injection. Moreover, in an acute N-methyl-D-aspartate-induced RGC death mouse model, the DHEA-loaded targeted unimolecular micelles exhibited a much better RGC protective effect than the DHEA-loaded non-targeted ones after 14 days (>40 % of RGC being preserved for the targeted micelles vs. <20% of RGC being preserved for the non-targeted micelles).
A unimolecular micelle-based drug delivery system was also developed for the treatment of vascular diseases. Restenosis is the recurrence of blood vessel narrowing, leading to a restricted blood flow rate [158]. Globally, millions of patients receive open vascular interventions every year, but a significant fraction of these interventions eventually fail due to restenosis [159]. A perivascular drug delivery system consisting of drug (rapamycin)-loaded unimolecular micelles made of PAMAM-PVL-PEG and a thermosensitive hydrogel formed by PLGA-PEG-PLGA triblock copolymer (Triblock gel) was designed to inhibit restenosis after open surgery [122]. This hybrid perivascular drug delivery system provides sustained drug release for four months, while Triblock gel alone or unimolecular micelles alone only provide sustained drug release for one and two months, respectively. Remarkably, this hybrid perivascular drug delivery system produced a rare feat of 3-month restenosis inhibition in an animal model. Specifically, rapamycin-loaded unimolecular micelles in the Triblock gel inhibited restenosis by 80% and induced a larger luminal area (60% bigger) compared to its respective drug-free control.
Stimuli-responsive characteristics have also been incorporated into the unimolecular micelles to achieve controlled release of payloads to potentially achieve better therapeutic efficacy. Since NPs are largely taken up by cells through endocytosis, their subcellular trafficking pathways typically involve acidic endosomes and lysosomes. Therefore, pH-responsive unimolecular micelles have been engineered to control the drug release from the unimolecular micelles. For example, Haag et al. reported a pH-responsive unimolecular micelle formed by the hyperbranched amphiphilic block copolymer HPG-octadecane-18-PEG (HPG-C18-PEG) [160]. The amphiphilic C18-PEG block copolymer molecules were conjugated to the HPG core via a pH-responsive imine linker. In the acidic endocytic compartments, the imine linkers were cleaved quickly, thereby causing a more rapid drug (DOX) release, and subsequently leading to a better therapeutic efficacy compared to unimolecular micelles lacking a pH-responsive drug release profile in A549 lung cancer cells. Since the concentration of glutathione (GSH) (approximately 2–10 mM) is drastically higher than in the extracellular spaces (approximately 2–20 μM) [161–163], GSH-responsive unimolecular micelles for redox-responsive drug release have also been reported [96, 164, 165].
A GSH-sensitive unimolecular micelle composed of H40-PLA (as the hydrophobic core) linked to poly(2-ethoxy-2-oxo-1,3,2-dioxaphospholane) (PEP) (as the hydrophilic shell) through disulfide bonds was engineered to achieve GSH-responsive drug release [164]. GSH (2–10 mM) in the cytosol triggered the detachment of the hydrophilic PEP shell from the unimolecular micelle, which resulted in a rapid drug (DOX) release due to the disruption of the micelle structure, thereby enhancing growth inhibition in tumor cells.
Drug molecules with certain types of functional groups, such as –OH, –NH2, and –C(O)–, can be covalently conjugated on unimolecular micelles to achieve stimuli-responsive drug release. We reported DOX-conjugated unimolecular micelles, where DOX was linked to the unimolecular micelles through a pH-sensitive hydrazone bond, thus taking advantage of the 13-keto position of the DOX molecule [69, 149]. Once taken up by cells through endocytosis, DOX can be easily cleaved from the unimolecular micelles in the acidic endocytic compartments. The DOX-conjugated unimolecular micelles exhibited a rapid drug release under acidic conditions. Specifically, 83% of the drug was released at a pH of 6.6 after 45 h, while only 15% of the drug was released at a pH of 7.4 after 45 h [149].
3.1.2. Water-Soluble Unimolecular NPs for Drug Delivery
Water-soluble unimolecular NPs are typically formed by employing polyelectrolytes to form the hydrophilic core and PEG as the hydrophilic shell (Figure 1). They have been used to deliver hydrophilic therapeutics, including small molecule drugs, genes, peptides, and proteins, mainly through electrostatic interactions and hydrogen bonding. More specifically, depending on the net charge of the biomacromolecule payload (e.g., nucleic acids, peptides, and proteins), unimolecular NPs are typically constructed with opposite-charged polyelectrolytes [70, 71, 137]. For small metal-based hydrophilic drugs, other interactions may also be utilized, such as ion–dipole interactions [136] and chelation [71]. Similar to self-assembled multi-molecular polymer micelles, multi-molecular polymeric NPs formed by electrostatic interactions, hydrogen bonding, and ion–dipole interactions possess insufficient in vivo stability because of their multi-molecular nature. Hence, the use of unimolecular NPs is more desirable.
As mentioned previously, a water-soluble PAMAM-PAsp-PEG unimolecular NP was engineered to deliver the platinum-based drug, carboplatin [136]. Carboplatin was complexed to the unimolecular NPs via pH-responsive ion–dipole interactions between the carboplatin and the carboxylate. The carboplatin-complexed unimolecular NPs exhibited excellent stability with a desirable pH-sensitive drug release profile. cRGD peptide (for integrin αvβ3-positive tumor targeting) was decorated on the surface of the unimolecular NPs for enhanced drug delivery efficiency. Liu et al. reported a unimolecular NP based on PAMAM (G3) that was conjugated with block copolymers poly(glutamic acid)-b-poly-(ethylene glycol) (PAMAM-PGA-PEG) to deliver 1,2-diaminocyclohexane-platinum(II) (DACHPt) through chelate complexation [71]. DACHPt-complexed unimolecular NPs displayed longer in vivo half-lives (13.2 h) than the self-assembled DACHPt-loaded micelles (7.1 h). The in vivo anticancer study showed that the tumor volume of the DACHPt-complexed unimolecular NP treated group was significantly smaller than that of the DACHPt-complexed self-assembled NP treated group (115 vs. 177 mm3; note the original tumor size was 50 mm3).
Delivery of nucleic acids is also of great interest to treat or prevent human diseases, such as genetic disorders and cancers [166, 167]. The polyplexes formed by the electrostatic interactions between cationic polymers and negatively charged genes have been widely explored to overcome the limitations associated with nucleic acids, such as limited cellular uptake due to their highly negatively charged nature, insufficient chemical stability, and short plasma half-life [168–170]. However, as aforementioned, polyplexes also exhibit poor in vivo stability due to various factors, including interactions with serum proteins (e.g., albumin) and in vivo dilution [171–174]. The use of unimolecular NPs could not only address the limitations with naked nucleic acids, but also provide excellent in vivo stability.
Chen et al. [137] engineered a pH and redox dual-sensitive unimolecular NP for siRNA delivery. The cationic core used for siRNA complexation contained GSH-cleavable disulfide bonds, thereby facilitating the decomplexation of siRNA from the unimolecular NPs. The cationic polymers were conjugated onto the H40 core via a pH-sensitive imine bond, which further facilitated siRNA decomplexation. The incorporation of imidazole in the unimolecular NP delivery system enhanced the endosomal escape capability of the NPs via the proton sponge effect. The resulting siRNA-complexed unimolecular NPs exhibited excellent stability. To enhance delivery efficiency, GE11 peptide was decorated on the surface of the unimolecular NPs, which was tested in EGFR-overexpressing TNBC cells. The in vitro gene silencing efficiency of the siRNA-complexed targeted unimolecular NPs (79 %) was comparable to the commercially available siRNA transfection agent, RNAiMAX (81 %). RNAiMAX is known to have severe cytotoxicity, whereas the unimolecular NPs exhibited a much better biocompatibility.
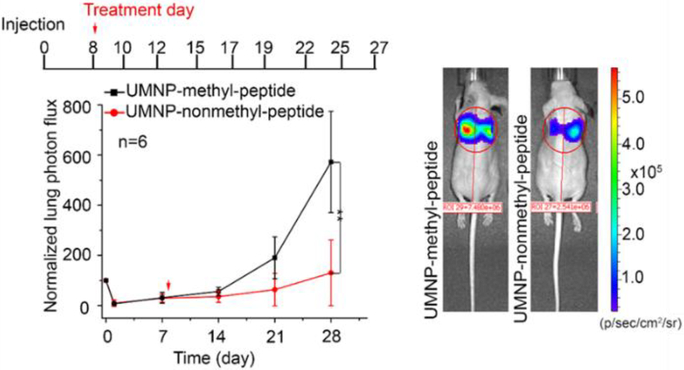
Unimolecular NPs can also be used to deliver peptides and small proteins. As mentioned earlier, a unique charge-conversional PAMAM-PAsp(DET-Aco-r-Im)-PEG unimolecular NP was developed for the delivery of a positively charged peptide, the pyruvate kinase isozyme M2 (PKM2) peptide (Figure 5) [70]. The PKM2 peptide is a fragment of the PKM2 protein that can specifically and efficiently bind with co-activator-associated arginine methyltransferase 1 (CARM1), and thus inhibits the interaction between PKM2 and CARM1 [70]. The non-methylated PKM2 peptide (nonmethyl-peptide), but not the methylated PKM2 peptide (methyl-peptide), can inhibit the CARM1-mediated methylation of PKM2. In a lung tumor metastasis mouse model, the bioluminescence intensities of lung-metastatic cells in the non-methyl PKM2 peptide-loaded unimolecular NP treated group were 5-fold lower than those in the methyl PKM2-peptide-loaded unimolecular NP treated group, indicating that the PKM2 peptide delivered by the unimolecular NPs successfully inhibited breast cancer lung metastasis by disrupting the metabolic energy balance in cancer cells (Figure 7).
Figure 7.
Inhibition of PKM2 methylation using a competitive PKM2 peptide reduces tumor lung metastasis in vivo. Bioluminescence at indicated times was measured in the lung when mice (n=6) were treated with unimolecular NPs loaded with methyl PKM2 peptide (UMNP-methyl-peptide) or unimolecular NPs loaded with non-methyl PKM2 peptide (UMNP-nonmethyl-peptide). Representative bioluminescence images of mice after four-week treatment with UMNP-methyl-peptide (n=4) or UMNP-nonmethyl-peptide (n=5) via retroorbital injection at the indicated times after tumor introduction. The color scale depicts the photon flux (photons per second) emitted from the xenografted mice. Reproduced with permission from Ref. [70].
Dai et al. [175] fabricated a dendritic poly(amido amine)-b-poly(ɛ-caprolactone)-b-poly(d-gluconamidoethyl methacrylate) (PAMAM-PCL-PGAMA) block copolymer for the delivery of Concanavalin A (Con A) protein. Con A is a carbohydrate-binding protein, and thus can bind to the sugar molecules in dendritic polymers. These unimolecular NPs were more stable than those self-assembled from their linear counterparts. Similarly, host–guest interactions (e.g., cyclodextrin and adamantane) or biotin/avidin interactions could also be potentially incorporated into the unimolecular NP systems.
3.2. Unimolecular NPs for Bioimaging
Bioimaging is a prominent area of research aiming at extending or developing novel tools for diagnosis with high specificity and quality [176, 177]. Most of the bioimaging probes currently used are small molecular compounds, such as organic fluorescent agents for optical imaging, metal ions for magnetic resonance imaging (MRI), and radiolabeled molecules for positron emission tomography (PET) [177, 178]. Although they have shown some utility, clinical applications of these bioimaging probes are still hindered by low specificity and sensitivity, instability, and potential toxicity [179–181]. Unimolecular NPs have emerged as an excellent candidate for bioimaging probes. Their high loading capacity and versatile chemistry allow encapsulation or conjugation of imaging probes for enhanced diagnosis.
Optical imaging utilizes photons emitted from fluorescence or bioluminescence probes. It is a fast and inexpensive approach [176, 182]. DOX, a common anticancer drug, is also fluorescent (red) and thus it is suitable for optical imaging. Hence, DOX-encapsulated unimolecular NPs can be used for optical imaging. For instance, Xu et al. reported an aptamer-conjugated and DOX-loaded unimolecular micelle to target prostate cancers [152]. A10 aptamer, which can specifically recognize the prostate-specific membrane antigen (PSMA) abundantly expressed on the surface of prostate cancer cells, was used as the targeting ligand. In tumor-bearing mice, the DOX fluorescence intensity measured by optical imaging suggests that targeted unimolecular micelles exhibited a much higher tumor accumulation than non-targeted micelles and free DOX. However, one concern with utilizing imaging probes loaded into NPs via physical encapsulation to localize the NPs in vitro and in vivo is that the imaging probes are continuously released from the NPs. Therefore, physically encapsulated imaging probes may not result in the precise localization of the NPs. Owing to the large number of surface functional groups on the unimolecular NPs, imaging probes can also be covalently linked to the NPs. For example, to track the unimolecular NPs, Cy5.5 molecules were conjugated onto the distal ends of the PEG arms [116, 118]. Another approach to endow the unimolecular NPs with optical imaging properties is to fabricate the NPs with fluorescent building segments. We also reported a self-fluorescent unimolecular micelle system for targeted drug delivery. The unimolecular NPs were formed by H40-biodegradable photo-luminescent polymer-poly(ethylene glycol) (PEG) (H40-BPLP-PEG) [183]. The hydrophobic BPLP segment was self-fluorescent, thereby making the unimolecular micelle self-fluorescent that is suitable for optical imaging. Self-fluorescent unimolecular micelles can also serve as drug carriers for cancer-targeted delivery. The combination of its intrinsic fluorescence properties and its capability of drug delivery holds great promise for cancer theranostics.
Pu et al. reported a bottom-up strategy to construct water-soluble fluorescent unimolecular NPs based on the cationic oligofluorene-conjugated POSS dendritic polymer for fluorescence amplification in cellular imaging [184]. The high quantum yields (e.g., 0.85 in water and 0.80 in PBS) and good signal amplification capability of POSS-based molecules allow for high quality biological imaging even with a small amount of indicator dyes, thereby avoiding the undesirable side effect of elevated dye concentrations. The emission wavelength, charged nature, and diameter of POSS-based fluorescent unimolecular NPs can be easily adjusted through chemical modification of the fluorescent arms so as to fulfill the different requirements of specific applications.
Another widely used bioimaging modality is MRI, which is an extremely versatile anatomical imaging technique that generates high quality images [185, 186]. Paramagnetic contrast agents (e.g., gadolinium (Gd)) can enhance the signal intensity of T1-weighted MRI images [187]. However, the commercial products for Gd-based agents, such as Gd-DTPA (Magnevist®; DTPA: diethylenetriaminepentaacetic acid), Gd-(DTPA-BMA) (Omniscan; DTPA-BMA: di-ethylenetriaininepentaacetic acid-bis(methylamide)), and Gd-DOTA Dotarem®; DOTA: 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid), often exhibit a rapid clearance rate and no specificity [188, 189].
Unimolecular NPs can be used to adequately address these concerns. Li et al. reported a unimolecular micelle formed by H40-star-(PCL-b-POEGMA/Gd/FA) for cancer-targeted MRI [148]. In vitro MRI experiments revealed considerably enhanced T1 relaxivity for Gd-loaded unimolecular micelles (18.14 s−1 mm−1) compared to that of small molecular DOTA–Gd complexes (3.12 s−1 mm−1). Further in vivo MRI experiments in rats showed prominently positive contrast enhancement in the major organs. Specifically, in the first 20 min, the contrast-to-noise ratio (CNR) in the heart, kidney, and liver dramatically increased. Both the heart and kidney reached the highest CNR at 20 min post-injection, while the CNR of the liver reached a maximum at 20 h post-injection.
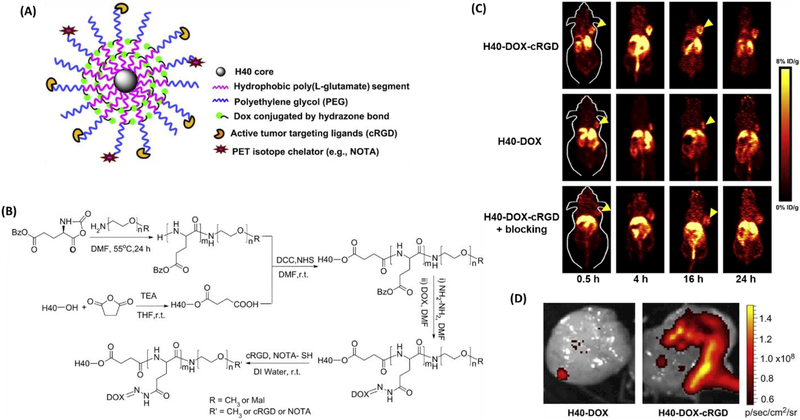
PET imaging has become increasingly popular in both preclinical and clinical settings as this non-invasive imaging modality is tomographic in nature, has excellent tissue penetration as well as high sensitivity and specificity, and can observe functional changes over a short time period [190, 191]. As such, PET-based nanomedicine has gained increasing attention during the last decade. The use of unimolecular NPs for PET imaging has also been reported. For instance, Xiao et al. [150] engineered a unimolecular micelle made of a hyperbranched amphiphilic block copolymer (Figure 8). NOTA (a macrocyclicchelator for 64Cu-labeling) and cRGD peptide (for integrin αvβ3-positive tumor targeting) were linked to the surface of the unimolecular micelles. In vivo PET imaging demonstrated a higher tumor accumulation of 64Cu-labeled targeted unimolecular micelles (e.g., 5.7 % ID/g at 4 h post injection) than the non-targeted ones (e.g., 2.6 % ID/g at 4 h post injection). Injection with a blocking dose of cRGD peptide, along with 64Cu-labeled targeted unimolecular micelles, significantly reduced tumor uptake (e.g., 3.2 % ID/g at 4 h post injection) to a level similar to the non-targeted ones, indicating integrin αvβ3-specific binding of 64Cu–labeled targeted unimolecular micelles. In addition, DOX was also conjugated to the unimolecular micelles via a pH-labile hydrazine bond, allowing for optical imaging as well. The ex vivo fluorescence imaging of the excised tumors also revealed a higher tumor accumulation of targeted micelles compared to the non-targeted control.
Figure 8:
(A) A schematic illustration of the multifunctional unimolecular micelle formed by H40-P(LG-Hyd-DOX)-b-PEG-OCH3/cRGD/NOTA(64Cu). (B) The synthetic scheme for H40-P(LG-Hyd-DOX)-b-PEG-OCH3/cRGD/NOTA(64Cu). (C) PET imaging of 64Cu-labeled nanocarriers in U87MG tumor-bearing mice. (D) Ex vivo fluorescence imaging of excised U87MG tumors, with the excitation and emission set for DOX fluorescence. Reproduced with permission from Ref. [150].
3.3. Unimolecular NPs for Nanotheranostics Applications
Nanotheranostics, the use of nanotechnology for the integration of therapy and diagnostics, may offer new opportunities for “personalized nanomedicine” [192–194]. Unimolecular micelles can conveniently incorporate therapeutic and diagnostic agents to achieve multifunctionality due to their excellent stability, high loading capacity, and versatile chemistry [67, 68]. Therefore, unimolecular micelles can be a desirable platform for nanotheranostics [115, 130, 150, 152, 183, 195–199].
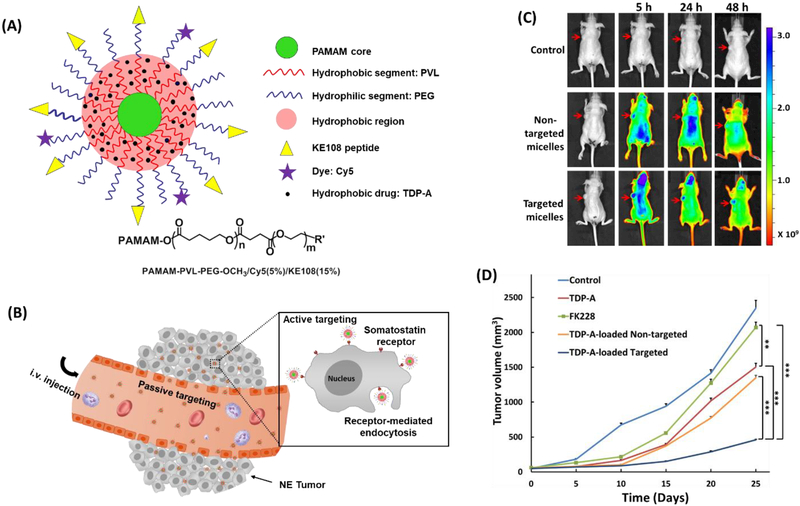
We developed a multifunctional unimolecular micelle for targeted neuroendocrine (NE) cancer theranostics [195]. The unimolecular micelle was formed by multi-arm star amphiphilic block copolymer PAMAM-PVL-PEG conjugated with KE108 peptide as a targeting ligand and Cy5 dye as an optical imaging probe (abbreviated as PAMAM-PVL-PEG-KE108/Cy5) (Figure 9). The targeted unimolecular micelles exhibited a much higher tumor accumulation than that of the non-targeted micelles in an NE-tumor-bearing mouse model based on in vivo optical imaging. Moreover, the drug (thailandepsin A (TDP-A))-loaded targeted unimolecular micelles induced the highest antitumor efficacy (92 % tumor reduction compared to the control group) without detectable systemic toxicity.
Figure 9:
(A) A schematic illustration of the multifunctional unimolecular micelle formed by the multi-arm star amphiphilic block copolymer PAMAM–PVL–PEG–OCH3/Cy5/KE108 for targeted NE cancer therapy. (B) A schematic illustration of the passive and active tumor targeting capabilities exhibited by the multifunctional unimolecular micelles after i.v. injection. (C) In vivo near-infrared fluorescence imaging of subcutaneous BON tumor-bearing mice treated with saline (control), non-targeted micelles conjugated with Cy5, and targeted micelles conjugated with KE108 and Cy5; arrows point to the tumor sites. (D) In vivo anticancer efficacy of different TDP-A formulations in BON tumor xenografts. Reproduced with permission from Ref. [195].
Hu et al. fabricated a theranostic unimolecular NP formed by hyperbranched polyprodrug amphiphiles consisting of a hyperbranched core conjugated with reduction-activatable camptothecin (CPT) prodrugs and MRI contrast agent (Gd-DOTA), and hydrophilic coronas functionalized with guanidine residues (abbreviated as H-P(CPTM-co-DOTA(Gd))-b-P(OEGMA-co-GPMA), thus enabling a combination of chemotherapy and MRI [196]. Upon cellular internalization, CPT in its active form was released from the polyprodrug unimolecular NPs under intracellular cytosol reductive conditions (e.g., cytosol GSH ~10 mM). Synchronously, the MR contrast performance was also enhanced (~9.6-fold) in the reductive environment due to the hydrophobic-to-hydrophilic transition of the hyperbranched cores. Furthermore, the unimolecular NP exhibited a long blood circulation time (t1/2 ~10.6 h), enabling this unimolecular NP to be a promising candidate for synergistic imaging/chemotherapy.
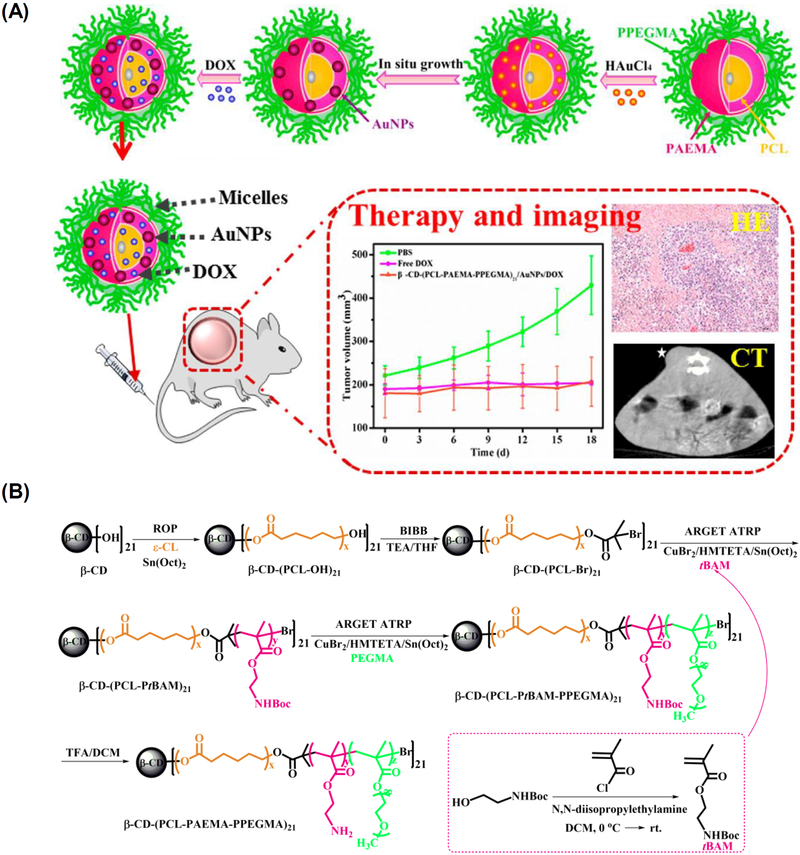
The tumor-targeted combination of chemotherapy and computed tomography (CT) imaging enabled by a unimolecular micelle system has also been demonstrated [197]. The unimolecular micelle was formed by a 21-arm star-like triblock polymer of β-cyclodextrin-[poly(ε-caprolactone)-poly(2-aminoethyl methacrylate)-poly(poly(ethylene glycol) methyl ether methacrylate)]21 [β-CD-(PCL-PAEMA-PPEGMA)21] (Figure 10). The micelles can encapsulate anticancer drugs, such as DOX, and serve as a template for fabricating small gold NPs AuNPs) for CT imaging. In vivo CT imaging revealed that the CT signals in the tumor sites treated by β-CD-(PCL-PAEMAPPEGMA)21/AuNPs/DOX unimolecular micelles were significantly higher than those of Omnipaque, a commercially available iodinated contrast agent for CT imaging. Specifically, compared to pre-injection, the magnitude of CT value improvement in the tumor sites was much greater in the unimolecular micelle treated group (32, 54, 65, or 71% of increase after 0.5 1, 2, or 4 h post injection, respectively) than in the Omnipaque treated group (18, 26, 36, or 43% of increase correspondingly). Moreover, the unimolecular micelles induced ~87% tumor reduction compared to the control group (PBS). Collectively, this platform holds great promise for combination CT imaging and chemotherapy.
Figure 10.
(A) Schematic representation of the fabrication of a dual-functional β-CD-(PCL-PAEMA-PPEGMA)21/AuNPs/DOX nanoplatform for cancer theranostics. (B) Synthetic route of β-CD-(PCL-PAEMA-PPEGMA)21. Reproduced with permission from Ref. [197].
4. Conclusions and Future Perspectives
In this review, we have surveyed recent progress on unimolecular NPs, including the architecture of water-dispersible unimolecular micelles and water-soluble unimolecular NPs, as well as their potential therapeutic and diagnostic applications. In contrast to multi-molecular self-assembled polymeric NPs, unimolecular NPs can offer excellent in vitro and in vivo stabilities due to their covalent nature. In addition, multifunctional unimolecular NPs can be conveniently fabricated due to their unique chemical structures and versatile chemistry. Unimolecular NPs, and in particular, unimolecular micelles, have been extensively investigated for targeted cancer therapy and, more recently, for targeted cancer theranostics. Unimolecular NPs have also been explored to treat a number of other diseases including vascular and eye diseases, as well as genetic disorders. Their diverse polymer chemistry makes it possible to design numerous desirable unimolecular NP platforms for different applications.
Despite their promise, some challenges exist for clinical translation. First, the synthesis process for the multi-arm polymer molecules used to form the unimolecular NPs may be more complex than for linear polymers. Thus, more facial polymer synthesis and conjugation strategies to fabricate well-defined multi-arm polymers are highly desirable. Of note, once a well-controlled scale-up synthesis process for the multi-arm polymers is established, it is expected that the unimolecular NPs will exhibit better reproducibility and quality assurance than multi-molecular self-assembled polymeric NPs. The unimolecular NPs can readily form in an aqueous solution and stay as intact NPs during freezing drying and the re-dispersion process, while the formation of multi-molecular nanoplatforms requires the optimization of various parameters, including concentrations, temperatures, solvents, and processing parameters. The stability of multi-molecular nanoplatforms is also affected by a number of processes (e.g., freezing drying and re-dispersion) and various factors (e.g., concentration, flow stress, interaction with serum proteins, etc.). The second challenge is a lack of fundamental understanding of the interactions between unimolecular NPs and cells/tissues. In contrast to some of the well-investigated drug delivery systems (e.g., liposomes and multi-molecular polymer micelles), exploration of core-shell structured unimolecular nanoparticles is still largely limited to small animal (e.g., mouse) experiments. Thus, more in-depth understanding of the interactions between unimolecular NPs and cells/tissues, and more comprehensive investigation on their in vivo behaviors including pharmacokinetics, pharmacodynamics, and biosafety needs to be carried out before clinical translation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Jo SD, Ku SH, Won Y-Y, Kim SH, Kwon IC, Targeted nanotheranostics for future personalized medicine: recent progress in cancer therapy, Theranostics, 6 (2016) 1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ma Y, Huang J, Song S, Chen H, Zhang Z, Cancer-Targeted Nanotheranostics: Recent Advances and Perspectives, Small, 12 (2016) 4936–4954. [DOI] [PubMed] [Google Scholar]
- [3].Sapiezynski J, Taratula O, Rodriguez-Rodriguez L, Minko T, Precision targeted therapy of ovarian cancer, Journal of Controlled Release, 243 (2016) 250–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ulbrich K, Hola K, Subr V, Bakandritsos A, Tucek J, Zboril R, Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies, Chemical Reviews, 116 (2016) 5338–5431. [DOI] [PubMed] [Google Scholar]
- [5].Wang S, Huang P, Chen X, Hierarchical Targeting Strategy for Enhanced Tumor Tissue Accumulation/Retention and Cellular Internalization, Advanced Materials, 28 (2016) 7340–7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dai Y, Xu C, Sun X, Chen X, Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment, Chemical Society Reviews, 46 (2017) 3830–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hartshorn CM, Bradbury MS, Lanza GM, Nel AE, Rao J, Wang AZ, Wiesner UB, Yang L, Grodzinski P, Nanotechnology Strategies To Advance Outcomes in Clinical Cancer Care, ACS Nano, 12 (2017) 24–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shi J, Kantoff PW, Wooster R, Farokhzad OC, Cancer nanomedicine: progress, challenges and opportunities, Nature Reviews Cancer, 17 (2017) 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sun Q, Zhou Z, Qiu N, Shen Y, Rational Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization, Advanced Materials, 29 (2017) 1606628. [DOI] [PubMed] [Google Scholar]
- [10].Wang Y, Sun S, Zhang Z, Shi D, Nanomaterials for Cancer Precision Medicine, Advanced Materials, 30 (2018) 1705660. [DOI] [PubMed] [Google Scholar]
- [11].Rosenblum D, Joshi N, Tao W, Karp JM, Peer D, Progress and challenges towards targeted delivery of cancer therapeutics, Nature communications, 9 (2018) 1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tao W, Zeng X, Liu T, Wang Z, Xiong Q, Ouyang C, Huang L, Mei L, Docetaxel-loaded nanoparticles based on star-shaped mannitol-core PLGA-TPGS diblock copolymer for breast cancer therapy, Acta biomaterialia, 9 (2013) 8910–8920. [DOI] [PubMed] [Google Scholar]
- [13].Tao W, Zeng X, Wu J, Zhu X, Yu X, Zhang X, Zhang J, Liu G, Mei L, Polydopamine-based surface modification of novel nanoparticle-aptamer bioconjugates for in vivo breast cancer targeting and enhanced therapeutic effects, Theranostics, 6 (2016) 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tao W, Zhang J, Zeng X, Liu D, Liu G, Zhu X, Liu Y, Yu Q, Huang L, Mei L, Blended nanoparticle system based on miscible structurally similar polymers: a safe, simple, targeted, and surprisingly high efficiency vehicle for cancer therapy, Advanced healthcare materials, 4 (2015) 1203–1214. [DOI] [PubMed] [Google Scholar]
- [15].Tao W, Zeng X, Zhang J, Zhu H, Chang D, Zhang X, Gao Y, Tang J, Huang L, Mei L, Synthesis of cholic acid-core poly (ε-caprolactone-ran-lactide)-b-poly (ethylene glycol) 1000 random copolymer as a chemotherapeutic nanocarrier for liver cancer treatment, Biomaterials Science, 2 (2014) 1262–1274. [DOI] [PubMed] [Google Scholar]
- [16].Zheng M, Tao W, Zou Y, Farokhzad OC, Shi B, Nanotechnology-Based Strategies for siRNA Brain Delivery for Disease Therapy, Trends in biotechnology, 36 (2018) 562–575. [DOI] [PubMed] [Google Scholar]
- [17].Marrache S, Dhar S, Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis, Proceedings of the National Academy of Sciences, 110 (2013) 9445–9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Anselmo AC, Modery-Pawlowski CL, Menegatti S, Kumar S, Vogus DR, Tian LL, Chen M, Squires TM, Gupta AS, Mitragotri S, Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries, ACS nano, 8 (2014) 11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lewis DR, Petersen LK, York AW, Zablocki KR, Joseph LB, Kholodovych V, Prud’homme RK, Uhrich KE, Moghe PV, Sugar-based amphiphilic nanoparticles arrest atherosclerosis in vivo, Proceedings of the National Academy of Sciences, 112 (2015) 2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kamaly N, Fredman G, Fojas JJR, Subramanian M, Choi W II, Zepeda K, Vilos C, Yu M, Gadde S, Wu J, Milton J, Carvalho Leitao R, Rosa Fernandes L, Hasan M, Gao H, Nguyen V, Harris J, Tabas I, Farokhzad OC, Targeted Interleukin-10 Nanotherapeutics Developed with a Microfluidic Chip Enhance Resolution of Inflammation in Advanced Atherosclerosis, ACS Nano, 10 (2016) 5280–5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Myerson JW, Anselmo AC, Liu Y, Mitragotri S, Eckmann DM, Muzykantov VR, Non-affinity factors modulating vascular targeting of nano-and microcarriers, Advanced drug delivery reviews, 99 (2016) 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang Y, Chen J, Yang B, Qiao H, Gao L, Su T, Ma S, Zhang X, Li X, Liu G, In vivo MR and fluorescence dual-modality imaging of atherosclerosis characteristics in mice using profilin-1 targeted magnetic nanoparticles, Theranostics, 6 (2016) 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang Y-J, Larsson M, Huang W-T, Chiou S-H, Nicholls SJ, Chao J-I, Liu D-M, The use of polymer-based nanoparticles and nanostructured materials in treatment and diagnosis of cardiovascular diseases: Recent advances and emerging designs, Progress in Polymer Science, 57 (2016) 153–178. [Google Scholar]
- [24].Beldman TJ, Senders ML, Alaarg A, Pérez-Medina C, Tang J, Zhao Y, Fay F, Deichmöller J, Born B, Desclos E, van der Wel NN, Hoebe RA, Kohen F, Kartvelishvily E, Neeman M, Reiner T, Calcagno C, Fayad ZA, de Winther MPJ, Lutgens E, Mulder WJM, Kluza E, Hyaluronan Nanoparticles Selectively Target Plaque-Associated Macrophages and Improve Plaque Stability in Atherosclerosis, ACS Nano, 11 (2017) 5785–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gao W, Zhao Y, Li X, Sun Y, Cai M, Cao W, Liu Z, Tong L, Cui G, Tang B, H2O2-responsive and plaque-penetrating nanoplatform for mTOR gene silencing with robust anti-atherosclerosis efficacy, Chemical Science, 9 (2017) 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Keliher EJ, Ye Y-X, Wojtkiewicz GR, Aguirre AD, Tricot B, Senders ML, Groenen H, Fay F, Perez-Medina C, Calcagno C, Carlucci G, Reiner T, Sun Y, Courties G, Iwamoto Y, Kim H-Y, Wang C, Chen JW, Swirski FK, Wey H-Y, Hooker J, Fayad ZA, Mulder WJM, Weissleder R, Nahrendorf M, Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischaemic heart disease, Nature communications, 8 (2017) 14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xiong M-H, Li Y-J, Bao Y, Yang X-Z, Hu B, Wang J, Bacteria-Responsive Multifunctional Nanogel for Targeted Antibiotic Delivery, Advanced Materials, 24 (2012) 6175–6180. [DOI] [PubMed] [Google Scholar]
- [28].Akin D, Sturgis J, Ragheb K, Sherman D, Burkholder K, Robinson JP, Bhunia AK, Mohammed S, Bashir R, Bacteria-mediated delivery of nanoparticles and cargo into cells, Nature nanotechnology, 2 (2007) 441–449. [DOI] [PubMed] [Google Scholar]
- [29].Lam SJ, O’Brien-Simpson NM, Pantarat N, Sulistio A, Wong EHH, Chen Y-Y, Lenzo JC, Holden JA, Blencowe A, Reynolds EC, Qiao GG, Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers, 1 (2016) 16162. [DOI] [PubMed] [Google Scholar]
- [30].Cagno V, Andreozzi P, D’Alicarnasso M, Jacob Silva P, Mueller M, Galloux M, Le Goffic R, Jones ST, Vallino M, Hodek J, Weber J, Sen S, Janeček E-R, Bekdemir A, Sanavio B, Martinelli C, Donalisio M, Rameix Welti M-A, Eleouet J-F, Han Y, Kaiser L, Vukovic L, Tapparel C, Král P, Krol S, Lembo D, Stellacci F, Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism, Nature Materials, 17 (2017) 195–203. [DOI] [PubMed] [Google Scholar]
- [31].Chin W, Zhong G, Pu Q, Yang C, Lou W, De Sessions PF, Periaswamy B, Lee A, Liang ZC, Ding X, Gao S, Chu CW, Bianco S, Bao C, Tong YW, Fan W, Wu M, Hedrick JL, Yang YY, A macromolecular approach to eradicate multidrug resistant bacterial infections while mitigating drug resistance onset, Nature Communications, 9 (2018) 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hussain S, Joo J, Kang J, Kim B, Braun GB, She Z-G, Kim D, Mann AP, Mölder T, Teesalu T, Carnazza S, Guglielmino S, Sailor MJ, Ruoslahti E, Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy, Nature Biomedical Engineering, 2 (2018) 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rigo S, Cai C, Gunkel-Grabole G, Maurizi L, Zhang X, Xu J, Palivan CG, Nanoscience-Based Strategies to Engineer Antimicrobial Surfaces, Advanced Science, 5 (2018) 1700892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Anselmo AC, Mitragotri S, Impact of particle elasticity on particle-based drug delivery systems, Advanced Drug Delivery Reviews, 108 (2017) 51–67. [DOI] [PubMed] [Google Scholar]
- [35].Alvarez MM, Aizenberg J, Analoui M, Andrews AM, Bisker G, Boyden ES, Kamm RD, Karp JM, Mooney DJ, Oklu R, Peer D, Stolzoff M, Strano MS, Trujillo-de Santiago G, Webster TJ, Weiss PS, Khademhosseini A, Emerging Trends in Micro- and Nanoscale Technologies in Medicine: From Basic Discoveries to Translation, ACS Nano, 11 (2017) 5195–5214. [DOI] [PubMed] [Google Scholar]
- [36].Chen G, Roy I, Yang C, Prasad PN, Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy, Chemical Reviews, 116 (2016) 2826–2885. [DOI] [PubMed] [Google Scholar]
- [37].Min Y, Caster JM, Eblan MJ, Wang AZ, Clinical Translation of Nanomedicine, Chemical Reviews, 115 (2015) 11147–11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nature biotechnology, 33 (2015) 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li F, Lu J, Kong X, Hyeon T, Ling D, Dynamic Nanoparticle Assemblies for Biomedical Applications, Advanced Materials, 29 (2017) 1605897. [DOI] [PubMed] [Google Scholar]
- [40].Chen H, Zhang W, Zhu G, Xie J, Chen X, Rethinking cancer nanotheranostics, Nature Reviews Materials, 2 (2017) 17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Webber MJ, Langer R, Drug delivery by supramolecular design, Chemical Society Reviews, 46 (2017) 6600–6620. [DOI] [PubMed] [Google Scholar]
- [42].Lu Y, Aimetti AA, Langer R, Gu Z, Bioresponsive materials, Nature Reviews Materials, 1 (2016) 16075. [Google Scholar]
- [43].Croy S, Kwon G, Polymeric micelles for drug delivery, Current pharmaceutical design, 12 (2006) 4669–4684. [DOI] [PubMed] [Google Scholar]
- [44].Nishiyama N, Kataoka K, Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery, Pharmacology & therapeutics, 112 (2006) 630–648. [DOI] [PubMed] [Google Scholar]
- [45].Kataoka K, Harada A, Nagasaki Y, Block copolymer micelles for drug delivery: design, characterization and biological significance, Advanced drug delivery reviews, 47 (2001) 113–131. [DOI] [PubMed] [Google Scholar]
- [46].Kim S, Shi Y, Kim JY, Park K, Cheng J-X, Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle–cell interaction, Expert opinion on drug delivery, 7 (2010) 49–62. [DOI] [PubMed] [Google Scholar]
- [47].Deng C, Jiang Y, Cheng R, Meng F, Zhong Z, Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: promises, progress and prospects, Nano Today, 7 (2012) 467–480. [Google Scholar]
- [48].Talelli M, Barz M, Rijcken CJ, Kiessling F, Hennink WE, Lammers T, Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation, Nano today, 10 (2015) 93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang Y, Li Q, Welsh WJ, Moghe PV, Uhrich KE, Micellar and structural stability of nanoscale amphiphilic polymers: implications for anti-atherosclerotic bioactivity, Biomaterials, 84 (2016) 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Heise A, Hedrick JL, Frank CW, Miller RD, Starlike block copolymers with amphiphilic arms as models for unimolecular micelles, JACS, 121 (1999) 8647–8648. [Google Scholar]
- [51].Lawrence MJ, Surfactant systems: their use in drug delivery, Chemical Society Reviews, 23 (1994) 417–424. [Google Scholar]
- [52].Kim S, Shi Y, Kim J, Park K, Cheng J, Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction, Expert Opin Drug Deliv., 7 (2010) 49–62. [DOI] [PubMed] [Google Scholar]
- [53].Takeda KM, Yamasaki Y, Dirisala A, Ikeda S, Tockary TA, Toh K, Osada K, Kataoka K, Effect of shear stress on structure and function of polyplex micelles from poly (ethylene glycol)-poly (l-lysine) block copolymers as systemic gene delivery carrier, Biomaterials, 126 (2017) 31–38. [DOI] [PubMed] [Google Scholar]
- [54].Sun X, Wang G, Zhang H, Hu S, Liu X, Tang J, Shen Y, The Blood Clearance Kinetics and Pathway of Polymeric Micelles in Cancer Drug Delivery, ACS nano, (2018). [DOI] [PubMed] [Google Scholar]
- [55].Maeda H, Khatami M, Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs, Clinical and translational medicine, 7 (2018) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Eugene D, Grayson S, Unimolecular micelles for Drug delivery, Encycl Supramolecular Chem, 1 (2010) 1e15. [Google Scholar]
- [57].Sunder A, Krämer M, Hanselmann R, Mülhaupt R, Frey H, Molecular nanocapsules based on amphiphilic hyperbranched polyglycerols, Angewandte Chemie International Edition, 38 (1999) 3552–3555. [DOI] [PubMed] [Google Scholar]
- [58].Newkome GR, Moorefield CN, Baker GR, Saunders MJ, Grossman SH, Unimolecular micelles, Angewandte Chemie International Edition in English, 30 (1991) 1178–1180. [Google Scholar]
- [59].Liu H, Jiang A, Guo J, Uhrich KE, Unimolecular micelles: synthesis and characterization of amphiphilic polymer systems, Journal of Polymer Science Part A: Polymer Chemistry, 37 (1999) 703–711. [Google Scholar]
- [60].Maniruzzaman M, Kawaguchi S, Ito K, Micellar copolymerization of styrene with poly (ethylene oxide) macromonomer in water: approach to unimolecular nanoparticles via pseudo-living radical polymerization, Macromolecules, 33 (2000) 1583–1592. [Google Scholar]
- [61].Chen S, Zhang X-Z, Cheng S-X, Zhuo R-X, Gu Z-W, Functionalized amphiphilic hyperbranched polymers for targeted drug delivery, Biomacromolecules, 9 (2008) 2578–2585. [DOI] [PubMed] [Google Scholar]
- [62].Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S, Folate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn H40, poly(L-lactide) and poly(ethylene glycol) for tumor-targeted drug delivery, Biomaterials, 30 (2009) 3009–3019. [DOI] [PubMed] [Google Scholar]
- [63].Luo S, Xu J, Zhu Z, Wu C, Liu S, Phase transition behavior of unimolecular micelles with thermoresponsive poly (N-isopropylacrylamide) coronas, The Journal of Physical Chemistry B, 110 (2006) 9132–9139. [DOI] [PubMed] [Google Scholar]
- [64].Haag R, Supramolecular drug-delivery systems based on polymeric core–shell architectures, Angewandte Chemie International Edition, 43 (2004) 278–282. [DOI] [PubMed] [Google Scholar]
- [65].Jones M-C, Ranger M, Leroux J-C, pH-sensitive unimolecular polymeric micelles: synthesis of a novel drug carrier, Bioconjugate chemistry, 14 (2003) 774–781. [DOI] [PubMed] [Google Scholar]
- [66].Liu M, Kono K, Fréchet JM, Water-soluble dendritic unimolecular micelles:: Their potential as drug delivery agents, Journal of Controlled Release, 65 (2000) 121–131. [DOI] [PubMed] [Google Scholar]
- [67].Fan X, Li Z, Loh XJ, Recent development of unimolecular micelles as functional materials and applications, Polymer Chemistry, 7 (2016) 5898–5919. [Google Scholar]
- [68].Jin X, Sun P, Tong G, Zhu X, Star polymer-based unimolecular micelles and their application in bio-imaging and diagnosis, Biomaterials, (2018). [DOI] [PubMed] [Google Scholar]
- [69].Yang X, Grailer JJ, Pilla S, Steeber DA, Gong S, Tumor-targeting, pH-responsive, and stable unimolecular micelles as drug nanocarriers for targeted cancer therapy, Bioconjugate chemistry, 21 (2010) 496–504. [DOI] [PubMed] [Google Scholar]
- [70].Liu F, Ma F, Wang Y, Hao L, Zeng H, Jia C, Wang Y, Liu P, Ong IM, Li B, PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis, Nat Cell Biol, 19 (2017) 1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Liu G, Gao H, Zuo Y, Zeng X, Tao W, Tsai H.-i., Mei L, DACHPt-loaded unimolecular micelles based on hydrophilic dendritic block copolymers for enhanced therapy of lung cancer, ACS applied materials & interfaces, 9 (2016) 112–119. [DOI] [PubMed] [Google Scholar]
- [72].Jiang R, Lu X, Yang M, Deng W, Fan Q, Huang W, Monodispersed brush-like conjugated polyelectrolyte nanoparticles with efficient and visualized SiRNA delivery for gene silencing, Biomacromolecules, 14 (2013) 3643–3652. [DOI] [PubMed] [Google Scholar]
- [73].Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S, Gold nanoparticles with a monolayer of doxorubicin-conjugated amphiphilic block copolymer for tumor-targeted drug delivery, Biomaterials, 30 (2009) 6065–6075. [DOI] [PubMed] [Google Scholar]
- [74].Chen G, Jaskula-Sztul R, Esquibel CR, Lou I, Zheng Q, Dammalapati A, Harrison A, Eliceiri KW, Tang W, Chen H, Gong S, Neuroendocrine Tumor-Targeted Upconversion Nanoparticle-Based Micelles for Simultaneous NIR-Controlled Combination Chemotherapy and Photodynamic Therapy, and Fluorescence Imaging, Advanced Functional Materials, (2017) 1604671–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chen H, Zou H, Paholak HJ, Ito M, Qian W, Che Y, Sun D, Thiol-reactive amphiphilic block copolymer for coating gold nanoparticles with neutral and functionable surfaces, Polymer chemistry, 5 (2014) 2768–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Budijono SJ, Shan J, Yao N, Miura Y, Hoye T, Austin RH, Ju Y, Prud’homme RK, Synthesis of stable block-copolymer-protected NaYF4: Yb3+, Er3+ up-converting phosphor nanoparticles, Chemistry of Materials, 22 (2009) 311–318. [Google Scholar]
- [77].Chen G, Ma B, Wang Y, Xie R, Li C, Dou K, Gong S, CuS-Based Theranostic Micelles for NIR-Controlled Combination Chemotherapy and Photothermal Therapy and Photoacoustic Imaging, ACS applied materials & interfaces, 9 (2017) 41700–41711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wang Y, Wang Y, Chen G, Li Y, Xu W, Gong S, Quantum-Dot-Based Theranostic Micelles Conjugated with an Anti-EGFR Nanobody for Triple-Negative Breast Cancer Therapy, ACS applied materials & interfaces, 9 (2017) 30297–30305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Liu Y, Inoue Y, Ishihara K, Surface functionalization of quantum dots with fine-structured pH-sensitive phospholipid polymer chains, Colloids and Surfaces B: Biointerfaces, 135 (2015) 490–496. [DOI] [PubMed] [Google Scholar]
- [80].Kim YH, Hyperbranched polymers 10 years after, Journal of Polymer Science Part A: Polymer Chemistry, 36 (1998) 1685–1698. [Google Scholar]
- [81].Zhu Q, Qiu F, Zhu B, Zhu X, Hyperbranched polymers for bioimaging, Rsc Advances, 3 (2013) 2071–2083. [Google Scholar]
- [82].Voit B, New developments in hyperbranched polymers, Journal of Polymer Science Part A: Polymer Chemistry, 38 (2000) 2505–2525. [Google Scholar]
- [83].Caminade A-M, Yan D, Smith DK, Dendrimers and hyperbranched polymers, Chemical Society Reviews, 44 (2015) 3870–3873. [DOI] [PubMed] [Google Scholar]
- [84].Feast WJ, Stainton NM, Synthesis, structure and properties of some hyperbranched polyesters, Journal of Materials Chemistry, 5 (1995) 405–411. [Google Scholar]
- [85].Bakhshi H, Agarwal S, Hyperbranched polyesters as biodegradable and antibacterial additives, Journal of Materials Chemistry B, 5 (2017) 6827–6834. [DOI] [PubMed] [Google Scholar]
- [86].Borgaonkar P, Sharma S, Chen M, Bhowmick S, Schmidt DF, A flexible approach to the preparation of polymer scaffolds for tissue engineering, Macromolecular bioscience, 7 (2007) 201–207. [DOI] [PubMed] [Google Scholar]
- [87].Žagar E, Žigon M, Characterization of a commercial hyperbranched aliphatic polyester based on 2, 2-bis (methylol) propionic acid, Macromolecules, 35 (2002) 9913–9925. [Google Scholar]
- [88].Kontoyianni C, Sideratou Z, Theodossiou T, Tziveleka LA, Tsiourvas D, Paleos CM, A novel micellar PEGylated hyperbranched polyester as a prospective drug delivery system for paclitaxel, Macromolecular bioscience, 8 (2008) 871–881. [DOI] [PubMed] [Google Scholar]
- [89].Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S, Folate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn® H40, poly (l-lactide) and poly (ethylene glycol) for tumor-targeted drug delivery, Biomaterials, 30 (2009) 3009–3019. [DOI] [PubMed] [Google Scholar]
- [90].Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S, Amphiphilic Multi-Arm Block Copolymer Based on Hyperbranched Polyester, Poly (L-lactide) and Poly (ethylene glycol) as a Drug Delivery Carrier, Macromolecular bioscience, 9 (2009) 515–524. [DOI] [PubMed] [Google Scholar]
- [91].Zhang S, Xu J, Chen H, Song Z, Wu Y, Dai X, Kong J, Acid-Cleavable Unimolecular Micelles from Amphiphilic Star Copolymers for Triggered Release of Anticancer Drugs, Macromolecular bioscience, 17 (2017). [DOI] [PubMed] [Google Scholar]
- [92].Nabid MR, Bide Y, H40-PCL-PEG unimolecular micelles both as anchoring sites for palladium nanoparticles and micellar catalyst for Heck reaction in water, Applied Catalysis A: General, 469 (2014) 183–190. [Google Scholar]
- [93].Rezaei SJT, Nabid MR, Niknejad H, Entezami AA, Multifunctional and thermoresponsive unimolecular micelles for tumor-targeted delivery and site-specifically release of anticancer drugs, Polymer, 53 (2012) 3485–3497. [Google Scholar]
- [94].Zeng X, Tao W, Wang Z, Zhang X, Zhu H, Wu Y, Gao Y, Liu K, Jiang Y, Huang L, Docetaxel-Loaded Nanoparticles of Dendritic Amphiphilic Block Copolymer H40-PLA-b-TPGS for Cancer Treatment, Particle & Particle Systems Characterization, 32 (2015) 112–122. [Google Scholar]
- [95].Rezaei SJT, Abandansari HS, Nabid MR, Niknejad H, pH-responsive unimolecular micelles self-assembled from amphiphilic hyperbranched block copolymer for efficient intracellular release of poorly water-soluble anticancer drugs, Journal of colloid and interface science, 425 (2014) 27–35. [DOI] [PubMed] [Google Scholar]
- [96].Pang Y, Liu J, Su Y, Zhu B, Huang W, Zhou Y, Zhu X, Yan D, Bioreducible unimolecular micelles based on amphiphilic multiarm hyperbranched copolymers for triggered drug release, Science China Chemistry, 53 (2010) 2497–2508. [Google Scholar]
- [97].Luo S, Han M, Cao Y, Ling C, Zhang Y, Temperature-and pH-responsive unimolecular micelles with a hydrophobic hyperbranched core, Colloid and Polymer Science, 289 (2011) 1243–1251. [Google Scholar]
- [98].Abbina S, Vappala S, Kumar P, Siren EM, La CC, Abbasi U, Brooks DE, Kizhakkedathu JN, Hyperbranched polyglycerols: recent advances in synthesis, biocompatibility and biomedical applications, Journal of Materials Chemistry B, 5 (2017) 9249–9277. [DOI] [PubMed] [Google Scholar]
- [99].Du F, Hönzke S, Neumann F, Keilitz J, Chen W, Ma N, Hedtrich S, Haag R, Development of biodegradable hyperbranched core-multishell nanocarriers for efficient topical drug delivery, Journal of Controlled Release, 242 (2016) 42–49. [DOI] [PubMed] [Google Scholar]
- [100].Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, Hanifehpour Y, Nejati-Koshki K, Pashaei-Asl R, Dendrimers: synthesis, applications, and properties, Nanoscale Research Letters, 9 (2014) 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Noriega-Luna B, Godínez LA, Rodríguez FJ, Rodríguez A, Larrea G, Sosa-Ferreyra C, Mercado-Curiel R, Manríquez J, Bustos E, Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection, Journal of Nanomaterials, 2014 (2014) 39. [Google Scholar]
- [102].Buhleier E, Wehner W, Vogtle F, Cascade and Nonskid Chain-like Synthesis of Molecular Cavity Topologies 1978, Synthesis, 55 155–158. [Google Scholar]
- [103].Grayson SM, Frechet JM, Convergent dendrons and dendrimers: from synthesis to applications, Chemical Reviews, 101 (2001) 3819–3868. [DOI] [PubMed] [Google Scholar]
- [104].Šebestík J, Reiniš M, Ježek J, Synthesis of Dendrimers: Convergent and Divergent Approaches, Biomedical Applications of Peptide-, Glyco-and Glycopeptide Dendrimers, and Analogous Dendrimeric Structures, Springer; 2012, pp. 55–81. [Google Scholar]
- [105].Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P, A new class of polymers: starburst-dendritic macromolecules, Polymer Journal, 17 (1985) 117. [Google Scholar]
- [106].Denkewalter R, Kolc J, Lukasavage W, US Pat. 4289872, 1981, Chem. Abstr, 1985, pp. P79324q.
- [107].Newkome GR, Yao Z, Baker GR, Gupta VK, Micelles. Part 1. Cascade molecules: a new approach to micelles. A [27]-arborol, The Journal of Organic Chemistry, 50 (1985) 2003–2004. [Google Scholar]
- [108].Carnahan MA, Grinstaff MW, Synthesis of generational polyester dendrimers derived from glycerol and succinic or adipic acid, Macromolecules, 39 (2006) 609–616. [Google Scholar]
- [109].Buhleier E, Wehner W, Vögtle F, ′CASCADE′‐AND ′NONSKID‐CHAIN‐LIKE′ SYNTHESES OF MOLECULAR CAVITY TOPOLOGIES, ChemInform, 9 (1978). [Google Scholar]
- [110].Hari BV, Kalaimagal K, Porkodi R, Gajula PK, Ajay J, Dendrimer: globular nanostructured materials for drug delivery, Int J PharmTech Res, 4 (2012) 432–451. [Google Scholar]
- [111].Sampathkumar SG, Yarema KJ, Dendrimers in cancer treatment and diagnosis, Nanotechnologies for the Life Sciences, (2007). [Google Scholar]
- [112].Dufes C, Uchegbu IF, Schätzlein AG, Dendrimers in gene delivery, Advanced drug delivery reviews, 57 (2005) 2177–2202. [DOI] [PubMed] [Google Scholar]
- [113].Bourne N, Stanberry L, Kern E, Holan G, Matthews B, Bernstein D, Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection, Antimicrobial agents and chemotherapy, 44 (2000) 2471–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Maiti PK, Çaǧın T, Wang G, Goddard WA, Structure of PAMAM dendrimers: Generations 1 through 11, Macromolecules, 37 (2004) 6236–6254. [Google Scholar]
- [115].Guo J, Hong H, Chen G, Shi S, Zheng Q, Zhang Y, Theuer CP, Barnhart TE, Cai W, Gong S, Image-guided and tumor-targeted drug delivery with radiolabeled unimolecular micelles, Biomaterials, 34 (2013) 8323–8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Brinkman AM, Chen G, Wang Y, Hedman CJ, Sherer NM, Havighurst TC, Gong S, Xu W, Aminoflavone-loaded EGFR-targeted unimolecular micelle nanoparticles exhibit anti-cancer effects in triple negative breast cancer, Biomaterials, 101 (2016) 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].He H, Wang Y, Wen H, Jia X, Dendrimer-based multilayer nanocarrier for potential synergistic paclitaxel–doxorubicin combination drug delivery, RSC Advances, 4 (2014) 3643–3652. [Google Scholar]
- [118].Zhao L, Chen G, Li J, Fu Y, Mavlyutov TA, Yao A, Nickells RW, Gong S, Guo L-W, An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration, Journal of Controlled Release, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Jaskula-Sztul R, Chen G, Dammalapati A, Harrison A, Tang W, Gong S, Chen H, AB3-loaded and tumor-targeted unimolecular micelles for medullary thyroid cancer treatment, Journal of Materials Chemistry B, 5 (2017) 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Chen G, Jaskula–Sztul R, Harrison A, Dammalapati A, Xu W, Cheng Y, Chen H, Gong S, KE108-Conjugated Unimolecular Micelles Loaded with a Novel HDAC Inhibitor Thailandepsin-A for Targeted Neuroendocrine Cancer Therapy, Biomaterials, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wang F, Bronich TK, Kabanov AV, Rauh RD, Roovers J, Synthesis and evaluation of a star amphiphilic block copolymer from poly (ε-caprolactone) and poly (ethylene glycol) as a potential drug delivery carrier, Bioconjugate chemistry, 16 (2005) 397–405. [DOI] [PubMed] [Google Scholar]
- [122].Chen G, Shi X, Wang B, Xie R, Guo L-W, Gong S, Kent KC, Unimolecular micelle-based hybrid system for perivascular drug delivery produces long-term efficacy for neointima attenuation in rats, Biomacromolecules, 18 (2017) 2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Yuan W, Yuan J, Zhang F, Xie X, Pan C, Synthesis, characterization, crystalline morphologies, and hydrophilicity of brush copolymers with double crystallizable side chains, Macromolecules, 40 (2007) 9094–9102. [Google Scholar]
- [124].Hu M, Xia Y, McKenna GB, Kornfield JA, Grubbs RH, Linear rheological response of a series of densely branched brush polymers, Macromolecules, 44 (2011) 6935–6943. [Google Scholar]
- [125].Milner ST, Witten T, Cates M, Theory of the grafted polymer brush, Macromolecules, 21 (1988) 2610–2619. [Google Scholar]
- [126].Gao H, Matyjaszewski K, Synthesis of molecular brushes by “grafting onto” method: combination of ATRP and click reactions, Journal of the American Chemical Society, 129 (2007) 6633–6639. [DOI] [PubMed] [Google Scholar]
- [127].Johnson JA, Lu YY, Burts AO, Lim Y-H, Finn M, Koberstein JT, Turro NJ, Tirrell DA, Grubbs RH, Core-clickable PEG-branch-azide bivalent-bottle-brush polymers by ROMP: grafting-through and clicking-to, Journal of the American Chemical Society, 133 (2010) 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Johnson JA, Lu YY, Burts AO, Xia Y, Durrell AC, Tirrell DA, Grubbs RH, Drug-loaded, bivalent-bottle-brush polymers by graft-through ROMP, Macromolecules, 43 (2010) 10326–10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zhao P, Liu L, Feng X, Wang C, Shuai X, Chen Y, Molecular Nanoworm with PCL Core and PEO Shell as a Non-spherical Carrier for Drug Delivery, Macromolecular rapid communications, 33 (2012) 1351–1355. [DOI] [PubMed] [Google Scholar]
- [130].Guo J, Hong H, Chen G, Shi S, Nayak TR, Theuer CP, Barnhart TE, Cai W, Gong S, Theranostic unimolecular micelles based on brush-shaped amphiphilic block copolymers for tumor-targeted drug delivery and positron emission tomography imaging, ACS applied materials & interfaces, 6 (2014) 21769–21779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Tu XY, Meng C, Wang YF, Ma LW, Wang BY, He JL, Ni PH, Ji XL, Liu MZ, Wei H, Fabrication of Thermosensitive Cyclic Brush Copolymer with Enhanced Therapeutic Efficacy for Anticancer Drug Delivery, Macromolecular rapid communications, 39 (2018) 1700744. [DOI] [PubMed] [Google Scholar]
- [132].Phillips SH, Haddad TS, Tomczak SJ, Developments in nanoscience: polyhedral oligomeric silsesquioxane (POSS)-polymers, Current Opinion in Solid State and Materials Science, 8 (2004) 21–29. [Google Scholar]
- [133].Fan X, Hu Z, Wang G, Synthesis and unimolecular micelles of amphiphilic copolymer with dendritic poly (l-lactide) core and poly (ethylene oxide) shell for drug delivery, RSC Advances, 5 (2015) 100816–100823. [Google Scholar]
- [134].Loftsson T, Duchêne D, Cyclodextrins and their pharmaceutical applications, International journal of pharmaceutics, 329 (2007) 1–11. [DOI] [PubMed] [Google Scholar]
- [135].Pang X, Feng C, Xu H, Han W, Xin X, Xia H, Qiu F, Lin Z, Unimolecular micelles composed of inner coil-like blocks and outer rod-like blocks crafted by combination of living polymerization with click chemistry, Polymer Chemistry, 5 (2014) 2747–2755. [Google Scholar]
- [136].Wang Y, Wang L, Chen G, Gong S, Carboplatin-Complexed and cRGD-Conjugated Unimolecular Nanoparticles for Targeted Ovarian Cancer Therapy, Macromolecular bioscience, 17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Chen G, Wang Y, Xie R, Gong S, Tumor-targeted pH/redox dual-sensitive unimolecular nanoparticles for efficient siRNA delivery, Journal of Controlled Release, 259 (2017) 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Tambe P, Kumar P, Karpe YA, Paknikar KM, Gajbhiye V, Triptorelin Tethered Multifunctional PAMAM-Histidine-PEG Nanoconstructs Enable Specific Targeting and Efficient Gene Silencing in LHRH Overexpressing Cancer Cells, ACS applied materials & interfaces, 9 (2017) 35562–35573. [DOI] [PubMed] [Google Scholar]
- [139].Duncan R, Kopeček J, Soluble synthetic polymers as potential drug carriers, Polymers in medicine, Springer; 1984, pp. 51–101. [Google Scholar]
- [140].Hajj KA, Whitehead KA, Tools for translation: non-viral materials for therapeutic mRNA delivery, Nature Reviews Materials, 2 (2017) 17056. [Google Scholar]
- [141].Kanasty R, Dorkin JR, Vegas A, Anderson D, Delivery materials for siRNA therapeutics, Nat Mater, 12 (2013) 967–977. [DOI] [PubMed] [Google Scholar]
- [142].Dahlman JE, Barnes C, Khan OF, Thiriot A, Jhunjunwala S, Shaw TE, Xing Y, Sager HB, Sahay G, Speciner L, Bader A, Bogorad RL, Yin H, Racie T, Dong Y, Jiang S, Seedorf D, Dave A, Singh Sandhu K, Webber MJ, Novobrantseva T, Ruda VM, Lytton-JeanAbigail KR, Levins CG, Kalish B, Mudge DK, Perez M, Abezgauz L, Dutta P, Smith L, Charisse K, Kieran MW, Fitzgerald K, Nahrendorf M, Danino D, Tuder RM, von Andrian UH, Akinc A, Panigrahy D, Schroeder A, Koteliansky V, Langer R, Anderson DG, In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight, Nat Nano, 9 (2014) 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Lu Y, Sun W, Gu Z, Stimuli-responsive nanomaterials for therapeutic protein delivery, Journal of Controlled Release, 194 (2014) 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Fahr A, Liu X, Drug delivery strategies for poorly water-soluble drugs, Expert opinion on drug delivery, 4 (2007) 403–416. [DOI] [PubMed] [Google Scholar]
- [145].Kalepu S, Nekkanti V, Insoluble drug delivery strategies: review of recent advances and business prospects, Acta Pharmaceutica Sinica B, 5 (2015) 442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Popeney CS, Lukowiak MC, Böttcher C, Schade B, Welker P, Mangoldt D, Gunkel G, Guan Z, Haag R, Tandem coordination, ring-opening, hyperbranched polymerization for the synthesis of water-soluble core–shell unimolecular transporters, ACS Macro Letters, 1 (2012) 564–567. [DOI] [PubMed] [Google Scholar]
- [147].Cao W, Zhou J, Mann A, Wang Y, Zhu L, Folate-functionalized unimolecular micelles based on a degradable amphiphilic dendrimer-like star polymer for cancer cell-targeted drug delivery, Biomacromolecules, 12 (2011) 2697–2707. [DOI] [PubMed] [Google Scholar]
- [148].Li X, Qian Y, Liu T, Hu X, Zhang G, You Y, Liu S, Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and MR imaging, Biomaterials, 32 (2011) 6595–6605. [DOI] [PubMed] [Google Scholar]
- [149].Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S, Amphiphilic multi-arm-block copolymer conjugated with doxorubicin via pH-sensitive hydrazone bond for tumor-targeted drug delivery, Biomaterials, 30 (2009) 5757–5766. [DOI] [PubMed] [Google Scholar]
- [150].Xiao Y, Hong H, Javadi A, Engle JW, Xu W, Yang Y, Zhang Y, Barnhart TE, Cai W, Gong S, Multifunctional unimolecular micelles for cancer-targeted drug delivery and positron emission tomography imaging, Biomaterials, 33 (2012) 3071–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Xu W, Burke JF, Pilla S, Chen H, Jaskula-Sztul R, Gong S, Octreotide-functionalized and resveratrol-loaded unimolecular micelles for targeted neuroendocrine cancer therapy, Nanoscale, 5 (2013) 9924–9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Xu W, Siddiqui IA, Nihal M, Pilla S, Rosenthal K, Mukhtar H, Gong S, Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer, Biomaterials, 34 (2013) 5244–5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Loaiza-Pérez AI, Kenney S, Boswell J, Hollingshead M, Alley MC, Hose C, Ciolino HP, Yeh GC, Trepel JB, Vistica DT, Aryl hydrocarbon receptor activation of an antitumor aminoflavone: basis of selective toxicity for MCF-7 breast tumor cells, Molecular Cancer Therapeutics, 3 (2004) 715–725. [PubMed] [Google Scholar]
- [154].Loaiza-Perez A, Kenney S, Boswell J, Hollingshead M, Hose C, Linehan W, Worrell R, Rubinstein L, Sausville E, Vistica D, Sensitivity of renal cell carcinoma to aminoflavone: role of CYP1A1, The Journal of urology, 171 (2004) 1688–1697. [DOI] [PubMed] [Google Scholar]
- [155].Moorkoth S, Synthesis and anti-cancer activity of novel thiazolidinone analogs of 6-aminoflavone, Chemical and Pharmaceutical Bulletin, 63 (2015) 974–985. [DOI] [PubMed] [Google Scholar]
- [156].Jia T, Huang S, Yang C, Wang M, Unimolecular micelles of pH-responsive star-like copolymers for co-delivery of anticancer drugs and small-molecular photothermal agents: a new drug-carrier for combinational chemo/photothermal cancer therapy, Journal of Materials Chemistry B, 5 (2017) 8514–8524. [DOI] [PubMed] [Google Scholar]
- [157].Zhao L, Chen G, Li J, Fu Y, Mavlyutov TA, Yao A, Nickells RW, Gong S, Guo L-W, An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration, Journal of Controlled Release, 247 (2017) 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Jukema JW, Ahmed TA, Verschuren JJ, Quax PH, Restenosis after PCI. Part 2: prevention and therapy, Nature Reviews Cardiology, 9 (2012) 79. [DOI] [PubMed] [Google Scholar]
- [159].Chaudhary MA, Guo L-W, Shi X, Chen G, Gong S, Liu B, Kent KC, Periadventitial drug delivery for the prevention of intimal hyperplasia following open surgery, Journal of Controlled Release, 233 (2016) 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Fleige E, Achazi K, Schaletzki K, Triemer T, Haag R, pH-responsive dendritic core–multishell nanocarriers, Journal of Controlled Release, 185 (2014) 99–108. [DOI] [PubMed] [Google Scholar]
- [161].Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z, Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery, J. Control. Release, 152 (2011) 2–12. [DOI] [PubMed] [Google Scholar]
- [162].Cheng R, Meng F, Deng C, Klok H-A, Zhong Z, Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery, Biomaterials, 34 (2013) 3647–3657. [DOI] [PubMed] [Google Scholar]
- [163].Lee Y, Mo H, Koo H, Park J-Y, Cho MY, Jin G.-w., Park J-S, Visualization of the degradation of a disulfide polymer, linear poly (ethylenimine sulfide), for gene delivery, Bioconjugate chemistry, 18 (2007) 13–18. [DOI] [PubMed] [Google Scholar]
- [164].Liu J, Pang Y, Huang W, Huang X, Meng L, Zhu X, Zhou Y, Yan D, Bioreducible micelles self-assembled from amphiphilic hyperbranched multiarm copolymer for glutathione-mediated intracellular drug delivery, Biomacromolecules, 12 (2011) 1567–1577. [DOI] [PubMed] [Google Scholar]
- [165].Porsch C, Zhang Y, Montañez MI, Malho J-M, Kostiainen MA, Nyström AM, Malmström, Disulfide-functionalized unimolecular micelles as selective redox-responsive nanocarriers, Biomacromolecules, 16 (2015) 2872–2883. [DOI] [PubMed] [Google Scholar]
- [166].Niidome T, Huang L, Gene therapy progress and prospects: nonviral vectors, Gene therapy, 9 (2002) 1647. [DOI] [PubMed] [Google Scholar]
- [167].Sinn P, Sauter S, McCray P Jr, Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors–design, biosafety, and production, Gene therapy, 12 (2005) 1089. [DOI] [PubMed] [Google Scholar]
- [168].Panyam J, Labhasetwar V, Biodegradable nanoparticles for drug and gene delivery to cells and tissue, Advanced drug delivery reviews, 55 (2003) 329–347. [DOI] [PubMed] [Google Scholar]
- [169].Pack DW, Hoffman AS, Pun S, Stayton PS, Design and development of polymers for gene delivery, Nature reviews Drug discovery, 4 (2005) 581. [DOI] [PubMed] [Google Scholar]
- [170].Kircheis R, Wightman L, Wagner E, Design and gene delivery activity of modified polyethylenimines, Advanced drug delivery reviews, 53 (2001) 341–358. [DOI] [PubMed] [Google Scholar]
- [171].Oba M, Miyata K, Osada K, Christie RJ, Sanjoh M, Li W, Fukushima S, Ishii T, Kano MR, Nishiyama N, Polyplex micelles prepared from ω-cholesteryl PEG-polycation block copolymers for systemic gene delivery, Biomaterials, 32 (2011) 652–663. [DOI] [PubMed] [Google Scholar]
- [172].Lee Y, Miyata K, Oba M, Ishii T, Fukushima S, Han M, Koyama H, Nishiyama N, Kataoka K, Charge-Conversion Ternary Polyplex with Endosome Disruption Moiety: A Technique for Efficient and Safe Gene Delivery, Angewandte Chemie, 120 (2008) 5241–5244. [DOI] [PubMed] [Google Scholar]
- [173].Wang Z, Zou H, Wang Z, Wu J, Xia Z, Feng M, Highly stable polyglutamate derivatives/siRNA polyplex efficiently down-relegate survivin expression and augment the efficacy of cisplatin, International journal of pharmaceutics, 505 (2016) 24–34. [DOI] [PubMed] [Google Scholar]
- [174].Huang H, Cao D, Qin L, Tian S, Liang Y, Pan S, Feng M, Dilution-stable PAMAM G1-grafted polyrotaxane supermolecules deliver gene into cells through a caveolae-dependent pathway, Molecular pharmaceutics, 11 (2014) 2323–2333. [DOI] [PubMed] [Google Scholar]
- [175].Dai X-H, Zhang H-D, Dong C-M, Fabrication, biomolecular binding, in vitro drug release behavior of sugar-installed nanoparticles from star poly (ɛ-caprolactone)/glycopolymer biohybrid with a dendrimer core, Polymer, 50 (2009) 4626–4634. [Google Scholar]
- [176].Balas C, Review of biomedical optical imaging—a powerful, non-invasive, non-ionizing technology for improving in vivo diagnosis, Measurement science and technology, 20 (2009) 104020. [Google Scholar]
- [177].Vadivambal R, Jayas DS, Bio-imaging: principles, techniques, and applications, CRC Press; 2015. [Google Scholar]
- [178].Qin L, Genant HK, Griffith JF, Leung KS, Advanced bioimaging technologies in assessment of the quality of bone and scaffold materials: techniques and applications, Springer Science & Business Media; 2007. [Google Scholar]
- [179].Sharma P, Brown S, Walter G, Santra S, Moudgil B, Nanoparticles for bioimaging, Advances in colloid and interface science, 123 (2006) 471–485. [DOI] [PubMed] [Google Scholar]
- [180].Erathodiyil N, Ying JY, Functionalization of inorganic nanoparticles for bioimaging applications, Accounts of chemical research, 44 (2011) 925–935. [DOI] [PubMed] [Google Scholar]
- [181].Wolfbeis OS, An overview of nanoparticles commonly used in fluorescent bioimaging, Chemical Society Reviews, 44 (2015) 4743–4768. [DOI] [PubMed] [Google Scholar]
- [182].Herranz M, Ruibal A, Optical imaging in breast cancer diagnosis: the next evolution, Journal of oncology, 2012 (2012) 863747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Chen G, Wang L, Cordie T, Vokoun C, Eliceiri KW, Gong S, Multi-functional self-fluorescent unimolecular micelles for tumor-targeted drug delivery and bioimaging, Biomaterials, 47 (2015) 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Pu KY, Li K, Liu B, Cationic Oligofluorene-Substituted Polyhedral Oligomeric Silsesquioxane as Light-Harvesting Unimolecular Nanoparticle for Fluorescence Amplification in Cellular Imaging, Advanced Materials, 22 (2010) 643–646. [DOI] [PubMed] [Google Scholar]
- [185].Symms M, Jäger H, Schmierer K, Yousry T, A review of structural magnetic resonance neuroimaging, Journal of Neurology, Neurosurgery & Psychiatry, 75 (2004) 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Sun C, Lee JS, Zhang M, Magnetic nanoparticles in MR imaging and drug delivery, Advanced drug delivery reviews, 60 (2008) 1252–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Reynolds CH, Annan N, Beshah K, Huber JH, Shaber SH, Lenkinski RE, Wortman JA, Gadolinium-loaded nanoparticles: new contrast agents for magnetic resonance imaging, Journal of the American Chemical Society, 122 (2000) 8940–8945. [Google Scholar]
- [188].Herborn CU, Honold E, Wolf M, Kemper J, Kinner S, Adam G, Barkhausen J, Clinical safety and diagnostic value of the gadolinium chelate gadoterate meglumine (Gd-DOTA), Investigative radiology, 42 (2007) 58–62. [DOI] [PubMed] [Google Scholar]
- [189].Caravan P, Strategies for increasing the sensitivity of gadolinium based MRI contrast agents, Chemical Society Reviews, 35 (2006) 512–523. [DOI] [PubMed] [Google Scholar]
- [190].Phelps ME, PET: the merging of biology and imaging into molecular imaging, The Journal of Nuclear Medicine, 41 (2000) 661. [PubMed] [Google Scholar]
- [191].Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, Humm J, Gönen M, Kalaigian H, Schöder H, Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe, Science translational medicine, 6 (2014) 260ra149–260ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Janib SM, Moses AS, MacKay JA, Imaging and drug delivery using theranostic nanoparticles, Advanced drug delivery reviews, 62 (2010) 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Mura S, Couvreur P, Nanotheranostics for personalized medicine, Advanced drug delivery reviews, 64 (2012) 1394–1416. [DOI] [PubMed] [Google Scholar]
- [194].Kievit FM, Zhang M, Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers, Advanced materials, 23 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Chen G, Jaskula–Sztul R, Harrison A, Dammalapati A, Xu W, Cheng Y, Chen H, Gong S, KE108-conjugated unimolecular micelles loaded with a novel HDAC inhibitor thailandepsin-A for targeted neuroendocrine cancer therapy, Biomaterials, 97 (2016) 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Hu X, Liu G, Li Y, Wang X, Liu S, Cell-penetrating hyperbranched polyprodrug amphiphiles for synergistic reductive milieu-triggered drug release and enhanced magnetic resonance signals, Journal of the American Chemical Society, 137 (2014) 362–368. [DOI] [PubMed] [Google Scholar]
- [197].Lin W, Zhang X, Qian L, Yao N, Pan Y, Zhang L, Doxorubicin-Loaded Unimolecular Micelle-Stabilized Gold Nanoparticles as a Theranostic Nanoplatform for Tumor-Targeted Chemotherapy and Computed Tomography Imaging, Biomacromolecules, 18 (2017) 3869–3880. [DOI] [PubMed] [Google Scholar]
- [198].Huang Y, Qiu F, Shen L, Chen D, Su Y, Yang C, Li B, Yan D, Zhu X, Combining two-photon-activated fluorescence resonance energy transfer and near-infrared photothermal effect of unimolecular micelles for enhanced photodynamic therapy, ACS nano, 10 (2016) 10489–10499. [DOI] [PubMed] [Google Scholar]
- [199].Liu T, Li X, Qian Y, Hu X, Liu S, Multifunctional pH-disintegrable micellar nanoparticles of asymmetrically functionalized β-cyclodextrin-based star copolymer covalently conjugated with doxorubicin and DOTA-Gd moieties, Biomaterials, 33 (2012) 2521–2531. [DOI] [PubMed] [Google Scholar]