Abstract
Background
The aim of this study was to investigate whether melatonin is involved in brain injury following subarachnoid hemorrhage (SAH).
Material/Methods
An SAH model was established and TUNEL assays were utilized to detect the effect of melatonin on cell apoptosis. Western blot analysis was used to detect the effect of melatonin on expression of autophagic markers and apoptotic factors. Real-time PCR, Western blot analysis, and luciferase assay were performed to study the effect of melatonin on nuclear factor erythroid-2 related factor 2 (NRF2) expression.
Results
The SAH group displayed a lower neurological score and a higher brain water content, while melatonin treatment increased the neurological score and decreased the brain water content. The administration of melatonin also inhibited the apoptosis of neurons in the brain. In addition, higher Beclin-1 expression and higher conversion ratio from LC3- II to LC3-I were observed in the SAH group. The activation of Beclin-1 and the conversion from LC3-II to LC3-I was further enhanced by melatonin treatment. Furthermore, in the SAH group, the level of Bcl-2 was decreased while the level of Bax and cleaved caspase-3 were increased. However, following melatonin treatment in the SAH group, the level of Bcl-2 was increased while the levels of Bax and cleaved caspase-3 were decreased.
Conclusions
Our study indicated that, by increasing the expression of NRF2, the mitophagy induced by melatonin provided protection against brain injury post-SAH.
MeSH Keywords: Endoplasmic Reticulum, Endoplasmic Reticulum Stress, Melatonin, Subarachnoid Hemorrhage
Background
As a frequently seen and devastating condition, subarachnoid hemorrhage (SAH) accounts for 5% of all stroke [1]. In North America, the incidence of aneurysmal SAH is about 1 in 10 000 persons every year, and its combined rate of morbidity and mortality is over 50% [2]. Although the surgical treatment and diagnosis of SAH seen major advances, the clinical outcomes of SAH remain poor and its therapeutic interventions still only show limited efficacy. For SAH, the delayed cerebral vasospasm (CVS) has always been deemed as the most critical cause for poor prognosis and delayed cerebral ischemia [3]. In addition, it has been shown in recent investigations that early brain injuries tend to occur in the first 72 h after SAH [4]. Furthermore, Sehba et al. (2011) showed that, in spite of the increasing knowledge and improved management of early brain injury (EBI) and SAH, SAH is still one of the most serious health problems worldwide [5].
As a form of cell death, autophagy has been observed in conjunction with necrosis and apoptosis after SAH. Nevertheless, although many researchers have extensively investigated the mechanism of autophagy in stroke, few have studied the relationship between autophagy and SAH [6]. For example, Zhao et al. (2013) discovered the presence of autophagy as early as 6 h after experimental SAH [7]. In addition, Lee et al. (2009a) found that the expression of beclin-1 and the conversion from light chain-3 I to light chain-3 II greatly increased after SAH, suggesting that autophagy is activated during EBI [8]. It has also been demonstrated that both neuronal apoptosis and inflammation contribute to EBI [9]. For example, a previous study discovered that post-SAH neuronal apoptosis could be induced by the factors downstream of endoplasmic reticulum (ER) stress [10]. Although ER stress is mainly a self-protective signal transduction pathway, a high level of ER stress could promote cell death through the activation of apoptosis and inflammation [11]. In addition, several recent studies have demonstrated that TXNIP mediates neuron apoptosis and inflammation during post-SAH EBI, while ER-stress-induced suppression in TXNIP expression could lead to reduced expression of some prognostic indicators and inhibited cell apoptosis and inflammation. Therefore, it was suggested that the suppression of TXNIP may become a potential therapeutic strategy for the treatment of SAH [12].
Melatonin was found to reverse tumour formation and to delay tumour progression in HCC patients [13]. In addition, the proapoptotic effects of melatonin have been demonstrated by many studies [14,15]. Interestingly, it has been shown in hepatoma cells that melatonin can reduce apoptosis under ER stress by inhibiting cyclooxygenase 2 (COX-2) and promoting CCAAT/enhancer binding protein homologues protein (CHOP) [16]. In particular, melatonin has been reported to protect the brain against post-SAH injury [17]. For example, it was found that the administration of melatonin could regulate the nuclear factor erythroid-2 related factor 2 (Nrf2) signaling pathway to reduce ER stress, which is considered a key contributor to post-SAH EBI [12,18–20]. In this study, we established an animal model of SAH and subsequently gave melatonin treatment to SAH animals. In this way, the possible involvement of Nrf2-autophagy as well as ER stress in post-SAH EBI was investigated.
Material and Methods
Animals
Healthy adult male C57BL/6J mice weighing 22 to 25 g were used in our study. These mice were obtained from the Experimental Center of the Fourth Military Medical University. All experiments were approved by the Ethics Committee and were conducted under NIH’s “Laboratory Animal Care and Use Guide”.
SAH model
SAH was induced using the endovascular perforation method. In brief, 50 mg/kg pentobarbital sodium was injected into the mice to trigger anesthesia. During the following procedures, the rectal temperature of mice was maintained at 37±0.5°C using a thermal blanket. Subsequently, the external carotid artery, internal carotid artery, and left common carotid artery were exposed by a neckline incision. The left lateral carotid artery was connected and dissected to leave a 3-mm stump. At the same time, a nylon suture was inserted into the left internal carotid artery through the arteries in the middle cerebral artery branch. Except for arterial perforation, the same procedure was performed for sham-operated mice.
Experimental protocol
The mice were subdivided into the following groups: (1) sham operation group, (2) SAH group, and (3) SAH + melatonin. For the melatonin treatment, melatonin was dissolved in 1 mL of saline containing 1% ethanol and subsequently injected intraperitoneally into the mice at a dose of 150 mg/kg, which was given at 2 and 12 h after SAH. In addition, 1 mg/kg/day of Luz was administered intraperitoneally for 6 consecutive days prior to the establishment of SAH.
Neurological score
An 18-point system was used to detect the neurological deficits at 24 h after the establishment of SAH. The 6 parameters to be considered by this system included: symmetry in the movement of all limbs (0–3), spontaneous activity (0–3), outstretching of forelimbs (0–3), body proprioception (1–3), ability to climb (1–3), and response to vibrissae touch (1–3). The overall score was the sum of all 6 parameters and a higher score indicated a better neurological function.
Brain water content
The brain water content was determined using a standard wet-dry method. In brief, the mice were sacrificed at 24 h following the establishment of SAH, and their brains were immediately collected and separated into the left and right cerebella, the cerebral hemispheres, and the brain stem. These specimens were weighed to get the total wet weight. Subsequently, the brain tissues were dried at 105°C for 24 h and weighed to get the total dry weight. Finally, the brain water content was calculated as total wet weight – total dry weight)/total wet weight ×100.
RNA isolation and real-time PCR
A miRNeasy Mini kit (Qiagen, Valencia, CA) was used to isolate the total RNA from tissue samples. NanoDrop2000 (Thermo-Fisher, Wilmington, DE) was used to quantify the amount of isolated RNA. Subsequently, 5 μg total RNA were reversely transcribed into the cDNA of Nrf2 and CHOP1 using a reverse transcription kit (Invitrogen, CA). The conditions of RT-PCR reactions were: 25°C for 30 min, 42°C for 40 min, and 85°C for 5 min. GAPDH was selected as the internal control for RT-PCR reactions. In addition, specific primers were designed and used to measure the relative expression of Nrf2 and CHOP1 on a Roche 480 quantitative real-time PCR system (Roche, Basel, Switzerland). The conditions of qRT-PCR reactions were: 94°C for 10 min, followed by 45 cycles of 94°C for 15 s and 60°C for 60 s. Finally, the expression levels of Nrf2 and CHOP1mRNA were calculated using the ΔΔCT method. All reactions were run 3 times.
Cell culture and transfection
SH-SY5Y and U251 cells were purchased from the Cell Bank of Chinese Academy of Sciences. The cells were maintained in a high glucose DMEM medium supplemented with 10% FBS and 1% penicillin-streptomycin. The cells were incubated in a 37°C humidified incubator with 5% CO2. For melatonin treatment, the cells were seeded into 48-well plates and treated with 1 μM of melatonin. For transfection experiments, the SH-SY5Y and U251 cells were cultured to 70–80% confluence. Subsequently, Lipofectamine 2000 (Invitrogen, CA) was utilized to transfect the cells with constructs. All reactions were carried out in triplicate.
Cell proliferation assay
A total number of 1000 SH-SY5Y and U251 cells were counted and seeded into a 96-well plate, followed by the addition of 100 nM 1.25 (OH) 2D3 once every 24 h for 5 consecutive days. Subsequently, the cells were incubated in 60 μL of 0.1 mg/ml melatoninT (Sigma-Aldrich, USA) for 4 h at 37°C, followed by 30 min of additional incubation in 180 μL of dimethylsulfoxide (DMSO) at room temperature. Finally, a Sienergy H2 microplate reader (Bio Tek Instruments, USA) was used to measure the absorbance in each well. All experiments were carried out in triplicate and repeated 3 times.
Vector construction/mutagenesis and luciferase assay
The full fragment of NRF2 was amplified by PCR. The PCR products were inserted into a pcDNA3.1 (+) vector upstream of the firefly luciferase reporter gene. Subsequently, SH-SY5Y and U251 cells were cultured in 96-well plates and transfected with the NRF2 constructs. After transfection, the cells were subjected to melatonin treatment at a final concentration of 100 nM, 1 μM, and 10 μM. Finally, the luciferase activity of the cells was determined using a dual luciferase reporter assay system (Promega, Madison, WI, USA) based on the manufacturer’s guidelines. Each test was repeated 3 times.
Determination of MDA, SOD, and GSH-Px levels
In cardiac tissues and cultured cells, the levels of SOD, MDA, and GSH-Px activity were determined using a commercially available kit and according to the manufacturer’s instructions. The SOD, MDA, and GSH-Px data were analyzed by a SpectraMax M5 (Molecular Devices, CA, USA) spectrophotometer.
Determination of NRF2 activity
A commercially available kit was used to assess the deacetylase activity of NRF2 in accordance with the manufacturer’s instructions. In brief, 40 μg of purified NRF2 were incubated with the substrates included in the kit at 37°C for 45 min, followed by the addition of 25 μL of developer reagent and 45 min of additional incubation. The NRF2 activity was subsequently determined using a SpectraMax M5 (Molecular Devices, CA, USA) spectrophotometer.
Western blot analysis
The collected tissues were lysed in a lysis buffer (150 mM Tris, 50 mM DTT, 8 M urea, 15% sucrose, 2% sodium dodecyl sulfate, 0.01% bromophenol blue, 2 mM EDTA, and a 1% cocktail of protease and phosphatase inhibitors) and sonicated for 3 min. In the next step, the lysate was centrifuged at 12 000 g for 15 min, and the supernatant was collected and subjected to boiling. Subsequently, an equal amount of proteins from the samples was separated by SDS-PAGE and transferred onto a PMSF membrane (Amersham Biosciences, Piscataway, NJ, USA), which was then blocked with TBST (50 mM Tris [pH 7.5], 150 mM NaCl, 0.1% Tween-20) containing 5% skim milk. In the next step, the membrane was incubated at 4°C overnight with diluted primary antibodies (1: 5000) against Nrf2, Beclin-1, LC3-I, LC3-II, Bax, Bcl-2, ac-SOD and Cleaved Caspase-3 (Cell Signaling Technologies, Danvers, MA, USA). The membrane was then rinsed and incubated with secondary antibodies (1: 5000 dilution, Sigma-Aldrich Co., LLC, USA) at room temperature for 1.5 h. Finally, the density of protein bands on the membrane was measured using a Bio-Rad imaging system (Bio-Rad, Hercules, CA, USA) and quantified with Quantity One software (West Berkeley, CA, USA). All experiments were carried out in triplicate.
Immunohistochemistry assay
The collected tissues were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, and sliced into 4-μm sections. In the next step, the sections were stained immunohistochemically using mouse anti-human Nrf2 monoclonal primary antibodies (Santa Cruz, Biotech) and anti-mouse IgG secondary antibodies. Finally, the staining was developed using peroxidase 3,3-diaminobenzidine (DAB) in accordance with the manufacturer’s instructions, while hematoxylin was used to perform counterstaining.
TUNEL assay
The apoptotic status of the brain cortex was evaluated using the TUNEL assay. In brief, 50 μL of TUNEL reaction buffer was mixed with the sample sections, which were then incubated at 37°C for 60 min in the dark in a humidified atmosphere. Subsequently, DAPI was utilized to stain the cell nuclei and flow cytometry was used to determine the number of apoptotic neurons. The ratio of apoptosis was calculated as the number of apoptotic neurons/the number of total neurons.
Statistical analysis
All results are shown as the mean ±SD (standard deviation). SPSS 18.0 (SPSS Inc., Chicago, IL, US) was utilized to perform all statistical analyses. The t test was used to compare the difference between 2 groups, and one-way analysis of variance (ANOVA) was used to compare the differences between 3 or more groups. A P value of less than 0.05 was considered statistically significant.
Results
Melatonin alleviated brain edema and ameliorated post-SAH neurological deficits
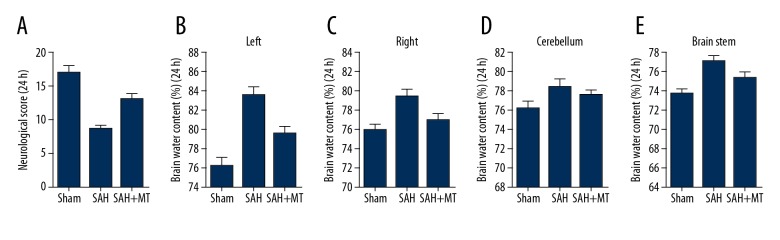
An SAH model was established using the endovascular perforation method. At 24 h following the establishment of SAH, brain water content and neurological scores were determined. The brain water content of mice in the SAH group showed an evident increase (Figure 1A), accompanied by an evident decline in neurological scores in different brain sections, including left cerebellum (Figure 1B), right cerebellum (Figure 1C), cerebral hemispheres (Figure 1D), and brain stem (Figure 1E). In addition, the melatonin treatment decreased the brain water content (Figure 1A) and ameliorated neurological deficits (Figure 1B–1E) at 24 h after the onset of SAH.
Figure 1.
The effect of melatonin on brain water content and neurological deficits. (A) The SAH group exhibited a lower level of brain water content, while the administration of melatonin increased the neurological score (P<0.05 compared with the control group, and analysis was performed using one-way ANOVA). (B–E) Evident increase in brain swelling, including left cerebellum, right cerebellum, and brain stem, was observed in the SAH group, and the magnitude of brain swelling was reduced by melatonin treatment. P <0.05 compared with the control group.
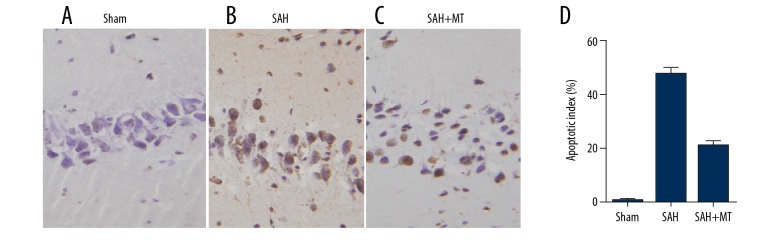
Melatonin inhibited neuronal apoptosis in the brain cortex
TUNEL assay was used to detect the apoptosis of neurons. As shown in Figure 2, the apoptotic index in the SAH group was much higher than that in the sham group and melatonin treatment remarkably inhibited the SAH-induced apoptosis of neurons at 24 h after the onset of SAH.
Figure 2.
(A–D) Melatonin inhibited SAH-induced apoptosis of neurons in the brain cortex (P<0.05 compared with the control group, and analysis was performed using one-way ANOVA).
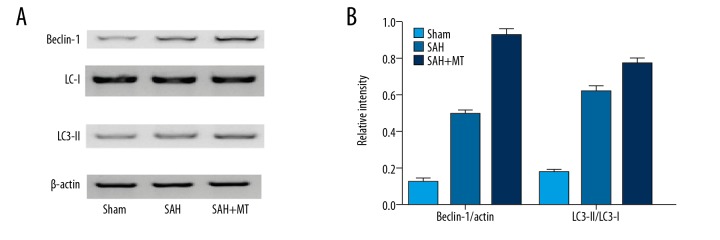
Melatonin treatment increased the expression of autophagic markers at 24 h after the onset of SAH
Autophagy is a cellular process responsible for the recycling of cellular constituents. In this study, Western blot analysis was carried out to compare the protein levels of Beclin-1, LC3-II (light chain-3 II), and LC3-I (light chain-3 I) among the sham, SAH, and SAH + melatonin groups. As shown in Figure 3, the SAH group exhibited a higher expression of Beclin-1, accompanied by an evident increase in the conversion ratio from LC3-II to LC3-I. Furthermore, SAH-induced autophagy activation was further enhanced by melatonin administration, suggesting that melatonin is involved in post-SAH EBI by influencing autophagy.
Figure 3.
(A, B) Melatonin administration enhanced the expression of autophagic markers after the onset of SAH, during which the expression of Beclin-1 and the conversion from LC3- II to LC3-I were increased. The above effect of SAH was further enhanced by melatonin treatment (P<0.05 compared with the control group, and analysis was performed using one-way ANOVA).
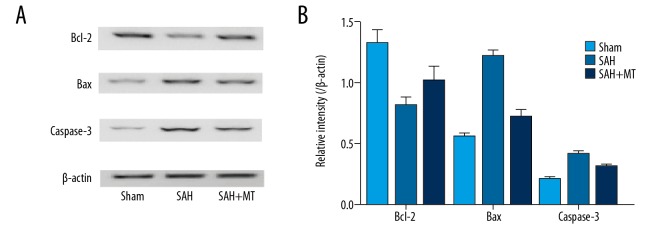
Melatonin treatment enhanced Bcl-2 expression but inhibited the expression of Bax and caspase-3 cleavage following the onset of SAH
Bcl-2, Bax, and cleaved caspase-3 are well-known regulators of cell proliferation and apoptosis. In this study, the effect of melatonin on cell survival and apoptosis was detected by measuring the expression levels of Bcl-2, Bax, and cleaved caspase-3 among the sham, SAH, and SAH + melatonin groups. As shown in Figure 4, the protein level of Bcl-2 in the SAH group was much higher than that in the sham group, but melatonin administration reduced the expression of Bcl-2 in the brain tissue. In addition, high protein levels of Bax and cleaved caspase-3 were observed in the SAH group, while the melatonin administration decreased the protein levels of Bax and cleaved caspase-3, indicating that melatonin prevented the apoptosis of neurons following the onset of SAH.
Figure 4.
(A, B) Western blot analysis was performed to detect the effect of melatonin on factors related to cell apoptosis. Melatonin administration upregulated the level of Bcl-2 but reduced the levels of Bax and cleavage caspase-3 following the onset of SAH (P<0.05 compared with the control group, and analysis was performed using one-way ANOVA).
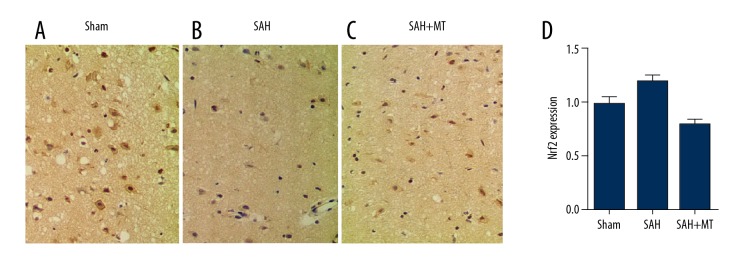
Different levels of NRF2 among different groups
Immunohistochemistry assay was carried out to measure the protein levels of NRF2 among the sham, SAH, and SAH + melatonin groups. As shown in Figure 5, the level of NRF2 protein (Figure 5A–5C) in the SAH group was much lower than that in the SHA+melatonin group, which was lower than in the sham group.
Figure 5.
(A–D) Immunohistochemistry assay was carried out to measure the protein level of NRF2, showing that melatonin administration elevated the level of NRF2 following the onset of SAH (P<0.05 compared with the control group, and analysis was performed using one-way ANOVA).
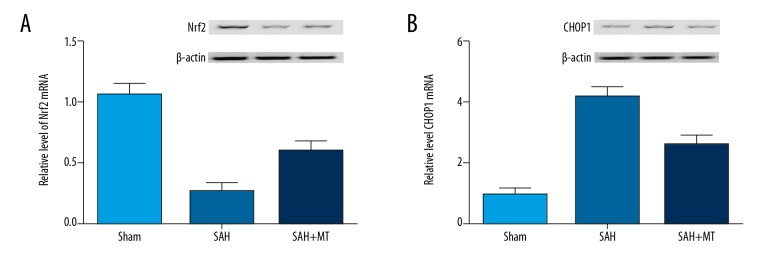
Melatonin affected post-SAH EBI by modulating REDOX and apoptosis
In this study, the expression of NRF2, a gene related to REDOX, and CHOP1, a gene related to apoptosis, was determined using real-time PCR and Western blot analysis. As shown in Figure 6, the mRNA and protein level of NRF2 (Figure 6A) in the SAH group was much lower than that in the SHA+melatonin group, which in turn was lower than in the sham group. In contrast, the mRNA and protein level of CHOP1 (Figure 6B) in the SAH group was substantially increased compared with that in the sham group, while the melatonin treatment reduced the CHOP1 expression.
Figure 6.
(A) Levels of NRF2 in the SAH and SAH+melatonin groups were much lower than that in the sham group, while the inhibition effect in the SAH group was much stronger than that in the 2 other groups. (P<0.05 compared with the control group, and analysis was performed using one-way ANOVA). (B) Levels of CHOP1 in the SAH and SAH+melatonin groups were much higher than that in the sham group, while the promotional effect in the SAH group was much stronger than in the 2 other groups (P<0.05 compared with the control group, and analysis was performed using one-way ANOVA).
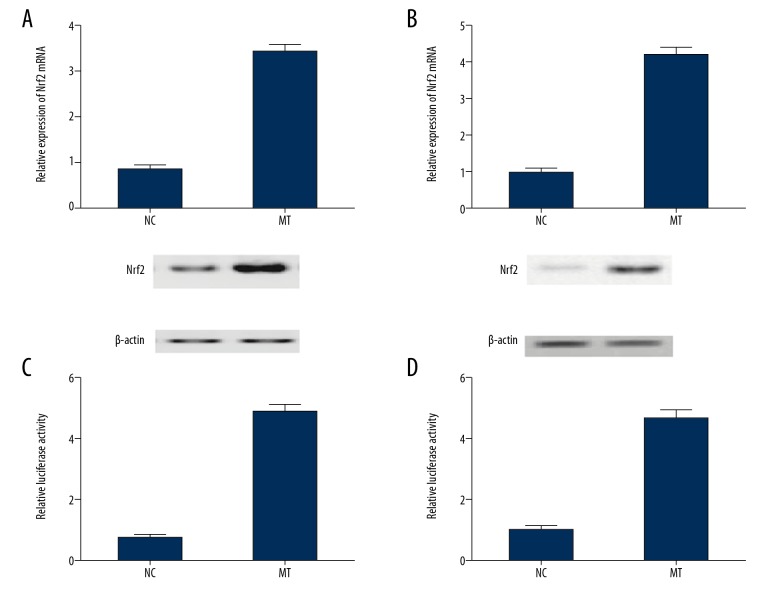
Melatonin affected NRF2 expression by influencing the transcription efficiency of NRF2 promoter
Real-time PCR, Western blot analysis, and luciferase assay were used to investigate the effect of melatonin on the transcriptional efficiency of NRF2. Different doses of melatonin (100 nM, 1uM, 10uM) were used to treat SH-SY5Y cells and U251 cells. As shown in Figure 7, the level of NRF2 in the SH-SY5Y (Figure 7A) and U251 (Figure 7B) cells was significantly increased after melatonin treatment. Meanwhile, the luciferase activity of SH-SY5Y (Figure 7C) and U251 (Figure 7D) cells transfected with NRF2 constructs was significantly increased following treatment with melatonin, suggesting that melatonin increased the transcription efficiency of NRF2 promoter.
Figure 7.
Melatonin regulated the expression of NRF2 by affecting the transcription efficiency of NRF2 promoter. (A) Level of NRF2 showed a stepwise increase at the increasing concentration of melatonin in SH-SY5Y cells (P<0.05 compared with the control group, and analysis was performed using the t test). (B) Level of NRF2 showed a stepwise increase at the increasing concentration of melatonin in U215 cells (P<0.05 compared with the control group, and analysis was performed using the t test). (C) Luciferase activity of SH-SY5Y cells transfected with NRF2 was dose-dependently upregulated subsequent to melatonin treatment (P<0.05 compared with the control group, and analysis was performed using the t test). (D) Luciferase activity of U251 cells transfected with NRF2 was dose-dependently upregulated subsequent to melatonin treatment. (P<0.05 compared with the control group, and analysis was performed using the t test).
Discussion
In SAH patients, EBI is the main cause of morbidity and mortality. Therefore, in the management of SAH survivors, the prevention of EBI is a major goal [21]. Melatonin reduces post-SAH apoptosis, oxidative stress, and inflammation by enhancing autophagy, thus providing a protective effect against EBI [22]. The role of melatonin in the regulation of autophagy has been previously shown in both healthy and disease states. For instance, it has been reported that melatonin can prevent rotenone-, morphine-, and methamphetamine-induced autophagic death of HeLa and SK-N-SH cells [23]. In this study, we established the animal models of SAH and demonstrated that the treatment with melatonin increased neurological scores and reduced brain swelling. In addition, we carried out TUNEL assays to determine the apoptotic status of neurons and revealed that melatonin treatment repressed SAH-induced neuronal apoptosis in the brain.
As a preserved pathway of lysosomal degradation, autophagy is critical for eliminating damaged organelles and obsolete cellular proteins, so as to sustain cytoplasmic homeostasis [24]. The autophagic activity is greatly augmented in post-SAH neurons and continues to increase during the entire EBI period [8]. At the same time, autophagy acts as a pro-survival mechanism and suppresses the neuronal apoptosis in post-SAH EBI [18,22,25,26]. As a member of the Cap ‘n’ Collar (CNC) family of basic leucine zipper (bZip) transcription factor [27], Nrf2 can accelerate the autophagic removal of toxic and ubiquitinated protein aggregates, thus providing protection against proteocytotoxicity in the heart [28]. It has also been shown that the activation of Nrf2 results in liver damage in a setting of autophagy impairment [29]. Moreover, in mouse CryABR120G (mCryABR120G)-induced cardiomyopathy, the insufficiency of autophagy plays a critical role [30]. As a basic leucine zipper redox-sensitive transcription factor, Nrf2 can also control the redox state of cells under harmful stresses and regulate the formation of autophagy [7,8]. In this study, we also examined the protein levels of Beclin-1, LC3-II and LC3-I among the sham, SAH, and SAH + melatonin groups, and found that the SAH group had a higher level of Beclin-1 and a higher ratio of LC3- II to LC3-I conversion. In addition, the effect of SAH on the activation of Beclin-1 expression and LC3-II to LC3-I conversion was further enhanced by melatonin treatment. We also found that the protein level of NRF2 in the SAH group was much lower than that in the SHA+melatonin group, which in turn was lower than in the sham group. This is not the first study to show that melatonin affects the expression of NRF2. For example, it was discovered previously that melatonin treatment led to an augmented protein expression of Nrf2 in the nucleus and the cytoplasm, thus exerting a potentially hepatoprotective effect against hepatic failure [31].
We found that the pathophysiological events occurred during the first 72 h after the onset of SAH are the most critical factors to determine the prognosis of SAH. Although the exact mechanisms of post-SAH EBI remain unknown, it is suspected that the neuronal apoptosis plays an essential role in this process [32,33]. ER stress is one of the leading contributors to apoptosis. It has been demonstrated that mild ER stress could promote autophagy and thus suppress neuronal death [34]. However, more severe and prolonged ER stress can lead to cell death [35]. It has also been shown that prolonged ER stress leads to reperfusion/ischemia neuronal damages [36]. Moreover, in an experimental model of neurodegenerative disease, the attenuation of ER stress alleviated neuronal apoptosis [37]. In particular, neuronal apoptosis plays a central role in SAH-induced EBI [38]. As an ER stress inhibitor, melatonin has been shown in previous studies to alleviate lipopolysaccharide-induced placental ER stress in mice [39]. In this study, we carried out Western blot analysis to measure the protein levels of Bcl-2, Bax, and cleaved caspase-3, and revealed that the melatonin treatment upregulated Bcl-2 expression but reduced the expression of Bax and caspase-3 cleavage. In addition, we also found that melatonin treatment increased NRF2 expression but decreased CHOP1 expression in post-SAH mice.
It was shown previously that melatonin greatly suppresses arsenite-induced activation of transcription factor-4, XBP-1, CHOP, and GRP78 in rat brains [40]. Recent studies also reported that melatonin reduced the expression of CHOP and GRP78, the phosphorylation of JNK and eIF2α, and the activation of XBP-1 in testes [41]. It has also been reported in a rabbit study of lethal fulminant hepatitis that melatonin greatly relieved ER stress through UPR signaling [42]. Furthermore, it was shown that melatonin could significantly inhibit the activation of UPR signaling and BLM-induced pulmonary ER stress [43,44].
Conclusions
Our study indicated that, by increasing the expression of Nrf2, melatonin-mediated mitophagy provided protection against EBI after the onset of SAH. Our study also found that the administration of melatonin could regulate the signaling pathway of Nrf2-autophagy as well as ER stress. Melatonin exerts its effect against post-SAH EBI by restricting the deregulation of autophagy and by decreasing the magnitude of ER stress.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–18. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 2.King JT., Jr Epidemiology of aneurysmal subarachnoid hemorrhage. Neuroimaging Clin N Am. 1997;7:659–68. [PubMed] [Google Scholar]
- 3.Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–72. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- 4.Cahill J, Zhang JH. Subarachnoid hemorrhage: Is it time for a new direction? Stroke. 2009;40:S86–87. doi: 10.1161/STROKEAHA.108.533315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sehba FA, Pluta RM, Zhang JH. Metamorphosis of subarachnoid hemorrhage research: from delayed vasospasm to early brain injury. Mol Neurobiol. 2011;43:27–40. doi: 10.1007/s12035-010-8155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leao AA. Further observations on the spreading depression of activity in the cerebral cortex. J Neurophysiol. 1947;10:409–14. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 7.Zhao H, Ji Z, Tang D, Yan C, et al. Role of autophagy in early brain injury after subarachnoid hemorrhage in rats. Mol Biol Rep. 2013;40:819–27. doi: 10.1007/s11033-012-2120-z. [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, He Y, Sagher O, et al. Activated autophagy pathway in experimental subarachnoid hemorrhage. Brain Res. 2009;1287:126–35. doi: 10.1016/j.brainres.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–53. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 10.He Z, Ostrowski RP, Sun X, et al. CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke. 2012;43:484–90. doi: 10.1161/STROKEAHA.111.626432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–62. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Q, Che X, Zhang H, et al. Thioredoxin-interacting protein links endoplasmic reticulum stress to inflammatory brain injury and apoptosis after subarachnoid haemorrhage. J Neuroinflammation. 2017;14:104. doi: 10.1186/s12974-017-0878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomov B, Popov D, Tomova R, et al. Therapeutic response of untreatable hepatocellular carcinoma after application of the immune modulators IL-2, BCG and melatonin. Anticancer Res. 2013;33:4531–35. [PubMed] [Google Scholar]
- 14.Ordonez R, Carbajo-Pescador S, Prieto-Dominguez N, et al. Inhibition of matrix metalloproteinase-9 and nuclear factor kappa B contribute to melatonin prevention of motility and invasiveness in HepG2 liver cancer cells. J Pineal Res. 2014;56:20–30. doi: 10.1111/jpi.12092. [DOI] [PubMed] [Google Scholar]
- 15.Fan L, Sun G, Ma T, et al. Melatonin reverses tunicamycin-induced endoplasmic reticulum stress in human hepatocellular carcinoma cells and improves cytotoxic response to doxorubicin by increasing CHOP and decreasing survivin. J Pineal Res. 2013;55:184–94. doi: 10.1111/jpi.12061. [DOI] [PubMed] [Google Scholar]
- 16.Zha L, Fan L, Sun G, et al. Melatonin sensitizes human hepatoma cells to endoplasmic reticulum stress-induced apoptosis. J Pineal Res. 2012;52:322–31. doi: 10.1111/j.1600-079X.2011.00946.x. [DOI] [PubMed] [Google Scholar]
- 17.Cao S, Shrestha S, Li J, et al. Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of NLRP3 inflammasome activation. Sci Rep. 2017;7:2417. doi: 10.1038/s41598-017-02679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Li J, Wang Z, et al. Attenuation of early brain injury and learning deficits following experimental subarachnoid hemorrhage secondary to Cystatin C: Possible involvement of the autophagy pathway. Mol Neurobiol. 2014;49:1043–54. doi: 10.1007/s12035-013-8579-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Wang H, Fan Y, et al. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways. Sci Rep. 2017;7:46763. doi: 10.1038/srep46763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topkoru BC, Altay O, Duris K, et al. Nasal administration of recombinant osteopontin attenuates early brain injury after subarachnoid hemorrhage. Stroke. 2013;44:3189–94. doi: 10.1161/STROKEAHA.113.001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–37. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Wang L, Wu C, et al. Melatonin-enhanced autophagy protects against neural apoptosis via a mitochondrial pathway in early brain injury following a subarachnoid hemorrhage. J Pineal Res. 2014;56:12–19. doi: 10.1111/jpi.12086. [DOI] [PubMed] [Google Scholar]
- 23.Chang CF, Huang HJ, Lee HC, et al. melatonin attenuates kainic acid-induced neurotoxicity in mouse hippocampus via inhibition of autophagy and alpha-synuclein aggregation. J Pineal Res. 2012;52:312–21. doi: 10.1111/j.1600-079X.2011.00945.x. [DOI] [PubMed] [Google Scholar]
- 24.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11:709–30. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Shi XY, Yin J, et al. Role of autophagy in early brain injury after experimental subarachnoid hemorrhage. J Mol Neurosci. 2012;46:192–202. doi: 10.1007/s12031-011-9575-6. [DOI] [PubMed] [Google Scholar]
- 26.Jing CH, Wang L, Liu PP, et al. Autophagy activation is associated with neuroprotection against apoptosis via a mitochondrial pathway in a rat model of subarachnoid hemorrhage. Neuroscience. 2012;213:144–53. doi: 10.1016/j.neuroscience.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 27.Luo B, Li B, Wang W, et al. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One. 2014;9:e104771. doi: 10.1371/journal.pone.0104771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 29.Komatsu M, Kurokawa H, Waguri S, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–23. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 30.Papandreou I, Denko NC, Olson M, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–14. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang HL, Xu M, Wei C, et al. Neuroprotective effects of pioglitazone in a rat model of permanent focal cerebral ischemia are associated with peroxisome proliferator-activated receptor gamma-mediated suppression of nuclear factor-kappaB signaling pathway. Neuroscience. 2011;176:381–95. doi: 10.1016/j.neuroscience.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 32.Sauzeau V, Rolli-Derkinderen M, Marionneau C, et al. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J Biol Chem. 2003;278:9472–80. doi: 10.1074/jbc.M212776200. [DOI] [PubMed] [Google Scholar]
- 33.Sehba FA, Hou J, Pluta RM, Zhang JH. The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol. 2012;97:14–37. doi: 10.1016/j.pneurobio.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fouillet A, Levet C, Virgone A, et al. ER stress inhibits neuronal death by promoting autophagy. Autophagy. 2012;8:915–26. doi: 10.4161/auto.19716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakka VP, Gusain A, Raghubir R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res. 2010;17:189–202. doi: 10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- 37.Tsujii S, Ishisaka M, Shimazawa M, et al. Zonisamide suppresses endoplasmic reticulum stress-induced neuronal cell damage in vitro and in vivo. Eur J Pharmacol. 2015;746:301–7. doi: 10.1016/j.ejphar.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 38.Yan F, Cao S, Li J, et al. Pharmacological inhibition of PERK attenuates early brain injury after subarachnoid hemorrhage in rats through the activation of Akt. Mol Neurobiol. 2017;54:1808–17. doi: 10.1007/s12035-016-9790-9. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Li L, Zhao M, et al. Melatonin alleviates lipopolysaccharide-induced placental cellular stress response in mice. J Pineal Res. 2011;50:418–26. doi: 10.1111/j.1600-079X.2011.00860.x. [DOI] [PubMed] [Google Scholar]
- 40.Lin AM, Fang SF, Chao PL, Yang CH. melatonin attenuates arsenite-induced apoptosis in rat brain: Involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of alpha-synuclein. J Pineal Res. 2007;43:163–71. doi: 10.1111/j.1600-079X.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- 41.Ji YL, Wang H, Meng C, et al. melatonin alleviates cadmium-induced cellular stress and germ cell apoptosis in testes. J Pineal Res. 2012;52:71–79. doi: 10.1111/j.1600-079X.2011.00921.x. [DOI] [PubMed] [Google Scholar]
- 42.Tunon MJ, San-Miguel B, Crespo I, et al. Melatonin treatment reduces endoplasmic reticulum stress and modulates the unfolded protein response in rabbits with lethal fulminant hepatitis of viral origin. J Pineal Res. 2013;55:221–28. doi: 10.1111/jpi.12063. [DOI] [PubMed] [Google Scholar]
- 43.Zhao H, Wu QQ, Cao LF, et al. melatonin inhibits endoplasmic reticulum stress and epithelial-mesenchymal transition during bleomycin-induced pulmonary fibrosis in mice. PLoS One. 2014;9:e97266. doi: 10.1371/journal.pone.0097266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu G, Liu Z. Nuclear factor erythroid 2-related factor 2 (Nrf2) mediates neuroprotection in traumatic brain injury at least in part by inactivating microglia. Med Sci Monit. 2016;22:2161–66. doi: 10.12659/MSM.896568. [DOI] [PMC free article] [PubMed] [Google Scholar]