Abstract
The aim of this study was to investigate the ameliorative effect of Lawsonia inermis Linn used traditionally against trypanosomosis. Twenty-five adult Wistar rats of both sex were individually infected intraperitoneally (IP) with 106Trypanosoma congolense per ml of blood. Following establishment of infection, the rats were randomly divided into five groups of 5 rats each. Rats in groups I, II, and III were treated with 125, 250 and 500 mg/kg of the extract, respectively, while rats in groups IV and V were treated with 3.5 mg/kg and 2 ml/kg of diminazene aceturate (DM) once and physiological buffered saline, respectively. All treatments except DM were given orally for 7 days IP. The antitrypanosomal effect of the plant was assessed by observing the level of parasitaemia daily, packed cell volume (PCV) weekly, erythrocyte osmotic fragility (EOF) and malondialdehyde (MDA) concentration on day 21. Phytochemical screening of the extract revealed the presence of alkaloids, carbohydrates, triterpenes, steroids, cardiac glycosides, saponins, tannins and flavonoids. The extract significantly (P < 0.05) reduced levels of parasitaemia at 250 mg/kg. PCV was higher (P > 0.05) in extract treated groups but significantly higher (P < 0.05) in group II at week 2 when compared to group V. Rats in group II had significantly lower values of EOF and MDA when compared with groups IV and V. Thus, the leaf of L. inermis has in addition to an antitrypanosomal effect against T. congolense in rats, an attenuating effect on the trypanosomosis pathology probably mediated via protection of the erythrocyte membrane against trypanosome-induced oxidative damage to the erythrocytes.
Abbreviations: ANOVA, analysis of variance; EOF, erythrocyte osmotic fragility; IP, intraperitoneal; kDNA, kinetoplast deoxyribonucleic acid; L, lawsonia; MDA, malondialdehyde; PCV, packed cell volume; PSS, physiological buffered saline; SEM, standard error of mean; T, trypanosoma; TBA, thiobarbituric acid; TCA, trichloroacetic acid; US, United States
Keywords: Antitrypanosomal, Antioxidant, Erythrocyte osmotic fragility, Malondialdehyde, Phytochemistry
1. Introduction
Trypanosomosis is a chronic debilitating haemoparasitic disease of man and domestic animals characterised by parasitaemia, pyrexia, anaemia, loss of condition, reduced productivity and death in some cases [1], [2]. The disease accounts for over 3 million livestock and 55,000 human deaths, respectively, annually [3], [4]. It has negative impact on food production and economic growth in many parts of the world, particularly, sub-Saharan Africa, with estimated direct annual losses to producers and consumers exceeding US$1 million [5], [6].
Oxidative stress, defined as imbalance between free radical generation and free radical scavenging, has been postulated to play a pivotal role in the pathogenesis of trypanosomosis [7], [8]. Free radicals cause lipoperoxidation in the body and an increase in the production of these radicals leads to overproduction of malondialdehyde (MDA). MDA is one of the best known degradation products of lipid peroxidation due to free radicals and therefore, can be used as a marker of cell membrane injury due to lipid peroxidation. The polyunsaturated fatty acids of the erythrocyte membrane are the main target for free radical lipoperoxidation [9], [10]. By-products of this lipid peroxidation have been reported to produce changes in the structures and functions of cell membrane, leading to decreased membrane fluidity, increased membrane permeability, inactivation of membrane-bound enzymes and loss of essential fatty acids [9], [11]. The end result of these changes on the surface of erythrocytes is increased erythrocyte osmotic fragility [12]. This may ultimately lead to anaemia and poor distribution of nutrient in the body. Control of anaemia in trypanosome-infected cattle is more important for survival, weight gain and productivity than control of parasitaemia [13]. Oxidative stress may be harmful to both the host and the trypanosome, but it is mainly due to a defensive reaction of the host (or vector) against the presence of the trypanosome. Both the host and the parasite dispose of an antioxidant defence system. However, the system of the mammalian host (having glutathione as the major intracellular antioxidant) is different from that of the trypanosomes (having trypanothione as the main defence system against oxidative stress).
Currently, only three trypanocides are available for controlling tsetse-transmitted trypanosomosis in domestic ruminants. These are isometamidium and homidium which have both prophylactic and therapeutic effects, and diminazene aceturate which has only therapeutic properties. It is estimated that 35 million doses of these drugs are used in Africa annually for the control of the disease [14]. The toxicity associated with the use of these drugs and the resistance developed against them by trypanosomes are some of the problems associated with the trypanosomosis. Drug-screening activities from plants have started decades back, and an emerging number of studies have now been developed and reported so far to discover drugs from medicinal plants that can help to combat trypanosomosis. The efforts have been geared towards medicinal plants with significant trypanocidal effect but fewer side effects.
Lawsonia inermis (Henna) is a branched glaborous shrub or a small tree (2–6 m high) belonging to the family Lythraceae [15]. It is widely used for traditional and prophetic medicine in Africa, Asia, and Middle East [16]. The plant is reputed to be used for treatment of plethora of diseases, such as trypanosomosis, malaria, fungal, viral, and bacterial infections; cancer and rheumatoid arthritis [17], [18], [19], [20], [21]. Since the search for a vaccine against trypanosomosis remains elusive for now [22], there is an urgent need to find more efficacious drugs, particularly, from traditional medicinal plants to combat the menace of the disease. Thus, the aim of this study was to elucidate the antitrypanosomal property of L. inermis against experimental Trypanosoma congolense infection in Wistar rats.
2. Materials and methods
2.1. Plant collection and identification
Fresh leaves of L. inermis were collected from the main campus of Ahmadu Bello University, Zaria, Nigeria. The leaves, flowers and seeds of the plants were sent to the Herbarium, Department of Biological Sciences, Ahmadu Bello University Zaria, Nigeria for identification. A specimen voucher Number (10461) was assigned to the plant. The leaves were dried to a constant weight at room temperature in the laboratory. The dried sample was ground into powder using mortar and pestle and kept in a polythene bag until required.
2.2. Plant extraction, concentration and phytochemical screening of the extract
Three hundred grams of the powdered leaf of L. inermis was cold extracted in a percolator using 900 ml of methanol as solvent. The mixture was allowed to stand in the percolator for 72 h. Thereafter, the liquid extract was drained into a clean bottle. The extracted powder was rinsed off with 100 ml of fresh solvent and added to the initial solution collected. The liquid extract was concentrated to dryness over water bath.
2.3. Phytochemical screening
Phytochemical screening to detect the presence of alkaloids, flavonoids, saponins, tannins, glycosides, triterpenes and steroids on the extract was carried out as described by Trease and Evans [23].
2.4. Experimental animals
Wistar rats of both sexes weighing between 90 and 100 g were obtained from Nigerian Institute for Trypanosomiasis Research (NITR), Kaduna. The animals were kept for 2 months in the animal house before the commencement of the experiment. They were housed in clean plastic cages with wood shavings as beddings. The beddings were changed twice in a week. The rats were fed on standard rat feed and given access to clean water ad libitum. The ethical approval for the use of rats was obtained from Ethical Committee on Animal Use and Care, Ahmadu Bello University, Zaria.
2.5. Test organism
T. congolense was obtained from the Department of Vector and Parasite, Nigerian Institute for Trypanosomiasis and Onchocerciasis Research (NITR), Kaduna. The parasite was maintained in the laboratory by continuous passage in rats until required. Each cycle of passage was done when parasitaemia was in the range of 35–40 parasites per field. For several passages, about 3 ml of blood was obtained from an infected rat by cardiac puncture after light chloroform anaesthesia into a 5 ml syringe and emptied into a vial containing 9 ml of physiological buffered saline (PBS). About 1 × 106 parasite in 0.2 ml blood/PBS solution was injected intraperitoneally (IP) into a rat previously unexposed to trypanosomal infection. Parasitaemia was monitored daily using the method of Herbert and Lumsden [24].
2.6. Inoculation of experimental animals
For inoculation of experimental rats, blood was obtained from a donor rat at peak parasitaemia by sacrificing the rat via jugular venisection after light chloroform anaesthesia into a vial containing PBS. About 1 × 106 parasite in 0.2 ml blood/PBS solution was injected IP into a rat previously unexposed to trypanosomal infection.
2.7. Determination of median lethal dose 50 (LD50)
The median lethal dose (LD50) was determined as described by Lorke [25]. In the initial phase, 9 rats of both sexes were randomly divided into three groups of 3 rats each. Groups 1, 2 and 3 were treated IP with crude methanol extract leaf extract of L. inermis at 10, 100 and 1000 mg/kg, respectively. The rats were then observed over 48 h for signs of toxicity and mortality. In the second phase of the study, 3 rats for each extract were assigned into 3 groups of 1 rat each. The animals were individually administered 2, 6, and 8 mg/kg of crude methanol leaf extract of L. inermis. The LD50 was then computed as geometric mean of highest dose that did not kill the rat and lowest dose that killed the rat.
2.8. Experimental grouping
Twenty-five adult Wistar rats of both sexes were individually infected, IP, with 106 of T. congolense per ml of blood. Immediately after the establishment of infection, the rats were randomly divided into five groups of 5 rats each. Rats in groups I, II, and III were treated with crude methanol extract of the leaf of L. inermis at 500, 250 and 125 mg/kg, respectively, per os for 7 consecutive days. Similarly, rats in group V were given PBS 2 ml/kg for 7-day; while rats in group IV were treated with diminazene aceturate 3.5 mg/kg IP once.
2.9. Determination of parasitaemia in experimental rats
The treated rats were observed daily for development of parasitaemia throughout the duration of the experiment. Parasitaemia was assessed using wet mount method. A drop of blood was collected on a clean microscope slide from the tail of each rat, covered with a cover slip and observed for parasitaemia under light microscope at ×400 magnification. The number of parasites per field was converted to the number of parasites per ml of blood using a standard chart [24].
2.10. Determination of packed cell volume (PCV)
The PCV of rats from all the groups was determined on days 8 and 15 by collecting blood from tail vein of each rat in a heparinized capillary tube and centrifuged the tube at 3000g for 10 min, where after the PCV was measured using a haematocrit reader. At the end of the experiment (day 21), the animals were sacrificed by severing the jugular vein. At that occasion blood was collected from each rat and the PCV determined.
2.11. Determination of erythrocyte osmotic fragility (EOF)
The EOF test was carried out as described by Faulkner and King [26]. Briefly, 5 ml of different concentrations (0.0%, 0.1%, 0.3%, 0.5%, 0.7% and 0.9%) of buffered sodium chloride pH 7.4 was poured into labelled test tubes and 0.02 ml of blood collected at day 21 from each rat was added into the respective test tubes. The contents were mixed gently and allowed to stand for 30 min at room temperature. The test tubes were centrifuged at 2000g for 5 min. Thereafter, 4 ml of the supernatant was transferred to a clean glass cuvette and the absorbance measured at 540 nm wavelength with spectrophotometer (Spectronic 20, Bausch and Lomb, USA). The percentage haemolysis for each sample was calculated as:
2.12. Determination of malondialdehyde (MDA)
Serum concentrations of MDA were measured as described by Draper and Hadley [27]. Briefly, 0.5 ml of serum from each infected rat was pipetted into a centrifuge tube and 2.5 ml of 100 g/L trichloroacetic acid (TCA) solution was added to it and then placed in boiling water bath for 15 min. Thereafter, it was cooled in tap water and centrifuged at 1000g for 10 min. Then 2 ml of the supernatant was taken and added to 1 ml of 6.7 g/L thiobarbituric acid (TBA) in a test tube and placed in boiling water bath for 15 min. It was then cooled in tap water and its absorbance was measured at 532 nm with spectrophotometer (spectrumlab 23A China). One ml of 10% TCA and 1 ml of 0.67% TBA was used as the blank. The intensity of the pink pigment formed from MDA-TBA condensation was measured by spectrometer and indicates the extent of lipid peroxidation.
2.13. Statistical analysis
Values obtained were expressed as mean ± standard error of mean (±SEM). Analysis of variance (ANOVA) was used followed by Tukey’s post hoc test for multiple comparisons of groups using GraphPad Prism version 5.03. Values of P < 0.05 were considered significant.
3. Results
3.1. Phytochemical screening
The crude methanol extract of L. inermis was positive for the presence of alkaloids, carbohydrates, triterpenes and steroids, cardiac glycosides, saponin glycosides, tannins and flavanoids.
3.2. Acute toxicity
The crude methanol leaf extract of L. inermis did not show any sign of toxicity or mortality even at highest dose of 5000 mg/kg.
3.3. Effect of treatment on the level of parasitaemia
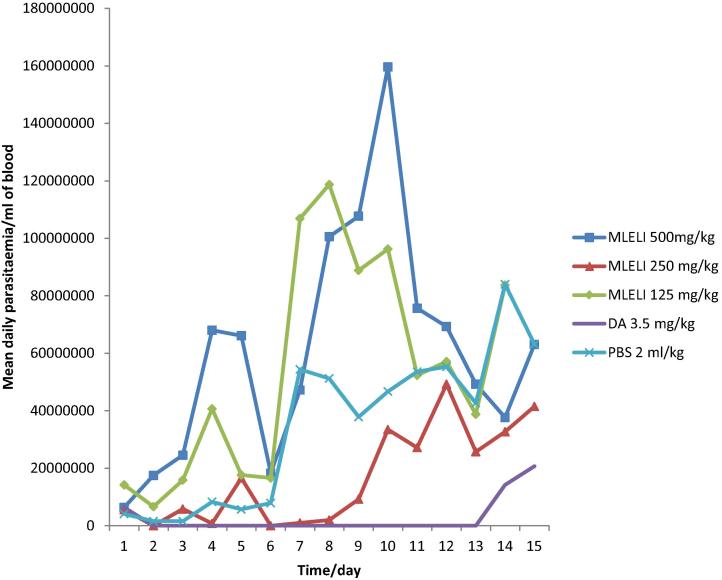
The effect of treatment on the level of parasitaemia in all the animals treated is shown in Fig. 1. From day six onwards, mean daily parasitaemia was significantly lower (P < 0.05) in rats treated with 250 mg/kg of the extract than rats administered PBS. However, rats treated with 500 and 125 mg/kg of the extract treated did not show significant suppression of parasitaemia. Nonetheless, diminazene treated group cleared parasites from the blood 24 h post-treatment until day 13 when parasitic relapse occurred.
Fig. 1.
Effect of methanol extract of the leaf of L. inermis on the level of parasitaemia in rats infected with T. congolense. MLELI = Methanol leaf extract of L. inermis; DA = Diminazene aceturate; PBS = Physiologic buffered phosphate saline.
3.4. Survival periods of rats treated with leaf extract of L. inermis
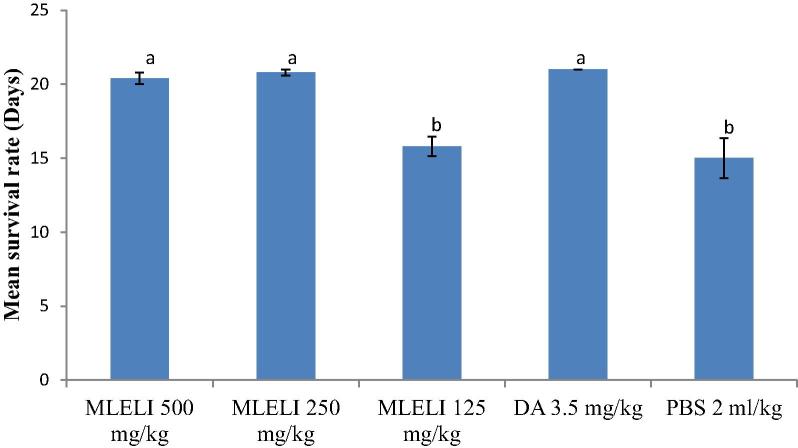
The effect of treatment with the methanol leaf extract of L. inermis (MLELI) on the survival periods of rats is shown in the Fig. 2. Rats treated with 500 and 250 mg/kg MLELI had significantly (P < 0.05) longer survival periods than the rats treated with 125 mg/kg LLELI and PBS. Mortality was first noticed on days 14 and 12 in the MLELI 125 mg/kg and PBS groups, respectively, culminating to the death of all the rats in MLELI 125 mg/kg and PBS on day 18.
Fig. 2.
Effect of treatment with extract of the leaf Lawsonia inermis on mean survival periods of experimental rats. a,bMeans with different superscript letters are statistically significant (P < 0.05). MLELI = Methanol leaf extract of L. inermis; DA = Diminazene aceturate; PBS = Physiologic buffered phosphate saline.
3.5. Effect of treatment with L. inermis extract on mean weekly packed cell volume (PCV)
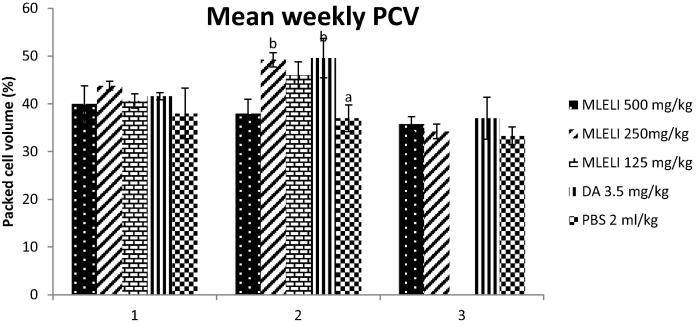
The PCV was higher in all the extract treated groups at weeks 1 and 2 than PBS treated group (Fig. 3). The PCV of rats treated with 250 mg/kg of the extract at week 2 was significantly higher (P < 0.05) than the PCV of rats in PBS group. Also, among the groups treated with the leaf extract, rats treated with 250 mg/kg of the extract had the highest PCV at weeks 1 and 2, though not statistically significant (P > 0.05) when compared to other extract treated groups.
Fig. 3.
Effect of treatment with extract of the leaf Lawsonia inermis on mean weekly PCV of experimental rats. a,bMeans with different superscript letters are statistically significant (P < 0.05). MLELI = Methanol leaf extract of L. inermis; DA = Diminazene aceturate; PBS = Physiologic buffered phosphate saline.
3.6. Effect of L. inermis extract on erythrocyte osmotic fragility (EOF) of treated rats
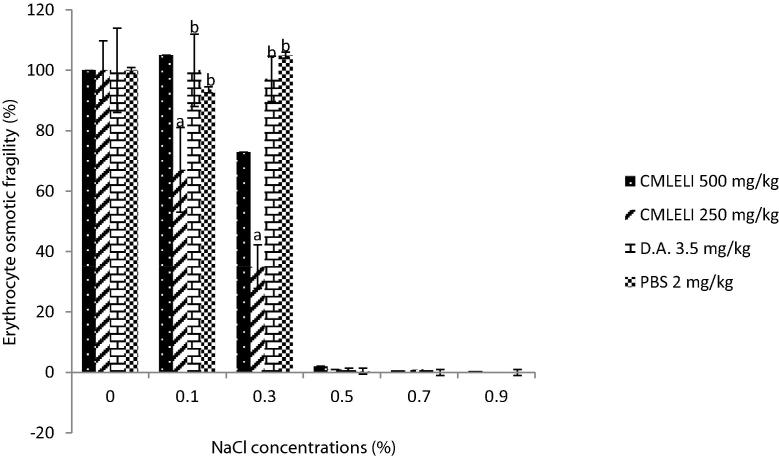
The ameliorative effect of the crude methanol extract of L. inermis on experimental T. congolense infection is shown in Fig. 4. The percentage EOF of extract treated groups was significantly lower (P < 0.05) at 0.1% and 0.3% NaCl concentrations when compared to PBS group. While rats treated with 500 mg/kg had lowest EOF at 0.3% NaCl concentration, rats treated with 250 mg/kg of extract had the lowest EOF at 0.1% NaCl concentration. However, diminazene (3.5 mg/kg) treated rats have significantly higher (P < 0.05) values of EOF when compared with extract treated groups.
Fig. 4.
Effect of Lawsonia inermis extract on EOF of treated rats experimentally infected with T. congolense. a,bMeans with different superscript letters are statistically significant (P < 0.05). MLELI = Methanol leaf extract of L. inermis; DA = Diminazene aceturate; PBS = Physiologic buffered phosphate saline.
3.7. Effect of L. inermis extract on serum malondialdehyde (MDA) concentrations of treated rats
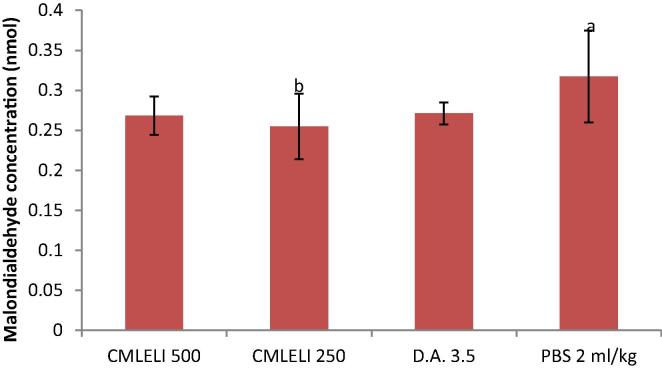
The effect of treatment on serum MDA concentration is shown in Fig. 5. There was an increase in the serum MDA concentration of rats in the control (PBS) group when compared to extract treated groups. The serum MDA concentration of rats treated with 250 mg/kg of the extract was significantly lower (P < 0.05) than in rats treated with PBS. Furthermore, serum concentration of rats treated with 250 mg/kg group was lower than that of rats treated with 500 mg/kg and diminazene 3.5 mg/kg groups though not statistically significant (P > 0.05). However, the serum concentration of MDA in diminazene aceturate treated group is lower (P > 0.05) than the PBS treated group.
Fig. 5.
Effect of Lawsonia inermis extract on serum MDA of treated rats experimentally infected with T. congolense. a,bMeans with different superscript letters are statistically significant (P < 0.05). MLELI = Methanol leaf extract of L. inermis; DA = Diminazene aceturate; PBS = Physiologic buffered phosphate saline.
4. Discussion
The absence of any apparent toxic signs in rats treated with the extract even at 5000 mg/kg showed that the leaf extract of L. inermis is relatively safe. Any substance that is not toxic to animals at a dose of up to 5000 mg/kg is relatively safe for use as therapeutic agent [25]. According to the World Health Organisation acute toxicity rankings [28], the extract could be described as unlikely to be associated with hazard when administered as a drug. This may underscore the reason why traditional herbalists use a wide range of doses of decoction and infusion made from the plant.
The L. inermis extract used in this study was able to suppress parasite growth. Surprisingly, the activity of the extract was higher at the dose of 250 mg/kg when compared with the dose of 500 mg/kg. This agrees with the finding of Das et al. [29], who reported better efficacy of extract of Argemone mexicana at lower dose than at higher dose. This effect may perhaps be attributed to the antioxidant action of the extract. It has been shown that antioxidants prevent the deleterious effects of Trypanosoma brucei [30]. Also, antioxidant action is maintained at certain dose above which it becomes a pro-oxidant with resultant lower pharmacological action and deleterious effect on the animal host. This may explain the poor antitrypanosomal effect observed at the higher dose of 500 mg/kg in this study. Bouayed and Bohn [31] and Martins [32] observed that at higher doses, an antioxidant becomes pro-oxidant thereby disrupting redox balance. Optimal doses of antioxidants are crucial for maintaining homeostasis [33], [34]. Besides, better antitrypanosomal effect at 250 mg/kg than 500 mg/kg suggests that the former is the optimal dose which conforms to the drug-receptor occupation theory. This theory states that drugs give their highest efficacy at full receptor occupation and no further effect is observed with further increase in dose [35]. Therefore, 250 mg/kg of the extract may be the optimal dose with full receptor occupation and also optimal dose for its antioxidant effect.
Trypanosoma brucei brucei has been shown to induce erythrocyte osmotic fragility (EOF) [36]. The increase in EOF in the infected untreated group in the present study could be attributed partly to generation of free radicals which could have overwhelmed the host body antioxidant defence system resulting in loss of integrity of erythrocyte membrane and their subsequent lysis. Similarly, Umar et al. [37] earlier reported that T. bruei brucei infection altered the host’s antioxidant defence against free radicals. In this study, rats treated with L. inermis extract at 250 mg/kg ameliorated oxidative stress as observed by significantly lower values of EOF. This implied that the membrane of erythrocytes was protected from the deleterious effects of the trypanosome on the erythrocyte membrane. Antioxidants stabilize free radicals and ameliorate free-radical-induced damage to the cells [38]. Anaemia in trypanosomosis has been attributed to injury to the membrane of erythrocytes resulting from either the lashing effect of the flagellum of trypanosomes on the erythrocyte membrane [39], [40]; or cleavage of sialic acid of the erythrocyte membrane by the sialidase of trypanosome [41]. The leaf extract of L. inermis has shown to protect the erythrocyte membrane against trypanosome-induced-EOF and therefore, may ameliorate anaemia associated with trypanosome infection in animals. Since anaemia but not level of parasitaemia in the trypanosome-infected animals is primarily responsible for decrease in the productivity of such animals and loss of their condition [13], [42], it is possible that the leaf of L. inermis could be used to improve productivity of trypanosome-infected animals.
One of the end products of lipid peroxidation is MDA, which is measured by concentration of MDA in body tissues or serum [8]. These products of lipid peroxidation produce changes in the structures and functions of the cell membrane, leading to decreased membrane fluidity, increased membrane permeability, inactivation of membrane-bound enzymes and loss of essential fatty acids [9], [11]. The end result of these changes on the surface of erythrocytes is increased erythrocyte osmotic fragility [12]. The increase in the concentration of MDA in PBS group suggests an increase in the production of free radicals by T. congolense, resulting from imbalance between radical generating and radical scavenging activities. This observation agrees with the findings of earlier workers [7], [36], [37]. The reduction in the concentration of MDA in L. inermis extract treated groups shows that the plant was able to ameliorate lipid peroxidation due to T. congolense infection. This observation further corroborates the results of EOF, which is an indirect index of measuring oxidative stress on erythrocyte membrane. It follows that methanol leaf extract of L. inermis enhances antioxidant defence against free radicals. Leaf of L. inermis has been reported to have an antioxidant effect against oxidative damage comparable to vitamin C [43]. Furthermore, reduced EOF and lipid peroxidation caused by the leaf extract of L. inermis lend credence to higher PCV values obtained in the extract treated groups, probably resulting from the protective effect of the extract on the erythrocyte membrane against oxidative stress.
The higher values of EOF and MDA in the diminazene aceturate (DA) treated group compared to the lower values in the 250 mg/kg extract treated group show that DA does not have a protective effect on the membrane of erythrocytes and probably lacks antioxidant effect. DA achieves its trypanocidal effect by binding to kinetoplast deoxyribonucleic acid (kDNA) and induces irreversible loss of the kDAN [44], [45]. On the other hand, the lower value of MDA in the DA treated group when compared with the PBS treated group may be a result of clearance of parasites from the blood of the treated rats, which precludes trypanosome-induced lipoperoxidation (oxidative damage) of the membrane of erythrocytes. This agrees with the finding of Kobo et al. [36] who reported lower values of MDA in T. brucei brucei infected rats treated with diminazene aceturate.
The antitrypanosomal effect of L. inermis vis-à-vis its antioxidant property observed in the present study could be attributable to the presence of one or combined effects of secondary metabolites detected. Antitrypanosomal effects of plant extracts are associated with the presence of one or more biologically active ingredients [17], [46]. Phytochemical constituents such as flavonoids and phenols are known to possess good antioxidant activities [47].
The antitrypanosomal activities of leaf of L. inermis observed in this study are not corroborated by the study of Wucherokke et al. [48]. This discrepancy could be attributed to differences in plant age/developmental stage, season and plant collection sites between both studies. In this study, the plant was collected in Zaria, Nigeria, which falls within the northern Guinea Savannah zone of Nigeria. Age, species, location, season of collection of plant and plant part affect the pharmacological activity of plant extracts [49]. Plants cultivated under different environmental conditions produce different phytochemical profiles or different amounts of individual components [50].
5. Conclusion
The results of this study validate the traditional use of the leaf of L. inermis in treating trypanosomosis. The ability of the leaf of L. inermis to enhance erythrocyte membrane integrity and maintain a high erythrocyte count in trypanosome-infected rats can be exploited to reduce anaemia and thus, improve productivity of trypanosome-ridden animals in trypanosome-endemic areas.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgement
We are grateful to L. Usman and H. Garba of the Department of Parasitology and Entomology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria for technical assistance.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Murray M., Morrison W.I., Whitelaw D.D. Host susceptibility to African trypanosomiasis: trypanotolerance. Adv Parasitol. 1983;21:1–68. doi: 10.1016/s0065-308x(08)60274-2. [DOI] [PubMed] [Google Scholar]
- 2.Nwosu C.O., Ikeme M.M. Parasitaemia and clinical manifestations in Trypanosoma brucei infected dogs. Rev Elev Med Vet Pays Trop. 1992;45:273–277. [PubMed] [Google Scholar]
- 3.Abenga J.N., Enwezor F.N.C., Lawani F.A.G., Ezebuiro C., Sule J., David K.M. Prevalence of trypanosomiasis in trade cattle at slaughter in Kaduna. Nig J Parasitol. 2002;23:107–110. [Google Scholar]
- 4.Mulumba K. Food and Agriculture Organization of the United Nations (FAO); Italy: 2003. (Socio-economic and cultural factors in the research and control of trypanosomiasis: paat technical and scientific series, 4). [Google Scholar]
- 5.Swallow B.M. Impacts of African trypanosomiasis on African agriculture. International Livestock Research Institute. 1999:1–46. [Google Scholar]
- 6.Kristjanson P.M., Swallow B.M., Rowland G.J., Krusoka R.L., Belew P.P. Measuring the cost of animal African trypanosomiasis, the potential benefit of control and returns to research. Agricult Syst1999;59:79–98. [Google Scholar]
- 7.Eze J.I., Anene B.M., Chukwu C.C. Determination of serum and organ malondialdehyde (MDA) concentration, a lipid peroxidation index in Trypanosoma brucei - infected rats. Comp Clin Pathol. 2008;17:67–72. [Google Scholar]
- 8.Umar I.A., Toma I., Akombum C.A., Nnaji C.J., Mahdi M.A., Gidado A. The role of intraperitoneally administered vitamin C during Trypanosoma congolense infection of rabbits. Afri J Biotechnol. 2010;9:5224–5228. [Google Scholar]
- 9.Ambali S.F., Abubakar M., Shittu M., Yaqub L.S., Anafi S.B., Abdullahi A. Chorpyrifos-induced alteration of haematological parameters in Wistar rats: ameriolative effect of zinc. Res J Env Toxicol. 2010;4:55–66. [Google Scholar]
- 10.Wahab A.A., Mabrouk M.A., Ayo J.O., Ambali S.F., Shittu M., Adenkola A.Y. Effects of co-administration of antioxidants on erythrocyte osmotic fragility of Wistar rats during the hot-fry season. Eur J Sci Res. 2010;46:73–79. [Google Scholar]
- 11.Kolanjiappan K., Manoharan S., Kayalvizhi M. Measurement of erythrocyte lipids, lipid peroxidation, antioxidants and osmotic fragility in cervical cancer patients. Clin Chim Acta. 2002;326:143–149. doi: 10.1016/s0009-8981(02)00300-5. [DOI] [PubMed] [Google Scholar]
- 12.Saluja P.S., Gupta S.L., Malhotra D.V., Ambawat H.K. Status of plasma malondialdehyde in experimental T. annulata infection in crossbred bovine calves. Ind Vet. 1999;76:379–781. [Google Scholar]
- 13.Naessens J. Bovine trypanotolerance: a natural ability to prevent severe anaemia and haemophagocytic syndrome? Int J Parasitol. 2006;36:521–528. doi: 10.1016/j.ijpara.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Geerts S., Holmes P.H., Diall O., Eisler M.C. African bovine trypanosomiasis: the problem of drug resistance. Trends Parasitol. 2001;17:25–28. doi: 10.1016/s1471-4922(00)01827-4. [DOI] [PubMed] [Google Scholar]
- 15.Orwa C, Mutua A, Kindt R, Jamnadass R, Anthony S. Agroforestree Database: a tree reference and selection guide version 4.0 2009; <http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp>.
- 16.Ali N.A.A., Julich W.D., Kusnick C., Lindequist U. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol. 2001;74:173–179. doi: 10.1016/s0378-8741(00)00364-0. [DOI] [PubMed] [Google Scholar]
- 17.Atawodi S.E., Ameh D.A., Ibrahim S., Andrew J.N., Nzelibe H.C., Onyike E. Indigenous knowledge system for treatment of trypanosomiasis in Kaduna state of Nigeria. J Ethnopharmacol. 2002;79:279–282. doi: 10.1016/s0378-8741(01)00351-8. [DOI] [PubMed] [Google Scholar]
- 18.Mikhaeil B.R., Badria F.A., Maatooq G.T., Amer M.M.A. Antioxidant and immunomodulatory constituents of henna leaves. Z Naturforsch C. 2004;59:468–476. doi: 10.1515/znc-2004-7-803. [DOI] [PubMed] [Google Scholar]
- 19.Endrini S., Rahmat A., Ismail P., Taufiq- Yap Y.H. Comparing of the cytotoxicity properties and mechanism of Lawsonia inermis and Strobilanthes crispus extract against several cancer cell lines. J Med Sci. 2007;7:1098–1102. [Google Scholar]
- 20.Zumrutdal M.E., Ozaslan M., Tuzcu M., Kalender M.E., Daglıoglu K., Akova A. Effect of Lawsonia inermis treatment on mice with sarcoma. Afr J Biotechnol. 2008;7:2781–2786. [Google Scholar]
- 21.Syamsudin I., Winarno H. The effects of Inai (Lawsonia inermis) leave extract on blood sugar level: An Experimental Study. Res J Pharmacol. 2008;2:20–23. [Google Scholar]
- 22.Aldhous P. Fighting parasites on a shoe string. Science. 1994;264:1857–1859. doi: 10.1126/science.8009209. [DOI] [PubMed] [Google Scholar]
- 23.Trease G.E., Evans W.C. Pharmacognosy. 12th ed. Ballire Tindal; United Kingdom: 1983. Drugs of biological origin. [Google Scholar]
- 24.Herbert W.J., Lumsden W.H. Trypanosoma brucei: a rapid “matching” method for estimating the host’s parasitemia. Exp Parasitol. 1976;40:427–431. doi: 10.1016/0014-4894(76)90110-7. [DOI] [PubMed] [Google Scholar]
- 25.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 26.Faulkner W.R., King J.W. Chemical Rubber Company; Cleveland, OH: 1970. Manual of clinical laboratory procedures. [Google Scholar]
- 27.Draper H.H., Hadley M. Malondialdehyde determination as index of lipid peoxidation. Met Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organisation . Pesticide Action Network, North America; San Francisco: 2001. PAN pesticides database. [Google Scholar]
- 29.Das P.K., Pillai S., Kar D., Pardhan D., Sahoo S. Pharmacological efficacy of Argemone mexicana plant extract, against cysteamine-induced duodenal ulceration in rats. Ind J Med Sci. 2011;65:92–99. [PubMed] [Google Scholar]
- 30.Eghianruwa I.K. The effect of supplemental antioxidants vitamin C and dimethyl sulfoxide on weight gain and survival in T. brucei infected and diminazene treated rats. Vet Arhiv. 2012;2:519. [Google Scholar]
- 31.Bouayed J., Bohn T. Exogenous antioxidants-double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3:228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins L.A., Coelho B.P., Behr G., Pettenuzzo L.F., Souza I.C., Moreira J.C. Resveratrol induces pro-oxidant effects and time-dependent resistance to cytotoxicity in activated hepatic stellate cells. Cell Biochem Biophys. 2014;68:247–257. doi: 10.1007/s12013-013-9703-8. [DOI] [PubMed] [Google Scholar]
- 33.Ratnam D.V., Ankola D.D., Bhardwaj V., Sahana D.K., Kumar M.N. Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Control Release. 2006;113:189–207. doi: 10.1016/j.jconrel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Pugh D.M. Qualitative and quantitative aspects of drug action. In: Brander G.C., Pugh D.M., Bywater R.J., Jenkkins W.L., editors. Veterinary applied pharmacology and therapeutics. 5th ed. Bailliére Tindall; London: 1991. pp. 9–27. [Google Scholar]
- 36.Kobo P.I., Ayo J.O., Aluwong T., Zezi A.U., Maikai V., Ambali S.F. Flavonoid mixture ameliorates increase in erythrocyte osmotic fragility and malondialdehyde concentration induced by Trypanosoma brucei-infection in Wistar rats. Res Vet Sci. 2014;96:139–142. doi: 10.1016/j.rvsc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Umar I.A., Ogenyi E., Okodaso D., Kimeng E., Stanecheva G.I., Omage J.J. Amelioration of anaemia and organ damage by combined intraperitoneal administration of vitamin A and C to Trypanosoma brucei brucei infected rats. Afr J Biotechnol. 2007;6:2083–2086. [Google Scholar]
- 38.Georgiva N.V. Oxidative stress as a factor of disrupted ecological oxidation balance in biological system – a review. Bulg J Vet Med. 2005;8:1–11. [Google Scholar]
- 39.Saror D.I. Proc first Natl Conf Tsetse Trypanosomiasis Res. 1982. Aspects of the anaemia of acute bovine trypanosomiasis; pp. 12–14. [Google Scholar]
- 40.Igbokwe I.O., Esievo K.A.N., Saror D.I., Obagaiye O.K. Increased susceptibility of erythrocytes to in vitro peroxidation in acute Trypanosoma brucei infection of mice. Vet Parasitol. 1994;55:279–286. doi: 10.1016/0304-4017(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 41.Esievo K.A.N. Proceedings of the 16th meeting of the International Scientific Council for Trypanosomiasis. Nairobi, Kenya. Organization of African Unity Scientific, Technical and Research and Control; 1979. In-vitro production of neuraminidase (sialidase) by Trypanosoma vivax; pp. 205–210. [Google Scholar]
- 42.Trail J.C.M., d’Ieteren G.D.M., Feron A., Kakiese O., Mulungo M., Pelo M. Effect of trypanosome infection, control of parasitaemia and control of anaemia development on productivity of N’Dama cattle. Acta Trop. 1991;48:37–45. doi: 10.1016/0001-706x(90)90063-6. [DOI] [PubMed] [Google Scholar]
- 43.Al-Demegh M.A. Evaluation of the antioxidant activity effect of Henna (Lawsonia inermis linn) leaves and or vitamin c in rats. Life Sci J. 2014;3:234–241. [Google Scholar]
- 44.Brack C., Delain E., Riou G., Festy B. Molecular organization of the kinetoplast DNA of Trypanosoma cruzi treated with berenil, a DNA interacting drug. J Ultrastruct Res. 1972;39:568–579. doi: 10.1016/s0022-5320(72)90122-0. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez V.M., Perez J.M., Alonso C. The berenil ligand directs the DNA binding of the cytotoxic drug Pt-berenil. J Inorg Biochem. 1997;68:283–287. doi: 10.1016/s0162-0134(97)00111-6. [DOI] [PubMed] [Google Scholar]
- 46.Mbaya A.W., Nwosu O.C., Onyeyili P.A. Toxicity and antitrypanosomal effects of Butryospermum paradoxum (Sapotaceae) stem bark in rats infected with Trypanosoma brucei and Trypanosoma congolense. J Ethnopharmacol. 2007;111:26–530. doi: 10.1016/j.jep.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 47.Zargar M., Azizah A.H., Roheeyati A.M., Fatimah A.B., Jahanshiri F., Pak-Dek M.S. Bioactive compounds and antioxidant activity of different extracts from Vitex negundo leaf. J Med Plants Res. 2011;5:2525–2532. [Google Scholar]
- 48.Wurochekke A.U., Chechet G., Nok A.J. In vitro and in vivo antitrypanosomal activity of the leaf of Lawsonia inermis against Trypanosoma brucei brucei infection in mice. J Med Sci. 2004;4:236–239. [Google Scholar]
- 49.Raskin I., Ribnicky D.M., Komarnytsky S., Ilic N., Poulev A., Borisjuk N. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20:522–531. doi: 10.1016/s0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- 50.Stevens L.H., Stoopen G.M., Elbers I.J.W., Molthoff J.W., Bakker H.A.C., Lommen A. Effect of climate conditions and plant developmental stage on the stability of antibodies expressed in transgenic tobacco. Plant Physiol. 2000;124:173–182. doi: 10.1104/pp.124.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]