Abstract
We report a case of acute massive pulmonary embolism in a patient with antithrombin III deficiency. The patient was treated with rivaroxaban. The patient responded well to the therapy, and contrast-enhanced computed tomography showed nearly complete disappearance of the pulmonary embolism. Patients with low antithrombin III activity may have resistance to heparin therapy, leading to insufficient anticoagulation during the acute phase of thromboembolism. This case suggests that direct oral anticoagulants, such as rivaroxaban, may be effective first-line agents for treating venous thromboembolism in patients with antithrombin III deficiency.
<Learning objective: Recently, direct oral anticoagulants represent a novel treatment option for venous thromboembolism with several practical advantages over conventional therapy. Antithrombin-III deficiency may lead to insufficient anticoagulation during the acute phase of thromboembolism. The present case suggests that rivaroxaban is a direct Factor Xa inhibitor and does not require cofactors such as antithrombin-III, thus it is suitable for anticoagulation therapy in patients with low antithrombin-III activity.>
Keywords: Venous thromboembolism, Direct oral anticoagulants, Antithrombin-III deficiency
Introduction
Pulmonary embolism (PE) is a common disease that can often be fatal. Establishing an accurate initial diagnosis and administering effective treatment are important for achieving the best possible prognosis for patients. For half a century, the standard therapy had been an intravenous heparin injection, along with overlapping administration of a vitamin K antagonist. However, direct oral anticoagulants (DOACs) targeted against factor Xa have recently demonstrated comparable efficacy to warfarin, with more favorable safety profiles [1]. Furthermore, DOACs overcome some limitations of standard therapy, such as the need for continuous heparin injection and for dose adjustments based on laboratory monitoring. Patients with low antithrombin III (AT-III) activity may have heparin resistance, which can lead to insufficient anticoagulation during the acute phase of thromboembolism [2]. We report here a case of acute massive PE successfully treated with rivaroxaban.
Case report
A 63-year-old man was referred to our hospital with shortness of breath and pain in both legs. He became aware of the symptoms two weeks earlier and noted that they had progressively worsened over time. He had a past medical history of hypertension for which he had been taking 2 antihypertensive agents for the past 10 years. He had not taken his medications for the last several days because of his illness. The patient was a heavy smoker of approximately 100 cigarettes per day over the last 30–40 years. Regarding his family medical history, his father died of old age, his mother died of heart failure and he had no siblings or children.
His vital signs on arrival were as follows: heart rate of 102 bpm, blood pressure of 113/89 mmHg, oxygen saturation of 95% with 10 L per minute of supplemental oxygen, and respiratory rate of 30 breaths per minute. A physical examination showed prominent IIp sound, systolic murmur on the left sternal border between the 4th and 5th rib, jugular venous distension, severe pitting edema in both limbs, and peripheral coldness in all extremities. An electrocardiogram showed sinus tachycardia (102 bpm) with ST elevation in leads V1-2, deep S wave in leads I, aVL, V5-6, and negative T waves in leads II, III, aVF, and V1-5. Echocardiography revealed preserved left ventricular (LV) contraction (with D-shaped LV), right ventricular (RV) strain and dysfunction with estimated RV systolic pressure of 61.6 mmHg and end-diastolic RV-LV diameter ratio of 1.2. Elevated levels of D-dimer (3.6 μg/mL), C-reactive protein (4.86 mg/dL), troponin I (42.5 pg/mL), and B-type natriuretic peptide (724 pg/mL) were detected by laboratory tests. The levels of blood urea nitrogen (10.9 mg/dL), creatinine (0.76 mg/dL), and creatinine clearance (104 mL/min) were within normal limits. His coagulation work-up at admission showed reduced levels of protein C activity (49%) and AT-III activity (38%), while contrast-enhanced computed tomography (CT) detected deep vein thrombosis (DVT) and PE (Fig. 1). Protein S level was normal. There were massive thrombi in both branches of the main pulmonary artery, and a small thrombus remaining in the left popliteal vein. There was also a patchy area of consolidation in the left upper lobe of the lung, which seemed to be caused by the pulmonary infarction. Because the remaining DVT was small, an inferior vena cava (IVC) filter was not inserted. Patients with high mortality risk typically require intensive therapy such as thrombolytic therapy, catheter embolectomy, or surgical embolectomy. However, the patchy consolidation in the upper lobe of the left lung prevented the exclusion of lung cancer as a possible comorbidity, therefore thrombolytic therapy was not conducted considering the risk for hemoptysis. After discussion of the treatment options with the patient and within the members of our department, he preferred anticoagulant therapy instead of intensive embolectomy. Administering exogenous AT-III and using heparin is recommended in the guideline of AT-III deficiency, but it may lead to insufficient anticoagulation during the acute phase of thromboembolism, which plays an important role in the treatment. As a result, the patient was treated with 30 mg per day of rivaroxaban.
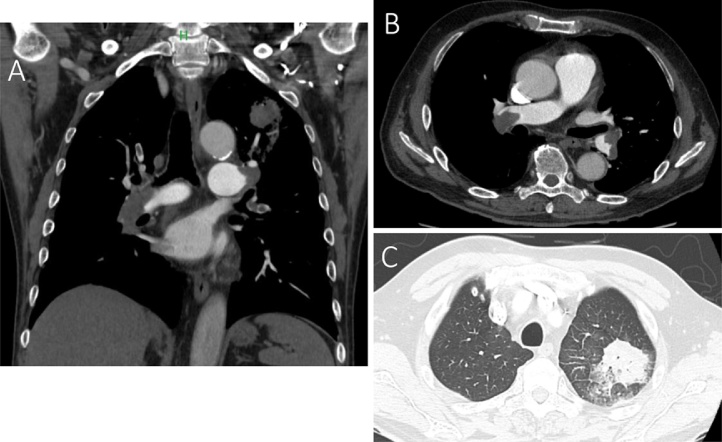
Fig. 1.
Coronal (A) and horizontal (B, C) section of contrast-enhanced computed tomography imaging at the time of admission. There were massive thrombi in both branches of the main pulmonary artery, and there was also a patchy area of consolidation in the upper lobe of the left lung, which appeared to be caused by the pulmonary infarction.
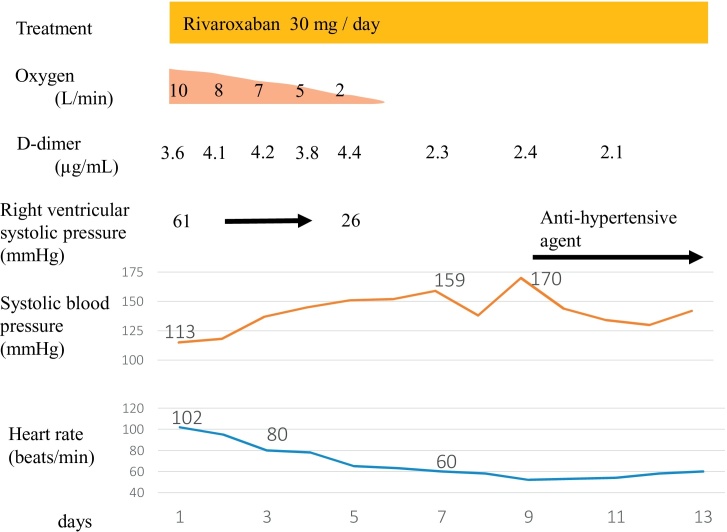
During the treatment course, his vital signs dramatically improved and were recorded 7 days after admission as follows: heart rate of 60 bpm, systolic blood pressure of 159 mmHg, oxygen saturation of 97% without supplemental oxygen, and respiratory rate of 16 bpm (Fig. 2). Simultaneously, his RV dysfunction improved with an estimated right ventricular systolic pressure of 26 mmHg 5 days after admission. Hemoptysis was not seen in the treatment course. Contrast-enhanced CT before discharge revealed that the thrombi remained in both branches of the main pulmonary artery, but were significantly reduced in size. There was also an improvement in enhancement of the peripheral pulmonary artery with contrast media. Oral rivaroxaban at a dose of 30 mg per day was continued for 3 weeks and then reduced to 15 mg per day. Four months after admission, a 12-lead electrocardiogram appeared normal and contrast-enhanced CT showed almost complete disappearance of the DVT and PE (Fig. 3). Simultaneously, the consolidation of left upper lobe completely disappeared.
Fig. 2.
Therapeutic course of the patient. The patient was treated with 30 mg per day of rivaroxaban. During the treatment course, the patient’s vital signs dramatically improved.
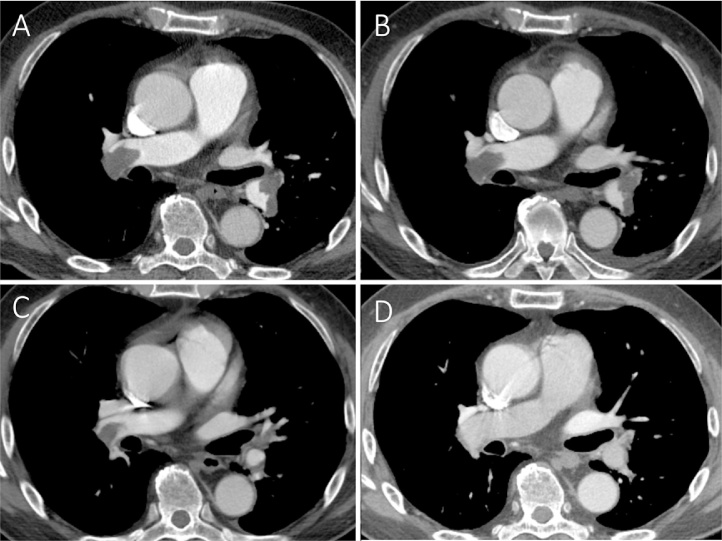
Fig. 3.
Horizontal section of contrast-enhanced computed tomography imaging at day 1 (A), day 3 (B), day 8 (C), and day 120 (D). Computed tomography at day 120 showed almost complete disappearance of the deep vein thrombosis and pulmonary embolism.
Because coagulation work-ups can be unreliable in the setting of an acute thromboembolic event, several tests were repeated after discharge. Although protein C activity returned to a normal level (83%), AT-III activity remained low (35%). The patient was therefore diagnosed with AT-III deficiency, and his thromboembolic event was considered to have been caused by his abnormal coagulation profile. Anticoagulation therapy has been continued to the present time and serious problems never happened after discharge.
Discussion
Intravenous heparin with oral warfarin is the standard treatment for DVT and PE. However, DOACs represent a novel treatment option with several practical advantages over conventional therapy. Individual studies have demonstrated that DOACs have comparable efficacy to warfarin, also showing more favorable safety profiles owing to their stable pharmacokinetics and pharmacodynamics [1], [3]. The efficacy and safety of rivaroxaban has also been demonstrated in the EINSTEIN study [4]. However, hemodynamically unstable PE patients or patients who required thrombolysis or pulmonary embolectomy were not included in the EINSTEIN study. Therefore, the efficacy and safety of rivaroxaban for those patients have not yet been established.
In the presently described case, the patient had a systolic blood pressure greater than 90 mmHg, but his other vital signs were unstable. His blood pressure was relatively low, considering that he had not taken his regular antihypertensive medications for several days before admission. The gradual elevation in his systolic blood pressure (by >50 mmHg) during the therapeutic course was therefore expected. Based on the European Society of Cardiology guideline classification for acute PE, this patient was considered to have high mortality risk because of his systolic blood pressure fall by 40 mmHg without new onset arrhythmia, hypovolemia, or sepsis [5]. Patients with high mortality risk typically require thrombolytic therapy, catheter embolectomy, or surgical embolectomy [6]. Patients with severe symptoms without hypotension are suggested to be treated with aggressive anticoagulation, not with thrombolytic therapy and need to be monitored to ensure that deteriorations are detected [7]. The patchy consolidation in the upper lobe of the left lung prevented the exclusion of lung cancer as a possible comorbidity. As a result, thrombolytic therapy was not conducted because of the risk for hemoptysis. After discussion of the treatment options with the patient and within the members of our department, he preferred anticoagulant therapy instead of intensive embolectomy.
Since the presence of AT-III is essential for unfractionated heparin to exert its anticoagulatory effect, patients with AT-III deficiency have resistance to heparin therapy. As a result, they may require higher doses of heparin or administration of exogenous AT-III with fresh frozen plasma to achieve a sufficient activated partial thromboplastin time (APTT) [2]. It has been previously reported from the retrospective study that almost 40% of patients with heparin therapy was subtherapeutic (APTT < 1.5), therefore it must be more difficult to control AT-III deficiency patients within the therapeutic range [8]. In this way, AT-III deficiency may lead to insufficient anticoagulation during the acute phase of thromboembolism. Acute phase treatment is already known to have a significantly important role in the treatment of PE. Since rivaroxaban is a direct Factor Xa inhibitor and does not require cofactors such as AT-III, it is suitable for anticoagulation therapy in patients with low AT-III activity [9].
AT-III deficiency can be inherited or acquired, and is known to be associated with an increased risk of thromboembolism. Potential causes of acquired AT-III deficiency should be excluded before making a classification of inherited deficiency. Such causes include cirrhosis of the liver, nephrotic syndrome, protein losing enteropathy, sepsis, burn injuries, polytrauma, hepatic veno-occlusive disease, thrombotic microangiopathies, cardiopulmonary bypass surgery, large hematomas, metastatic tumors, extra corporeal membrane oxygen therapy, l-asparaginase therapy, and heparin therapy [10]. Hereditary AT-III deficiency is rare, with an estimated prevalence in the general population of approximately 0.02%–0.2% worldwide, including in Japan. AT-III deficiency is divided into type I and type II, which is further divided into type IIa, IIb, and IIc, according to their place of defect from genetic analysis. When evaluating for AT-III deficiency, it is appropriate to start with an AT-III functional assay and there is no need to routinely check an AT-III antigen assay [10]. It is because that AT-III antigen cannot identify the type of AT-III deficiency and also cannot detect AT-III deficiency of its own. AT-III antigen is low in patients with type I and IIc, while it is often normal in patients with type IIa and IIb. The diagnosis of AT-III deficiency inevitably requires repeated demonstration of reduced plasma AT-III activity in a patient who is not in the midst of an acute illness or surgery. Although genetic analysis can identify the various specific mutations causing AT-III deficiency, it is typically not performed in routine clinical practice and is not necessary to diagnose AT-III deficiency [10]. Genetic analysis can be considered in rare cases in which a fetus may be expected to be homozygous or compound heterozygous for coagulation inhibitor defects.
In the presently described case, repeated measurements of AT-III activity levels were low. The patient did not have a history of heparin administration, and none of the other causes of acquired AT-III deficiency were applicable based on results from contrast-enhanced full-body CT imaging, blood tests (including tumor marker tests), and his treatment course. Although his plasma AT-III activity tests were conducted while taking rivaroxaban, the drug has been previously shown to have no effect on AT-III activity [3]. Based on this information, the patient’s AT-III deficiency was considered to be inherited. The patient had no children, siblings, or other close relatives, and his parents’ medical histories did not give an indication of AT-III deficiency.
In conclusion, a coagulation work-up including assessment of potential AT-III deficiency is essential in the treatment of acute thromboembolic events. DOACs may be regarded as first-line therapeutic agents in patients with AT-III deficiency.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Van der Hulle T., Kooiman J., den Exter P.L., Dekkers O.M., Klok F.A., Huisman M.V. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12:320–328. doi: 10.1111/jth.12485. [DOI] [PubMed] [Google Scholar]
- 2.Spiess B.D. Treating heparin resistance with antithrombin or fresh frozen plasma. Ann Thorac Surg. 2008;85:2153–2160. doi: 10.1016/j.athoracsur.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 3.Kubitza D., Becka M., Voith B., Zuehlsdorf M., Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412–421. doi: 10.1016/j.clpt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Bauersachs R., Berkowitz S.D., Brenner B., Buller H.R., Decousus H., Gallus A.S. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinides S.V., Torbicki A., Agnelli G., Danchin N., Fitzmaurice D., Galiè N. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3080. [Google Scholar]
- 6.Sobieszczyk P. Catheter-assisted pulmonary embolectomy. Circulation. 2012;126:1917–1922. doi: 10.1161/CIRCULATIONAHA.110.963041. [DOI] [PubMed] [Google Scholar]
- 7.Kearon C., Akl E.A., Ornelas J., Blaivas A., Jimenez D., Bounameaux H. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura M., Miyata T., Ozeki Y., Takayama M., Komori K., Yamada N. Current venous thromboembolism management and outcomes in Japan. Circ J. 2014;78:708–717. doi: 10.1253/circj.cj-13-0886. [DOI] [PubMed] [Google Scholar]
- 9.Samama M.M. The mechanism of action of rivaroxaban – an oral, direct Factor Xa inhibitor – compared with other anticoagulants. Thromb Res. 2011;127:497–504. doi: 10.1016/j.thromres.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Patnaik M.M., Moll S. Inherited antithrombin deficiency: a review. Haemophilia. 2008;14:1229–1239. doi: 10.1111/j.1365-2516.2008.01830.x. [DOI] [PubMed] [Google Scholar]