Abstract
The chelating behavior of the thiosemicarbazone derivatives of 2-hydroxy-8-R-tricyclo[7.3.1.0.2,7]tridecane-13-one (where R = H, CH3, C6H5) towards Co(II), Ni(II) and Cu(II) has been investigated by elemental analysis, molar conductivity measurements, UV-VIS, IR, ESR spectroscopy and thermal studies. It was deduced from the experiments performed that the ligands coordinate to metal ions in different ways – neutral bidentate or mononegative bidentate – depending on the nature of R. Also, if metal acetates are used instead of metal chlorides, the ligands coordinate in a mononegative bidentate fashion, regardless of the nature of R or the thiosemicarbazone type ligand. The antimicrobial activity of the ligands and of the complexes towards samples of Acinetobacter boumanii, Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa was determined.
Keywords: Thiosemicarbazone complexes, copper complexes, thiosemicarbazones, antimicrobial activity
Introduction
Thiosemicarbazones are known for their capacity to act as polydentate ligands, as well as for their biological activity. Collins et al. have reported the correlation between structure and anti-myco-bacterial activity in a series of 2-acetylpyridine thiosemicarbazones [1]. In many cases, by coordination to different transition metal ions that can be found in biological systems, it is possible to obtain complexes that are more efficient drugs than the corresponding free ligands [2a,b,c,d,e].
Copper(II) complexes possess a wide range of biological activity and are among the most potent antiviral, antitumor and anti-inflammatory agents. For example, a copper(II) complex of 2-formyl-pyridine thiosemicarbazone has been shown to inhibit the RNA-dependent DNA polymerases and the transforming ability of Rous sarcoma virus (RSV) [3]. In addition, copper(II) complexes of 2-acetyl-pyridine thiosemicarbazones are active anti-malarial agents [4]. They possess strong antineoplastic activity against a number of transplantable tumors, spontaneous murine tumors and human tumors. The mechanism of their antitumor action is thought to involve either inhibition of the enzyme ribonucleotide reductase, an obligatory enzyme in DNA synthesis [5,6,7], or creation of lesions in DNA strands [8]. The antifungal activity of Ni(II), Cu(II) and Zn(II) complexes with 5-nitro-2-furfural thiosemicarbazone has been demonstrated [9,10]. In addition, Agarwal et al. reported in 2006 the synthesis, magnetospectral, antibacterial, and antifungal properties of Cu(II) complexes of 4[N-(benzylidene)amino]-, 4[N-(4-methoxybenzylidene)amino]-, 4[N-(4-dimethylaminobenzylidene) amino]- and 4[N(cinnamalidene)amino] antipyrine thiosemicarbazone [11].
This paper describes the synthesis and characterization of some complexes of transition metal ions Co(II), Ni(II) and Cu(II) with a series of thiosemicarbazone derivatives 2-hydroxy-8-R-tricyclo-[7.3.1.0.2,7 ]tridecane-13-one (R = H, CH3, C6H5).
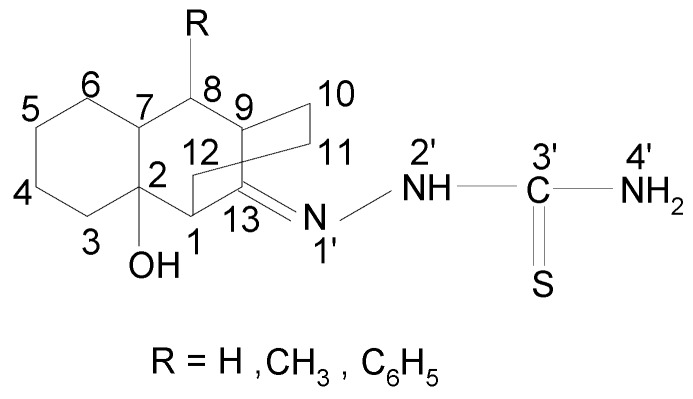
Figure 1.
The structures of the ligands ( LH, LCH3, LC6H5).
Characterization of the newly prepared complexes was accomplished by IR, UV-VIS and ESR spectroscopy, thermal analysis and molar electrical conductivity measurements. From the experimental data it was inferred that depending on the nature of R, the ligand coordinates to metal ions in different ways: neutral bidentate or mononegative bidentate. Also, if metal acetates are used instead of metal chlorides, then the ligands coordinate in a mononegative bidentate fashion, regardless of the nature of R or type of thiosemicarbazone ligand. In addition, taking into consideration the use of copper complexes in the treatment of some diseases, mentioned above, we tested the antimicrobial activity of the prepared combinations using strains of Acinetobacter boumanii, Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa isolated from different pathological products obtained from patients with infections associated with the use of cardiovascular prosthetic devices.
Results and Discussion
Synthesis
The ligands LH, LCH3 and LC6H5 used in this work were obtained by the method described elsewhere [12]. The [M(LH)2]Cl2, [M(LR)2] complexes (M = Cu(II), Ni(II), Co(II); R = CH3, C6H5) and [MLR(H2O)2ac] complexes (M = Cu(II), Ni(II), Co(II); R = H, CH3, C6H5) were prepared by reaction of the corresponding ligands with methanol solutions of a suitable M(II) salt.
Properties
The molar conductivity values (Ω-1·cm2·mol-1) of the complexes in nitrobenzene [M(LH)2]Cl2 (55.7 for [Cu(LH)2]Cl2, 53.9 for [Co(LH)2]Cl2 and 58.4 for [Ni(LH)2]Cl2, respectively) indicate that these complexes are 1:2 type electrolytes. The complexes display low solubility in chloroform, acetone and water, but they are soluble in methanol and dimethylformamide (DMF). The color of aqueous solutions changes after some 10 minutes, indicating that the complexes decompose in water. The molar conductibility values below 10 Ω-1·cm2·mol-1 for the other complexes, in nitrobenzene, indicate that these complexes are non-electrolytes.
Thermal Decomposition
The complexes of this study were investigated by thermogravimetry. The TG and DTG curves for all the complex combinations are similar, with the exception of the loss of weight temperatures. The decomposition takes place in two steps for [M(LH)2]Cl2, [M(LCH3)2] and [M(LC6H5)2] type complexes (220 – 450 and 430 – 690°C) and in three steps for [MLR (H2O)2ac] type complexes (112 – 153, 174 – 357 and 452 – 697°C).
The weight loss between 112 – 153°C is due to the loss of two water molecules per molecule of complex. Experimental data for the thermogravimetric analyses are presented in Table 1. The final residues were analyzed by IR spectroscopy and identified as CuO, Co2O3 and NiO, and the %M corresponded to the calculated one.
Table 1.
Thermogravimetric analysis data of the prepared complex combinations.
| Compound | Temperature range (oC) | Eliminated fragment | Weight loss % |
|---|---|---|---|
| [M(LH)2]Cl2 | 220-355 | Cl2+LH | 49.50-51.00 |
| 430-680 | LH | 39.00-41.00 | |
| 680 | MO | 10.70-12.50 | |
| M(LCH3)2] | 240-420 | LCH3 | 44.00-46.00 |
| 450-685 | LCH3 | 44.00-45.00 | |
| 685 | MO | 11.00-13.00 | |
| [M(LC6H5)2] | 248-450 | LC6H5 | 45.93-46.00 |
| 453-690 | LC6H5 | 45.93-46.20 | |
| 690 | MO | 9.50-10.25 | |
| [M(LH)(H2O)2ac] | 112-137 | H2O | 7.50-8.50 |
| 174-346 | Ac | 12.93-13.70 | |
| 452-657 | LH | 63.00-64.23 | |
| 657 | MO | 16.90-18.45 | |
| [M(LCH3)(H2O)2ac] | 115-145 | H2O | 7.40-8.50 |
| 180-354 | Ac | 12.65-13.47 | |
| 463-668 | LCH3 | 64.10-65.23 | |
| 668 | MO | 16.43-17.85 | |
| [M(LC6H5)(H2O)2ac] | 119-153 | H2O | 6.73-7.55 |
| 182-357 | Ac | 11.00-12.32 | |
| 467-697 | LC6H5 | 69.86-72.13 | |
| 697 | MO | 14.20-15.69 |
IR Spectra
Relevant IR data are given in Table 2. The IR spectra of the ligand LH shows an absorption band due to νC=S at 740 cm-1, associated with the νC=S + νC=N absorption band at 1291 cm-1, both of medium intensity. In the IR spectra of [M(LH)2] , the specific band for νC=S has a negative shift associated with a positive shift of the absorption band for νC=S+νC=N. This suggests that the ligand coordinates through the thiocarbonyl sulfur. The νC=N1’ frequency values decreased and the values of the νN1’-N2’ frequencies increased in the IR spectra of the complexes, compared with the values of the ligand spectrum, without a significant change in the νN2’H frequencies, suggesting that the ligand LH coordinates to metal ions through the N1’ atom.
Table 2.
IR spectral data for the ligands and their complexes (cm)-1.
| Compound | νC=N1’ | νN1’-N2’ | νN2’H | νN4’H | νC=S; νC=S + νC=O(s) |
νC=O(as) νC=N |
νC=N2’ | νC-S | νC-O | νOH |
|---|---|---|---|---|---|---|---|---|---|---|
| LH | 1599 | 1016 | 3230 | 3319 | 740 | - | - | - | - | 3426 |
| 1291 | 1144 | |||||||||
| [M(LH) 2]Cl2 | ||||||||||
| (CuC28H48N6O2S2)Cl2 | 1556 | 1056 | 3232 | 3322 | 712 | - | - | - | - | 3424 |
| 1315 | 1143 | |||||||||
| (CoC28H48N6O2S2)Cl2 | 1564 | 1060 | 3229 | 3318 | 716 | - | - | - | - | 3425 |
| 1320 | 1142 | |||||||||
| (NiC28H48N6O2S2)Cl2 | 1560 | 1058 | 3233 | 3320 | 718 | - | - | - | - | 3420 |
| 1317 | 1140 | |||||||||
| LCH3 | 1621 | 1060 | 3213 | 3330 | 782 | - | - | - | - | 3425 |
| 1296 | 1140 | |||||||||
| [M(LCH3) 2] | ||||||||||
| Cu(C30H50N6O2 S2) | 1580 | 1094 | - | 3329 | - | - | 1595 | 547 | - | 3422 |
| 1142 | ||||||||||
| Co(C30H50N6O2S2) | 1578 | 1103 | - | 3332 | - | - | 1598 | 562 | - | 3420 |
| 1138 | ||||||||||
| Ni(C30H50N6O2S2) | 1575 | 1098 | - | 3328 | - | - | 1593 | 554 | - | 3424 |
| 1138 | ||||||||||
| LC6H5 | 1633 | 1075 | 3191 | 3338 | 798 | - | - | - | - | 3423 |
| 1302 | 1132 | |||||||||
| [M(LC6H5) 2] | ||||||||||
| Cu(C40H54N6S2) | 1591 | 1108 | - | 3340 | - | - | 1615 | 590 | - | 3430 |
| 1138 | ||||||||||
| Co(C40H54N6O2S2) | 1593 | 1113 | - | 3336 | - | - | 1619 | 601 | - | 3426 |
| 1130 | ||||||||||
| Ni(C40H54N6O2S2) | 1586 | 1109 | - | 3340 | - | - | 1613 | 595 | - | 3428 |
| 1136 | ||||||||||
| [MLH (H2O)2ac] | ||||||||||
| Cu(C16H30N3O5S) | 1559 | 1047 | - | 3325 | - | 1532 | 1586 | 552 | - | 3418 |
| 1469 | 1137 | |||||||||
| Co(C16H30N3O5S) | 1568 | 1050 | - | 3321 | - | 1526 | 1589 | 571 | - | 3419 |
| 1452 | 1138 | |||||||||
| Ni(C16H30N3O5S) | 1565 | 1049 | - | 3323 | - | 1517 | 1581 | 564 | - | 3415 |
| 1448 | 1139 | |||||||||
| [MLCH 3(H2O)2ac] | ||||||||||
| Cu(C17H32N3O5S) | 1572 | 1078 | - | 3332 | - | 1528 | 1603 | 562 | - | 3420 |
| 1462 | 1141 | |||||||||
| Co(C17H32N3O5S) | 1569 | 1087 | - | 3336 | - | 1521 | 1598 | 567 | - | 3419 |
| 1448 | 1139 | |||||||||
| Ni(C17H32N3O5S) | 1567 | 1082 | - | 3334 | - | 1514 | 1592 | 561 | - | 3422 |
| 1437 | 1137 | |||||||||
| [MLC6H 5(H2O)2ac] | 1583 | 1098 | - | 3342 | - | 1522 | 1608 | 583 | - | 3425 |
| Cu(C22H34N3O5S) | 1458 | 1135 | ||||||||
| Co(C22H34N3O5S) | 1580 | 1101 | - | 3339 | - | 1518 | 1602 | 594 | - | 3430 |
| 1442 | 1134 | |||||||||
| Ni(C22H34N3O5S) | 1578 | 1097 | - | 3341 | - | 1509 | 1595 | 580 | - | 3428 |
| 1431 | 1137 |
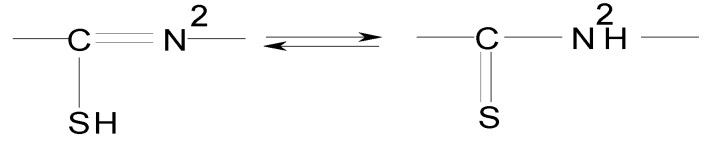
In the IR spectra of the [M(LCH3)2] and [M(LC6H5)2] complexes, the νN2’H band disappears and the νC=N1’ and νN1’-N2’ bands act in the same way as in the [M(LH) 2]Cl2 complexes. Also, the νC=S band and the associated νC=S + νC=N band disappeared and νC-S band appears in the 547-600 cm-1 range. This behavior confirms the coordination of ligand by sulfur atoms in the deprotonated thioenolic form. The ligand LH coordinates differently to metallic ions, as compared to the LCH3 and LC6H5 ligands. This behavior is probably due to the influence of the R radical from the ligand LR upon the unstable electronic pair of sulfur atom in the presence of the adjacent H atom, making possible the thioketo-thioenol equilibrium shown in Figure 2.
Figure 2.
In the IR spectra of [MLR(H2O)2ac] complexes the νC=N1’ , νN1’-N2’, νN2’H, νN4’H, νC=S, νC=S, νC=N, νC=N2’ and νC-S frequencies display almost the same behavior as in the spectra of the [M(LCH3s)2] and [M(LC6H5)2] complexes. Additionally, two absorption bands in the 1431-1469 cm-1 and 1509-1532 cm-1 ranges, assigned to νc=o(s) and νc=o(as) frequencies, confirm the bidentate nature of coordinated acetate [13,14]. In addition, a band appears in the 805-913 cm-1 range, which can be attributed to the ρr vibration mode specific to water molecules coordinated to metal ions [13,14].
Electronic spectra
The electronic spectroscopy data for the complexes in solid state are presented in Table 3. The values of the electronic transitions specific for the complex combinations [Cu(LR)] are very close (±30 cm-1). For [CoLR(H2O)2ac] and [NiLR(H2O)2ac] complexes, the values of the B, β and Dq parameters are also very close. These values indicate a similar ligand field strength. The electronic transitions of [MLR(H2O)2ac] complexes suggest an octahedral geometry around the metal ion.
Table 3.
Electronic spectral data for complexes in solid state (cm-1).
| Compound | Transitions d - d | B | β | Dq | Geometry | ||
|---|---|---|---|---|---|---|---|
| [Cu(LH)2] Cl2 | - | - | - | Pseudo-tetrahedral | |||
| [Cu(LCH3)2] | Pseudo-tetrahedral | ||||||
| [Co(LH)2] Cl2 | 4A2 → 4T1(P) | 919 | 0,947 | Pseudo-tetrahedral | |||
| [Co(LCH3)2] | 13030 | 808 | 0,833 | - | Pseudo-tetrahedral | ||
| [Ni(LH)2] Cl2 | ν3 | ||||||
| [Ni(LCH3)2] | 24350 | Sq - pl | |||||
| [CuLR(H2O)2ac] | |||||||
| [CoLR(H2O)2ac] | 4T1 g → 4T1 g (P) | ||||||
| [NiLR(H2O)2ac] | 3A2 g → 4T1 g (P) | ||||||
The same phenomenon may be seen for the [Cu(LR)]Cl2 complexes. A minor change in electronic transition values can be seen also for Co (II) and Ni (II) complexes. This behavior proves that the ligand field strength is essentially the same for this type of complexes with the same metal ions, regardless of the nature of R. The inter-electronic repulsion values could be calculated only for some of the complexes prepared, using the d-d transition energy values. The values of the B and β parameters indicate a covalent bond between M-L in [Co(LR)2] complexes, compared to [Co(LR)2]Cl2 complexes.
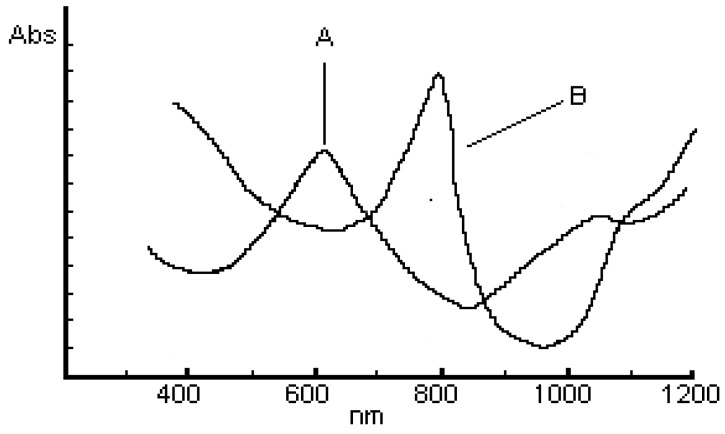
For [M(LR)2] and [M(LR)2]Cl2 (M=Cu, Co) complexes, the energy values of d-d transitions suggest a distorted tetrahedral arrangement for the metal ion, and a square-planar arrangement for [Ni(LR)2] and [Ni(LR)2]Cl2 complexes [10,13,15]. Figure 3 shows the electronic spectrum for [Co(LR)2]Cl2, (A) and [Co(LR)2], (B) complexes.
Figure 3.
Electronic spectra of Co(LH)2]Cl2, (A) and [Co(LR)2], (B) complexes.
ESR Spectra
X-band ESR spectra for Cu(II) complexes obtained from CuCl2 and LR were recorded using methanol solutions at 77K. The values of the {g} tensor confirm the uniaxial symmetry that corresponds to the following sequence: gz>gy; gz>gx [16]. The g values for [Cu(LR)2]n complexes, where n = +2, for R=H and n = 0, for R=CH3, C6H5 are presented in Table 4 .
Table 4.
The g values determined from ESR spectra.
| Compound | gx | gy | gz |
|---|---|---|---|
| [Cu(LH)2]Cl2 | 2.054 | 2.107 | 2.211 |
| [Cu(LCH3)2] | 2.035 | 2.115 | 2.203 |
| [Cu(LC6H5)2] | 2.040 | 2.116 | 2.210 |
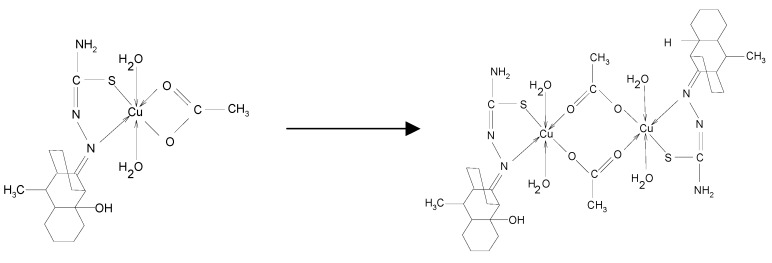
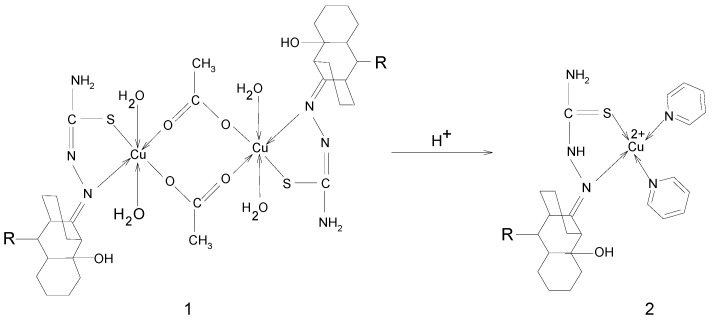
If a buffer solution of acetic acid-sodium acetate is added to a methanol solution of [CuLCH3(H2O)2ac], a brown precipitate can then be obtained. A strong band around 16230 cm-1 specific to octahedral Cu2+ characterizes the electronic spectra of this compound. Combining this experimental data with elemental analysis and IR spectra, the reaction mechanism of Scheme 1 can be proposed:
Scheme 1.
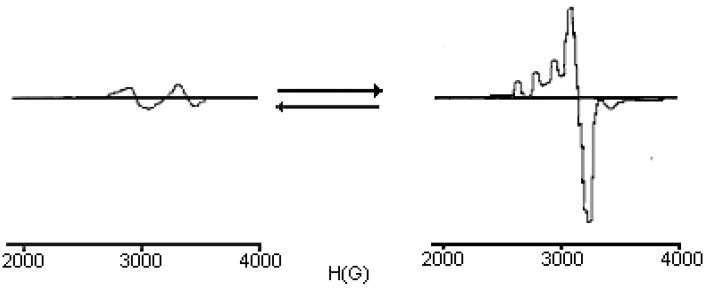
If the brown precipitate is dissolved in pyridine, the resulting brown solution becomes blue-green after 24 hours. ESR spectra of the two solutions recorded at 77K (Figure 4) suggest a transformation of compound 1 into compound 2, as shown in Scheme 2 [17,18].
Figure 4.
ESR for complexes 1 and 2 in pyridine at 77K.
Scheme 2.
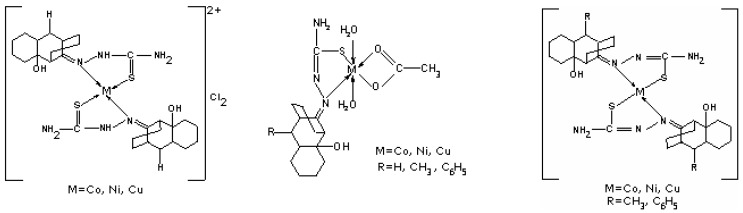
The ESR spectrum of a frozen solution of compound 2, recorded at 77 K, shows that the dinuclear complex 1 dissociates to form a mononuclear species with g⊥ =2.08, g// =2.25 and A// =155 Gauss. The precise structure of this mononuclear species is still unknown, but the g// and A// values indicate that three nitrogens are probably coordinated to the Cu(II) ion [19]. From the experimental data, different spatial arrangements can be proposed for the prepared complexes (Figure 5).
Figure 5.
It was found that the ligand with R=H is coordinated in a bidentate neutral mode, while the ligand containing R=CH3, C6H5 is coordinated in a bidentate mononegative way. The presence of an electron donating group (CH3 or C6H5) changes the charge density and influences the coordination mode of the ligand to metal ion. In the case of M(II) acetates, the ligand coordinates in a bidentate mononegative way, no matter the nature of R.
Antimicrobial activity assays
The antimicrobial activities of the complexes and ligands were screened by adapted qualitative, diffusimetric methods (i.e. distribution of the tested solutions on filter paper disks, in agar wells or in spots on solid media that have been inoculated with test microbial strains) and quantitative methods based on serial two-fold dilutions of the tested compounds in order to establish the corresponding Minimal Inhibitory Concentrations (MIC). Five bacterial strains, i.e. Acinetobacter boumanii, Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa, freshly isolated from patients with infections associated with cardiovascular devices from different clinical sources and identified by conventional methods were cultivated on solid media and incubated at 37°C for 24 hours prior to testing.
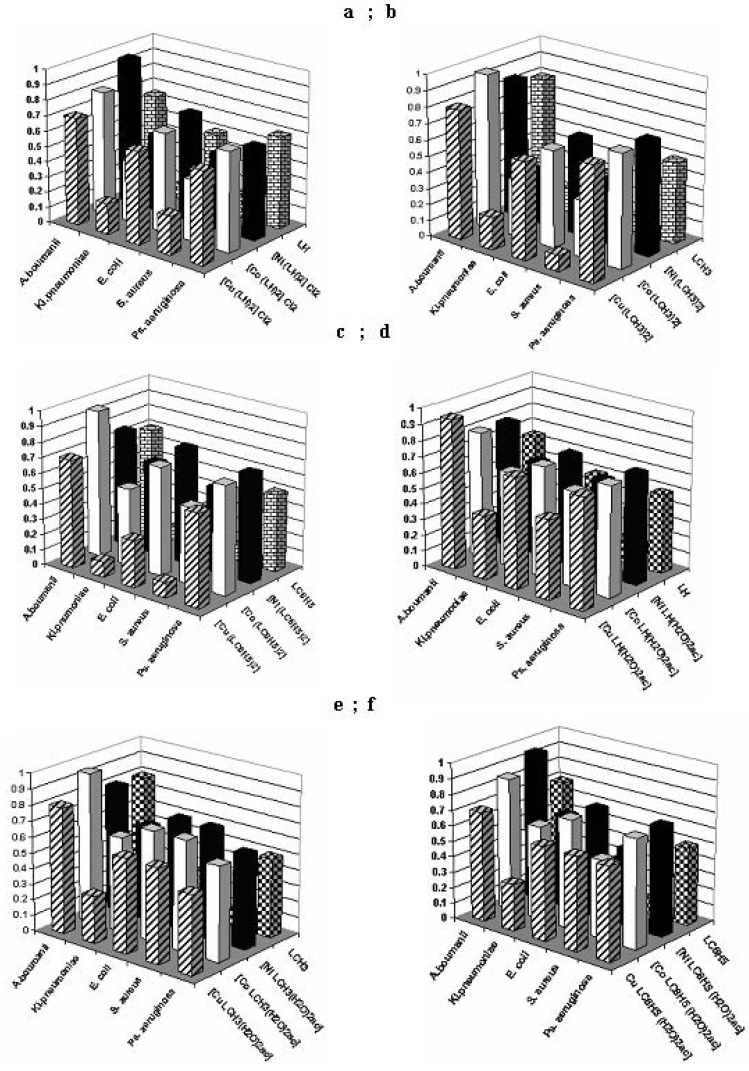
The qualitative screening results demonstrated that the three examined diffusion methods exhibited different sensitivities in detecting the antimicrobial potential of the tested compounds, with the most efficient one for different bacterial strains proving to be the spot method. The results of the quantitative assays (Figure 6) showed that the tested compounds exhibited variable MICs and selective antimicrobial activity, depending on the microbial strains. All tested compounds [M(LH)2]Cl2 proved to be active on Klebsiella pneumoniae and on Staphylococcus aureus, with the complex [Cu(LH)2]Cl2 and the LH ligand being the most active ones. The complexes of the [M(LCH3)2] type also show activity towards the same bacteria, and moreover, the LCH3 ligand is active on Escherichia coli too. Among the compounds of the [M(LC6H5)2] class, the complex [Cu(LC6H5)2] and the LC6H5 ligand show a pronounced activity towards Staphylococcus aureus, Klebsiella pneumoniae and Escherichia coli. From the compounds of the [MLR (H2O)2ac] class, only the [CuLR (H2O)2ac] complex shows activity towards Klebsiella pneumoniae, whereas the rest of the complexes present very low activities against the other bacterial strains.
Figure 6.
Graphical representation of the MIC values (mg/mL) of the tested compounds towards different bacterial strains.
Conclusions
The geometry of the studied complexes is influenced by the nature of ligand, the nature of R, which determines a variation of charge density at the coordination site and by the nature of the metal salts used in their preparation.
The sensitivity spectrum of the microbial strains towards the ligands and the corresponding complexes was determined by qualitative and quantitative methods and the following conclusions were reached:
-
■
the qualitative anti-microbial activity screening results of the tested compounds proved that the most efficient method is the spot method;
-
■
the quantitative anti-microbial activity test results proved that both the ligands and the complex combinations have specific anti-microbial activity, depending on the microbial species tested;
-
■
the complex combinations of the [MLR (H2O)2ac] type, except for the [CuLR (H2O)2ac] complex, show very low activity towards the tested bacterial strains.
Experimental
General
The metallic ion contents were determined by atomic absorption spectroscopy with a Carl Zeiss Jena AAS 1N spectrometer; Cl was determined by a gravimetric method; C, H, and N were analyzed with a Carlo Erba elemental analyzer. The prepared complexes and their elemental analysis are given in Table 5. Elemental analyses were performed after drying the complexes at 105 °C. The molar conductivity was determined with CONSORT–C533 conductometer. Electronic spectra were recorded by the diffuse-reflectance technique, using MgO as diluting matrix, on a JASCO V-550 spectrophotometer. IR spectra were recorded with a BioRad FTS 135 spectrophotometer in the 4000-400 cm-1 region using KBr pellets. All the complexes were studied by thermogravimety (TG) in static air atmosphere, with a sample heating rate of 10 °C/min. using a DuPont 2000 ATG thermo balance. ESR spectra were recorded on polycrystalline powders and solutions at room temperature and 77K with an ART-6, model IFA-Bucharest, X-band spectrometer (9.01 GHz) connected to a PC equipped with a 100KHz field modulation unit. The required chemicals were purchased from Merck and Chimopar Bucharest and all manipulations were performed using materials as received. The ligands LH (m.p. 183-184 oC), LCH3 (m.p. 194-195 oC), LC6H5 (m.p. 204-205 oC) were obtained by the method described elsewhere [12].
Table 5.
Elemental analyses.
| Compound | %H | %C | %N | %M | %Cl | Color | Dec. | Yield |
|---|---|---|---|---|---|---|---|---|
| exp. | exp. | exp. | exp. | exp. | Point | |||
| (calc) | (calc) | (calc) | (calc) | (calc) | (0C) | (%) | ||
| [M(LH)2]Cl2 | ||||||||
| (CuC28H48N6O2S2)Cl2 | 6.31 | 48.66 | 12.25 | 9.22 | 10.03 | light-green | 218 | 81 |
| (698.5) | (6.87) | (48.10) | (12.02) | (9.11) | (10.16) | |||
| (CoC28H48N6O2S2)Cl2 | 6.25 | 48.85 | 12.43 | 8.63 | 10.10 | dark-green | 245 | 79 |
| (694) | (6.91) | (48.41) | (12.10) | (8.50) | (10.23) | |||
| (NiC28H48N6O2S2)Cl2 | 6.40 | 48.80 | 12.50 | 8.42 | 10.18 | brown | 231 | 85 |
| (694) | (6.91) | (48.41) | (12.10) | (8.50) | (10.23) | |||
| [M(LCH3) 2] | ||||||||
| Cu(C30H50N6O2S2) | 7.30 | 55.40 | 12.56 | 9.62 | light-green | 239 | 67 | |
| (653.5) | (7.65) | (55.08) | (12.85) | (9.71) | ||||
| Co(C30H50N6O2S2) | 7.28 | 55.87 | 12.72 | 8.90 | dark-green | 261 | 72 | |
| (649) | (7.70) | (55.46) | (12.94) | (9.09) | ||||
| Ni(C30H50N6O2S2) | 7.31 | 55.73 | 12.67 | 8.89 | brown | 259 | 75 | |
| (649) | (7.70) | (55.46) | (12.94) | (9.09) | ||||
| [M(LC6H5) 2] | ||||||||
| Cu(C40H54N6O2 S2) | 6.58 | 62.18 | 10.48 | 8.00 | light-green | 245 | 63 | |
| (777.5) | (6.94) | (61.76) | (10.80) | (8.16) | ||||
| Co(C40H54N6O2S2) | 6.54 | 62.48 | 10.53 | 7.50 | dark- green | 263 | 62 | |
| (773) | (6.98) | (62.09) | (10.86) | (7.63) | ||||
| Ni(C40H54N6O2S2) | 6.57 | 62.57 | 10.66 | 7.52 | brown | 260 | 68 | |
| (773) | (6.98) | (62.09) | (10.86) | (7.63) | ||||
| [MLH (H2O)2ac] | ||||||||
| Cu(C16H30N3O5S) | 6.59 | 43.91 | 9.68 | 14.27 | dark-green | 179 | 87 | |
| (439.5) | (6.83) | (43.68) | (9.55) | (14.44) | ||||
| Co(C16H30N3O5S) | 6.47 | 44.58 | 9.38 | 13.40 | dark-green | 163 | 79 | |
| (435) | (6.89) | (44.13) | (9.65) | (13.56) | ||||
| Ni(C16H30N3O5S) | 6.63 | 44.52 | 9.43 | 13.39 | green | 197 | 81 | |
| (435) | (6.89) | (44.13) | (9.65) | (13.56) | ||||
| [MLCH 3(H2O)2ac] | ||||||||
| Cu(C17H32N3O5S) | 6.80 | 45.31 | 9.51 | 13.87 | green | 198 | 86 | |
| (453.5) | (7.05) | (44.98) | (9.26) | (14.00) | ||||
| Co(C17H32N3O5S) | 6.84 | 45.94 | 9.07 | 13.00 | dark-green | 198 | 83 | |
| (449) | (7.12) | (45.43) | (9.35) | (13.14) | ||||
| Ni(C17H32N3O5S) | 6.75 | 45.87 | 9.11 | 12.98 | green | 208 | 85 | |
| (449) | (7.12) | (45.43) | (9.35) | (13.14) | ||||
| [MLC6H 5(H2O)2ac] | green | |||||||
| Cu(C22H34N3O5S) | 6.27 | 51.63 | 8.37 | 12.02 | 194 | 73 | ||
| (515.5) | (6.59) | (51.21) | (8.14) | (12.13) | ||||
| Co(C22H34N3O5S) | 6.32 | 51.97 | 8.00 | 11.37 | dark-green | 185 | 79 | |
| (511) | (6.65) | (51.66) | (8.21) | (11.54) | ||||
| Ni(C22H34N3O5S) | 6.37 | 51.86 | 7.93 | 11.45 | green | 202 | 82 | |
| (511) | (6.65) | (51.66) | (8.21) | (11.54) |
Synthesis of [M(LH)2]Cl2 and [M(LR)2] complexes; M = Cu(II), Ni(II), Co(II); L= 2-hydroxy-8-R-tricyclo[7.3.1.0.2,7]tridecane-13-one-thiosemicarbazone; R = CH3, C6H5
An alcoholic solution of ligand LH (2 mmol in 20 mL) or LR was added to an alcoholic solution of metal chloride (1 mmol in 20 mL). The resulted solution was heated at 60oC with vigorous stirring for 3-4 hours. After cooling to room temperature and allowing the solution to stand in dark for 10-12 hours, precipitates of different colors were formed, which were filtered, washed with alcohol to remove the residue and vacuum dried.
Synthesis of [MLR(H2O)2ac] complexes; M = Cu(II), Ni(II), Co(II)
To an alcoholic solution of LR (1 mmol in 50 mL) an alcoholic solution of a metal acetate (1 mmol in 30 mL) was added. The [MLR(H2O)2ac] complexes with different colors were obtained following the same procedure described earlier. All these final products were obtained as microcrystalline powders. If a buffer solution of acetic acid-sodium acetate of pH=3.5 (15 mL) is added to a methanol solution of [Cu(LR)2] complex (2 mmol in 25 mL) then a light brown precipitate can be obtained, which corresponds to the molecular formula [Cu2(LR)2(H2O)4(ac)2].
Biological assays
In order to determine the antimicrobial activity, the working technique used was similar to that described in [20]. Thus, the fresh cultures obtained from clinical isolates were suspended in sterile saline and adjusted to a standard density of 0.5 MacFarland. The microbial suspensions were plated on solid Mueller Hinton medium and solutions of the test compounds (10 µL) prepared in DMF (1 mg/mL) were added on filter paper disks, in agar wells or as spots. Concomitantly, the disks were impregnated with the same concentration of gentamycin, which was used as reference standard for reporting the antibiotic sensitivity. The plates were incubated at 37 °C for 24 hours. During incubation, the tested compounds diffused around the test area creating a concentration gradient. The antimicrobial activity was recorded as any area of microbial growth inhibition that occurred in the diffusion area. The quantitative antimicrobial activity assay was performed by the two-fold serial microdilution method in liquid medium (nutrient broth for bacterial and liquid YPG for fungal strains). Serial two-fold dilutions of a stock solution in DMF (from 1000 to 62.5 µg/mL) were performed in 60 multi-well plates, in a total volume of 200 μL medium and a standard microbial suspension (50 μL) was added in each well. After 20-24 hours, the plates are examined visually for evidence of bacterial growth. Results are recorded as minimum inhibitory concentrations (MIC) at the lowest concentration of the tested compound that completely inhibited microbial growth.
Acknowledgements
The authors would like to thank the Organic Chemistry Department - ICECHIM, Bucharest for microanalysis and LCS-Bucharest, Romania, for antimicrobial activity assays.
Supplementary Materials
Footnotes
Sample availability: Available from MDPI. See the supporting information.
References
- 1.Collins F. M., Klayman D. L., Morrison N. E. Correlation between structure and anti-mycobacterial activity in a series of 2-acetylpyridine thiosemicarbazones. J. Gen. Microbiol. 1982;128:1349–1356. doi: 10.1099/00221287-128-6-1349. [DOI] [PubMed] [Google Scholar]
- 2.a) Williams D. R. Metals, ligands and cancer. Chem. Rev. 1972;72:203–213. doi: 10.1021/cr60277a001. [DOI] [PubMed] [Google Scholar]; b) John R. P., Sreekanth A., Rajakannan V., Ajith T. A., Kurup M. R. P. New copper(II) complexes thiosemicarbazones and polypyridyl co-ligands: structural, electrochemical of 2-hydroxyacetophenone N(4)-substituted and antimicrobial studies. Polyhedron. 2004;23:2549–2559. [Google Scholar]; c) Belicchi-Ferrari M., Bisceglie F., Casoli C., Durot S., Morgenstern-Badarau I., Pelosi G., Pilotti E., Pinelli S., Tarasconi P. Copper(II) and Cobalt(III) Pyridoxal Thiosemicarbazone Complexes with Nitroprusside as Counterion: Syntheses, Electronic Properties, and Antileukemic Activity. J. Med. Chem. 2005;48(5):1671–1675. doi: 10.1021/jm049529n. [DOI] [PubMed] [Google Scholar]; d) Gulea A., Poirier D., Roy J., Spinu S., Coziri N., Birca M., Tapcov V. Anticancer effect of sulfanilamide complexes of Cu (II) with thiosemicarbazones of substituted salicylic aldehydes. COF-rRoCA 2004, Actes du Colloque Franco-Roumain de Chimie Applique, 3rd Bacau, Romania, Sept.22-26. 2004;68(Fr) Rom ISBN: 973-8392-36-5. [Google Scholar]; e) Birca M., Tapcov V., Prisacari V., Gulea A., Buraciova S. Synthesis and antimicrobial properties of Cu complexes with thiosemicarbazones of substituted salicylic aldehydes. COFrRoCA 2004, Actes du Colloque Franco-Roumain de Chimie Applique, 3rdBacau, Romania, sept. 22-26. 2004;44-45(Fr) Rom ISBN: 973-8392-36-5. [Google Scholar]
- 3.Kaska W. C., Carrans C., Michalowski J., Jackson J., Levinson W. Inhibition of the RNA dependent DNA polymerase and the malignant transforming ability of Rous sarcoma virus by Thiosemicarbazone-transition metal complexes. Bioinorg. Chem. 1978;8:245–254. doi: 10.1016/s0006-3061(00)80198-2. [DOI] [PubMed] [Google Scholar]
- 4.a) Klayman D. L., Bartosevich J. E., Griffith T. S., Mason C. J., Scovill J. P. 2-Acetylpyridine thiosemicarbazones. 1. A new class of potential antimalarial agents. J. Med. Chem. 1979;22:855–862. doi: 10.1021/jm00193a020. [DOI] [PubMed] [Google Scholar]; b) Klayman D. L., Scovill J. P., Bartosevich J. P., Mason C. J. 2-Acetylpyridine thiosemicarbazones. 2. N4,N4-Disubstituted derivatives as potential antimalarial agents. J. Med. Chem. 1979;22:1367–1373. doi: 10.1021/jm00197a017. [DOI] [PubMed] [Google Scholar]
- 5.Chan-Stier C. H., Minkel D. T., Petering D. H. Reactions of Bis(thiosemicarbazonato) Copper(II) complexes with tumor cells and mitochondria. Bioinorg. Chem. 1976;6:203–217. doi: 10.1016/s0006-3061(00)80227-6. [DOI] [PubMed] [Google Scholar]
- 6.Antholine W.E., Knight J.M., Petering D. H. Inhibition of tumor cell transplantability by iron and copper complexes of 5-substituted 2-formylpyridine thiosemicarbazones. J. Med. Chem. 1976;19:336–341. doi: 10.1021/jm00224a030. [DOI] [PubMed] [Google Scholar]
- 7.Saryan L. A., Ankel E., Krishanamurti C., Petering D. H., Elford H. Comparative cytotoxic and biochemical effects of alpha-N-heterocyclic carboxaldehyde thiosemicarbazones. J. Med. Chem. 1979;22:1218–1221. doi: 10.1021/jm00196a013. [DOI] [PubMed] [Google Scholar]
- 8.Karon M., Benedict W.F. Chromatid Breakage: Cytosine Arabinoside-Induced Lesions Inhibited by Ultraviolet Irradiation. Science. 1972;178:62. doi: 10.1126/science.171.3972.680. [DOI] [PubMed] [Google Scholar]
- 9.Wengel A., Kolind-Andersen H., Jacobsen N., Svendsen A. K., Klemmensen P. D. A biocidal, particularly fungicidal and/or bactericidal composition and thiocarbazones and metal complexes thereof for use in the composition. W085/00955. PCT Int. Appl. 1985
- 10.West D. X., Liberta E. A., Padhye S. B., Chikate R. C., Sonawane P. B., Kumbhar A. S., Yerande R. G. Thiosemicarbazone complexes of copper (II): structural and biological studies. Coord. Chem. Rev. 1993;123:49–71. [Google Scholar]
- 11.Agarwal R. K., Singh L., Sharma D. K. Synthesis, Spectral, and Biological Properties of Copper(II) Complexes of Thiosemicarbazones of Schiff Bases Derived from 4-Aminoantipyrine and Aromatic Aldehydes. Chem. Appl. 2006:1–10. doi: 10.1155/BCA/2006/59509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolae A., Draghici C. Semicarbazone si tiosemicarbazone in seria 2-hydroxy-8-R Tricycle[7.3.1.0.2,7]tridecane-13-one, substante cu potentiala activitate biologica. Rev. Chim. 2004;55:179–182. [Google Scholar]
- 13.Dhumwad S. D., Gudasi K. B., Goudar T. R. Synthesis and Structural Characterization of Biologically-Active Metal-Complexes of N(1)-(N-Morpholinoacetyl)-N(4)-Phenyl Thiosemi-carbazide and 3,4-Methylenedioxybenzaldehyde Thiosemicarbazone with Oxovanadium(IV), Chromium(III), Manganese(II), Iron(III), Cobalt(II), Nickel(II), Copper(II), Cadmium(II), Uranium(VI), Thorium(IV) and Silicon(IV) Indian J. Chem. 1994;33A:320–324. [Google Scholar]
- 14.Nakamoto K. Infrared Spectra of Inorganic and Coordination Compounds. J. Wiley and Sons; New York: 1986. p. 233. [Google Scholar]
- 15.Lever A. B. P. Inorganic Electronic Spectroscopy, 2nd Edn. Elsevier Science; New York: 1984. [Google Scholar]
- 16.Mabbs F. E., Collison D. Electron Paramagnetic Resonance of d Transition Metal Compounds. Elsevier; Amsterdam: 1992. [Google Scholar]
- 17.Koolhaas G. J. A. A. Ph.D. Thesis. University of Leiden; 1996. Cooper Coordination Complexes with poly-imidazole ligands. [Google Scholar]
- 18.Abragam A., Bleaney B. Electron Paramagnetic Resonance of Transition Ions. Claredon Press; London: 1970. p. 492. [Google Scholar]
- 19.Hathaway B. J. In: Comprehensive Coordination Chemistry. Wilkinson G., Gillard R.D., McCleverty J.A., editors. vol. 5. Pergamon Press; London: 1987. p. 652. [Google Scholar]
- 20.Rosu T., Pasculescu S., Lazar V., Chifiriuc C., Cernat R. Copper(II) Complexes with Ligands Derived from 4-Amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one: Synthesis and Biological Activity. Molecules. 2006;11:904–914. doi: 10.3390/11110904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.