Abstract
Three new pentacyclic triterpenoids: (20R)-3-oxolupan-30-al (1), (20S)-3-oxolupan-30-al (2) and (20R)-28-hydroxylupen-30-al-3-one (3), along with (20S)-3β-hydroxylupan-30-al (4), the latter previously described as a constituent of an epimeric mixture, were isolated from Acacia mellifera. In addition, the known metabolites 30-hydroxylup-20-(29)-en-3-one (5), 30-hydroxylup-20-(29)-en-3β-ol (6), atranorin, methyl 2,4-dihydroxy-3,6 dimethyl benzoate, sitosterol-3β-O-glucoside and linoleic acid were found in the analyzed plant species for the first time. The structures of the new metabolites were elucidated by extensive spectroscopic analyses and their relative stereochemistry was determined by NOESY experiments. The new metabolite 3 exhibited significant cytotoxic activity against the NSCLC-N6 cell line, derived from a human non-small-cell bronchopulmonary carcinoma.
Keywords: Acacia mellifera, lupanes, cytotoxicity, NSCLC-N6 cell line, (20R)-28-hydroxylupen-30-al-3-one, (20R)-3-oxolupan-30-al, (20S)-3-oxolupan-30-al
Introduction
Acacia is the second largest genus in the Leguminosae family, comprising more than 1200 species worldwide [1], with members found in almost all habitats. Out of the 1200 Acacia species, approximately 800 are found in Australia [2], 130 in Africa [3], 20 in India [4,5], a smaller number in Asia, and the remaining species in the New World. The stem bark of Acacia mellifera (Leguminosae) is used in traditional African ethnomedicine for the treatment of pneumonia, malaria, primary infection of syphilis, sterility and stomach-ache [6]. Chemical investigations on other Acacia species have led to the isolation of alkaloids [7], chalcone glycosides [8], diterpenes [9] and flavonoids [10]. The isolation of the triterpenes acacigenin B [11], lupeol, lupenone, lupenyl palmitate and lupenyl cinnamate [12] has also been reported. Several triterpenoids, related to the above mentioned, have also been discovered during investigations of other species, namely Gymnosporia wallichiana and Pseudocyphellaria rubella [13,14].
In the course of our investigations towards the isolation of bioactive metabolites from terrestrial and marine organisms [15,16] we started a chemical study on the bark extract of A. mellifera, traditionally used in African medicine. Nine lupane triterpenoids, including the new natural product 28-hydroxy-3-oxo-lup-20-(29)-en-30-al, all bearing functionalised isopropylidene side chains were isolated in our previous study from the non polar fractions of A. mellifera extract [17]. In the present investigation we report the isolation of three new triterpenes, (20R)-3-oxolupan-30-al (1), (20S)-3-oxolupan-30-al (2) and (20R)-28-hydroxylupen-30-al-3-one (3), along with (20S)-3β-hydroxylupan-30-al (4) this last previously isolated together with its (20R) epimer as an inseparable mixture [14], the known metabolites 30-hydroxylup-20-(29)-en-3-one (5) [18], 30-hydroxylup-20-(29)-en-3β-ol (6) [19,20], atranorin, methyl-2,4-dihydroxy-3,6 dimethyl benzoate, sitosterol-3β-O-glucoside, and linoleic acid all isolated for the first time from tissues of A. mellifera. The structure elucidation of the new natural products was accomplished by extensive analyses of their spectral data. In addition the cytotoxicity evaluation of the isolated metabolites on the NSCLC-N6 cell line showed significant activity levels for metabolite 3.
Results and Discussion
After evaporation under vacuum the stem bark extract of A. mellifera was subjected to a series of chromatographic purifications, including several HPLC separations, to afford metabolites 1-6, along with quantities of atranorin, methyl-2,4-dihydroxy-3,6-dimethyl benzoate, sitosterol-3β-O-glucoside, and linoleic acid.
Metabolite 1 was obtained as a colourless gum and its HRFAB-MS spectrum showed an ion peak at m/z 439.3596, consistent with the molecular formula C30H47O2 ([M+Η]+). The IR spectrum of 1 showed an absorption band at 1,704 cm-1 indicative of the presence of carbonyl functionalities, a fact supported by 13C-NMR data. The 13C-NMR spectrum of 1, measured in CDCl3, showed signals of 30 carbon atoms, which were identified with the assistance of its DEPT spectrum as seven methyls, ten methylenes, seven methines, and six quaternary carbons (Table 1). Two of these appearing at δC 207.1 and 218.5 were attributed to the carbons of an aldehyde and a ketone, respectively. Furthermore, from the NMR spectra it was clear that the molecule did not contain any double bonds. From the above findings, the five degrees of unsaturation consistent for a pentacyclic structure and the spectral similarities with the triterpenes previously isolated from the non polar fractions, metabolite 1 was suspected to be a lupane-type triterpene with a saturated side chain.
Table 1.
NMR spectral data for metabolites 1 – 4 a.
| # | 1 (δH) | 1 (δC) | 2 (δH) | 2 (δC) | 3 (δH) | 3 (δC) | 4 (δH) | 4 (δC) |
|---|---|---|---|---|---|---|---|---|
| 1 | α,1.40 (m) β,1.91 (m) |
39.4 | α, 1.90 (ddd, 4.4, 7.6, 13.2) β, 1.38 (m) |
39.5 | α,1.39 (m) β,1.91 (m) |
39.5 | α, 0.90(m) β, 1.63 (m) |
38.7 |
| 2 | α, 2.40 (ddd, 4.7, 7.8, 15.7) β, 2.47 (ddd, 7.5, 9.6, 15.7) |
34.0 | α, 2.40 (m), β, 2.47 (ddd, 7.3, 9.7, 15.9) |
34.1 | α, 2.42 (ddd, 4.4, 7.9, 15.8) β, 2.46 (ddd, 7.5, 9.8, 15.8) |
34.1 | α, 1.58 (m) β, 1.63 (m) |
27.4 |
| 3 | 218.5 | 217.8 | 218.0 | 3.18 (dd, 4.4, 10.6) | 79.0 | |||
| 4 | 47.3 | 47.3 | 47.3 | 38.9 | ||||
| 5 | 1.33 (m) | 54.7 | 1.32 (m) | 54.8 | 1.34 (m) | 54.7 | 0.67 (dd, 2.72, 9.20) | 55.2 |
| 6 | α,1.44 (m) β,1.56 (m) |
19.6 | 2H, 1.41-1.53 (m) | 19.6 | 2Η ,1.48 (m) | 19.7 | α, 1.38 (m) β, 1.52 (m) |
18.3 |
| 7 | 2H, 1.44 (m) | 33.4 | 2H, 1.45 (m) | 33.6 | 2Η , 1.44 (m) | 33.5 | 2H, 1.37 (m) | 34.3 |
| 8 | 40.6 | 40.8 | 40.9 | 40.8 | ||||
| 9 | 1.38 (m) | 49.2 | 1.38 (m) | 49.3 | 1.40 (m) | 49.3 | 1.28 (m) | 50.0 |
| 10 | 36.7 | 36.8 | 36.8 | 37.1 | ||||
| 11 | α, 1.55 (m) β, 1.40 (m) |
21.3 | α, 1.06 (m) β, 1.52 (m) |
21.2 | α, 1.51 (m) β, 1.35 (m) |
21.3 | α, 1.47 (m) β, 128 (m) |
20.7 |
| 12 | α, 1.38 (m) β, 1.56 (m) |
27.4 | α, 1.35 (m) β, 1.65 (m) |
26.5 | α, 1.34 (m) β, 1.55 (m) |
27.6 | α, 1.65 (m) β, 1.38 (m) |
26.5 |
| 13 | 1.74 (m) | 37.8 | 1.79 (ddd, 4.1, 11.7, 11.7) | 37.8 | 1.70 (m) | 37.0 | 1.77 (ddd 4.4, 11.6 , 11.6) | 37.7 |
| 14 | 42.9 | 43.1 | 42.9 | 42.8 | ||||
| 15 | α, 1.05 (m) β, 1.69 (m) |
27.1 | α, 1.03 (m) β, 1.69 (m) |
27.2 | α, 1.07 (m) β, 1.72 (m) |
26.8 | α, 1.03 (m) β, 1.70 (m) |
27.2 |
| 16 | 2H, 1.47 (m) | 35.1 | a, 1.46 (m) b, 1.53 (m) |
35.2 | α, 1.19 (m) β, 1.92 (m) |
29.1 | α, 1.48 (m) β, 1.59 (m) |
35.3 |
| 17 | 43.0 | 42.9 | 47.8 | 43.0 | ||||
| 18 | 1.43 (m) | 48.9 | 1.26 (m) | 47.0 | 1.64 (m) | 49.3 | 1.24 (m) | 47.1 |
| 19 | 1.89 (m) | 42.6 | 2.35 (m) | 37.3 | 1.91 (m) | 42.7 | 2.35 (m) | 37.4 |
| 20 | 2.60 (m) | 48.9 | 2.63 (dq, 2.9, 6.8) | 49.7 | 2.57 (m) | 49.0 | 2.64 (dq, 3.1, 6.8) | 49.7 |
| 21 | α, 1.51 (m) β, 1.88 (m) |
25.0 | α, 1.15 (m) β, 1.68 (m) |
23.6 | α, 1.55 (m) β, 1.92 (m) |
24.9 | α, 1.15 (m) β, 1.68 (m) |
23.6 |
| 22 | α, 1.36 (m) β, 1.47 (m) |
39.9 | α, 1.39 (m) β, 1.45 (m) |
40.4 | α, 0.98(m) β,1.92 (m) |
33.7 | α, 1.08 (m) β, 1.38 (m) |
40.4 |
| 23 | 1.06 (s) | 26.6 | 1.06 (s) | 26.6 | 1.06 (s) | 26.7 | 0.96 (s) | 28.0 |
| 24 | 1.01 (s) | 21.0 | 1.01 (s) | 21.1 | 1.01 (s) | 21.1 | 0.75 (s) | 15.4 |
| 25 | 0.91 (s) | 15.7 | 0.93 (s) | 15.9 | 0.91 (s) | 16.0 | 0.82 (s) | 16.0 |
| 26 | 1.06 (s) | 15.7 | 1.07 (s) | 15.8 | 1.06 (s) | 15.8 | 1.03 (s) | 15.9 |
| 27 | 0.92 (s) | 14.2 | 0.93 (s) | 14.3 | 0.94 (s) | 14.5 | 0.92 (s) | 14.3 |
| 28 | 0.75 (s) | 17.5 | 0.78 (s) | 17.9 | α, 3.26 (d, 10.9) β, 3.76 (d, 10.9) |
60.1 | 0.78 (s) | 17.9 |
| 29 | 1.07 (d, 7.2) | 14.4 | 1.01 (d, 6.8) | 7.4 | 1.09 (d, 7.20) | 14.5 | 1.01 (d, 6.8) | 7.4 |
| 30 | 9.84 (d, 2.0) | 207.1 | 9.60 (s) | 205.1 | 9.83 (d, 2.0) | 206.7 | 9.60 (s) | 205.1 |
a 1H (400 MHz) and 13C (50.3 MHz) NMR spectra were recorded in CDCl3 with TMS as internal standard, δ (ppm), (J) in Hz
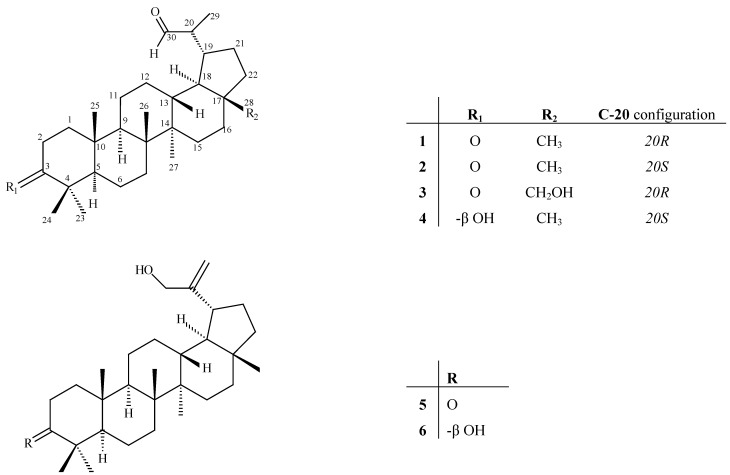
The latter assumption was also supported by the presence of a methyl doublet at δH 1.07 ppm. Comparison of the spectral data of 1 with those of 28-hydroxy-3-oxo-lup-20-(29)-en-30-al, a metabolite that had been earlier isolated by us from A. mellifera showed great similarity, with the exception of the absence of a double bond on the side chain and the reduction of C-28. The assignment of the proton and carbon signals on the side chain was assisted by the correlations observed in the HMBC spectrum between H-30 (9.84 ppm) and both C-29 (14.4 ppm) and C-19 (42.6 ppm) as well as by the correlation between H-29 (1.07 ppm) and C-19 (δC 42.6, s). Finally, the quaternary carbon signal at δC 218.0 was readily assigned to C-3 on the basis of its long-range correlations with H-23 and H-24. Additional information from the COSY and HMBC spectra allowed full assignments of all signals for metabolite 1. The H-19 resonating at δH 1.89 (m) was found to have nOe interactions with H- 28 (δH 0.75, s) indicating a β-orientation for both. Thus, the isopropanal group located at C-19 should be α-oriented. Additionally the H-28 protons showed nOe interactions with H-13 (δH 1.74 ppm), confirming the orientation of H-13β. The absence of nOe between H-18 and H-28 is indicative of the trans fusion of rings D and E. The remaining correlations that were similar to those observed with related compounds and in agreement with the biosynthesis of lupane triterpenes allowed the determination of relative stereochemistry (Figure 1).
Figure 1.
Isolated lupane triterpenes from A. mellifera.
The second isolated lupane metabolite 2 had the same molecular formula C30H49O2, the same functional groups and very similar spectral data (Table 1) as compound 1, therefore they were considered to be stereoisomers. The most significant spectral differences between metabolites 1 and 2 were observed for C-30 (δC/δΗ: 207.1/9.80 d, 2.0 Hz for 1; δC/δΗ: 205.1/9.60, s for 2), C-29 (δC/δΗ: 14.4/1.07 d, 7.17 Hz for 1; δC/δΗ: 7.4/1.01, d, 6.76 Hz for 2) and C-19 (δC/δΗ: 42.6/1.89 m, for 1; δC/δΗ: 37.3/2.35, m for 2). According to the method developed by Corbett and coworkers for the definition of absolute configuration at C-20 in similar lupane-type triterpenoids, carbons C-30, C- 29 and C-19 in 20R centers resonate in lower fields than those of the 20S epimers. Subsequently, on this basis the absolute configuration of metabolite 1 was assigned as 20R and the corresponding of metabolite 2 as 20S. This assumption is further supported by the optical rotations of compounds 1 and 2 that follow the trend of the 20R epimers having negative values whereas the 20S epimers appear dextrorotatory in analogous lupane-type compounds [21,22,23].
Metabolite 3 was also isolated as a colourless gum and on the basis of its HRFAB-MS spectrum (m/z 457.3672, [M-H]+), along with the 1H- and 13C-NMR spectral data, the molecular formula C30H48O3 was established. The IR spectrum of 3 revealed the presence of a hydroxy, and a keto-carbonyl functionality by the absorptions at vmax 3,625 and 1,702 cm-1, respectively. The 13C-NMR spectrum of 1 measured in CDCl3, showed signals of 30 carbon atoms, which were identified by the assistance of DEPT spectrum as six methyls, eleven methylenes, seven methines, and six quaternary carbons (Table 1). The signals appearing at δC 206.7 and 218.0 were attributed to the carbons of an aldehyde and a ketone, also confirmed by IR and DEPT spectra. The presence of a hydroxymethylene in the molecule was obvious from the chemical shift of the sole oxygenated sp3 carbon at δC 60.1 ppm, the IR and DEPT spectra. Comparison of the spectral data of 3 with those of metabolites 1 and 2 showed great similarity, with the exception of C-17 methyl being replaced by a hydroxymethylene. The HMBC experiment confirmed the position of the hydroxyl group by the correlations observed in the spectrum between H-28 (3.26 d, 3.76 d) with both C-16 (29.1 ppm) and C-22 (33.7 ppm). The correlations on the NOESY spectrum confirmed the retention of the relative configuration as described for metabolites 1 and 2. The spectral similarity of metabolite 3 with compound 1, especially the δC 14.5 ppm for C-29 (14.4 for 1) and the δH 9.83 ppm d, J= 2.0 Hz for C-30 (9.84, d, J= 2.0 Hz for 1) undoubtly indicated the 20R absolute configuration for metabolite 3.
Metabolite 4, that was isolated in pure form as a single epimer, showed spectral characteristics supporting the structure shown in Figure 1. Compound 4 has been described in the past as a constituent of a mixture along with the 20R epimer. The full assignment of the proton and carbon resonances for this metabolite is shown in Table 1. The present study showed that the 20R and 20R epimers of 4, in contrast to what was previously believed can be isolated in pure form [14,23].
Chromatographic separations led also to the isolation of 30-hydroxylup-20-(29)-en-3-one (5) [24] and 30-hydroxylup-20-(29)-en-3β-ol (6) [20,25], previously isolated from Catha cassinoides and Cassine papillosa, respectively, along with small amounts of atranorin [26], methyl 2,4-dihydroxy-3,6- dimethyl benzoate [27], sitosterol-3β-O-glucoside [28], and linoleic acid [29]. The structure elucidation of the known metabolites was based on comparison of their spectral data with literature values.
The antitumor activity of lupane derived triterpenoid compounds was first discovered over 20 years ago, when extracts from the stem barks of various plants were tested for cytostatic activity using different in vivo cancer model systems [30,31]. This has led to the isolation of betulinic acid, the best-known representative of the lupane-derived compounds with antiproliferative properties [32]. In a study on relationships between structure and activity of lupane triterpenes on the induction of B16 cell 2F2 cell differentiation and apoptosis, it was demonstrated that the keto function at C-3 enhanced their differentiation-inducing activities [33].
In the present study metabolites 1-6 were evaluated for cytotoxic activity against the NSCLC-N6 cell line. The new metabolite 3 showed noteworthy levels of activity (IC50= 39.5 ± 1.2 μM) whereas the activity of all other tested metabolites was not significant.
Structure-activity correlations for 1-4 and similar lupanes tested earlier on the same cell line [17] show that the hydroxyl group on C-28 is necessary for expression of cytotoxicity. Even though the potencies of the assayed metabolites are two orders of magnitude lower than those expressed by the anticancer drug navelbin on the same biological systems, the significantly lower toxicity of the tested metabolites is an indispensable advantage.
Experimental
General
Optical rotations were measured on a Perkin-Elmer model 341 polarimeter with a 10 cm cell. UV spectra were obtained in spectroscopic grade C6H14 on a Shimadzu UV-160A spectrophotometer. IR spectra were obtained using a Paragon 500 Perkin-Elmer spectrophotometer. NMR spectra were recorded using a Bruker AC 200 or a Bruker DRX 400 spectrometers. Chemical shifts are given in δ (ppm) scale using TMS as internal standard. The 2D experiments (1H–1H COSY, HMQC, HSQC, HMBC) were performed using standard Bruker programs. High Resolution Mass Spectra data were provided by the University of Notre Dame, Department of Chemistry and Biochemistry, Notre Dame, Indiana, USA. EIMS data were recorded on a Hewlett Packard 5973 Mass Selective Detector. Column chromatography was performed with Kieselgel 60 (Merck), HPLC was conducted on an Agilent 1100 series with refractive index detector, with Kromasil Sil 100, 5 um, 250 x 8 mm column. TLC was performed with Kieselgel 60 F254 (Merck aluminum support plates).
Plant Material
The stem bark of A. mellifera was collected in Machakos territory in Kenya. The plant was identified by Mr Onesimus Mwangangi at the East African Herbarium-Museum and a voucher specimen is deposited at the collection of the same institute in Nairobi.
Extraction and Isolation
Air-dried, powdered stem bark of A. mellifera (2.25 kg, wet wt) was extracted with CH2Cl2 (100%) and MeOH (100%), filtered and the extracts separately reduced to dryness under vacuum. The CH2Cl2 residue was subjected to column chromatography on silica gel, using mixtures of cyclohexane with increasing amounts of EtOAc as eluents to afford sixty one fractions that were combined into fractions I-IX on the basis of their spectral similarities, as deduced from their NMR spectra. Fraction III, eluted with cyclohexane-EtOAc (98:2-92:8), was purified on silica gel using cyclohexane-EtOAc (90:10) and further subjected to a series of normal phase HPLC separations to yield 1 (3.5 mg) and 2 (2.7 mg). Fraction VI, eluted with cyclohexane-EtOAc (75:25-70:30), was purified by normal phase HPLC using cyclohexane-EtOAc (80:20) to afford metabolites 3 (3 mg), 4 (2.7 mg) and atranorin (3.4 mg). Fraction V, eluted with cyclohexane:(90:10 - 80:20), was further chromatographed over silica gel using n-hexane-EtOAc (70:30) to yield metabolites 5 (32 mg), 6 (12 mg) and impure metabolites that were further purified by normal phase HPLC using n-hexane-EtOAc (80:20) to afford methyl 2,4-dihydroxy-3,6-dimethylbenzoate (2.0 mg) and sitosterol-3β-Ο-glycoside (1.2 mg). Finally, fraction IV was chromatographed by normal phase HPLC using as eluent mixtures of cyclohexane-EtOAc (5:11) to yield linoleic acid (5mg).
(20R)-3-Oxolupan-30-al (1). Colourless gum; [α]D20 -3.14° (CHCl3; c 0.64); IR (CHCl3) νmax cm-1: 2,942, 2,348, 1,704, 1,526, 1,234 cm-1 ; EIMS 70eV, m/z (rel. int. %): 382 (100), 163 (40), 205 (28), 81 (22), 55 (21), 107 (18), 189 (12), 135 (12), 425 (4), 273 (4) ; HRFAB-MS (m/z): 439.3596 [M-Η]+ (calcd. for C30H47O2, [M-Η]+: 439.35781); 1H-NMR (400 MHz, CDCl3) and 13C-NMR (50.3 MHz, CDCl3): see Table 1.
(20S)-3-Oxolupan-30-al (2). Colourless gum; [α]D20 +5.5° (CHCl3; c 0.18); IR (CHCl3) νmax cm-1: 2,942, 2,348, 1,704, 1,526, 1,234 cm-1; EIMS 70eV, m/z (rel. int. %): 382 (100), 163 (40), 205 (28), 81 (22), 55 (21), 107 (18), 189 (12), 135 (12), 425 (4), 273 (4); HRFAB-MS (m/z): 441.3735 [M+Η]+ (calcd. for C30H49O2, [M+Η]+: 441.3734); 1H-NMR (400 MHz, CDCl3) and 13C-NMR (50.3 MHz, CDCl3): see Table 1.
(20R)-28-Hydroxy-3-oxo-lupan-30-al (3). Colourless gum; [α]D20 -5.0° (CHCl3; c 0.60); IR (CHCl3) νmax cm-1: 3,625, 2,946, 2,360, 1,702, 1,521, 1,446, 1,249, 1,025 cm-1; EIMS 70eV, m/z (rel. int. %): 154 (100), 136 (90), 307 (25), 289 (15), 220 (10), 259 (5), 391 (5), 367 (4).; HRFAB-MS (m/z): 457.3682 [M+Η]+ (calcd. for C30H49O3, [M+Η]+: 457.3684); 1H-NMR (400 MHz, CDCl3) and 13C- NMR (50.3 MHz, CDCl3): see Table 1.
Evaluation of Cytotoxicity
The NSCLC-N6 cell line [34], derived from a human non-small-cell broncho-pulmonary carcinoma (moderately differentiated, rarely keratinizing, classified as T2N0M0) was used for all experiments. The cell were cultured at 37 oC in an air/carbon dioxide (95:5, v/v) atmosphere in RPMI 1640 medium with 5 % fetal calf serum, to which were added penicillin (100 IU·mL-1), streptomycin (100 μg·mL-1) and glutamine (2 mM). Under these conditions, cell doubling time was 48 h. Cells used in the experiment never exceeded 35 passages. Experiments were performed in 96 wells microtiter plates (2x105 cells·mL-1). Cell growth was estimated by colorimetric assay based on the conversion of tetrazolium dye (MTT) to a blue formazan product by live mitochondria [35]. Eight repeats were performed for each concentration. Control growth was estimated from 16 determinations. Optical density at 570 nm corresponding to solubilized formazan was read for each well on Titertek Multiskan MKII.
Acknowledgments
We wish to thank the Director of Kenya Medical Research Institute for granting a study leave to Mr. C. Mutai and State Scholarships Foundation of Greece for a scholarship (to C. Mutai). We also wish to thank Dr. Leonard Hagmann (Syngenta Agro, Basel, Switzerland) for the performance of some NMR experiments.
Footnotes
Sample Availability: Samples of the compounds 1-6 are available from the corresponding author.
References
- 1.Harden G.J., editor. Flora of New South Wales. Vol. 2. New South Wales Univ. Press; NSW Australia: 1991. [Google Scholar]
- 2.Doran J.C., Turnbull J.W., Boland D.J., Gunn B.V. Handbook on seeds of dry zone Acacias. FAO; Rome: 1983. [Google Scholar]
- 3.Ross J.H. A conspectus of the African Acacia species. Mem. Bot. Soc. South Africa. 1979;44:1–155. [Google Scholar]
- 4.Hooker J.D. The flora of British India. Vol. 3. L. Reeve and Co; London: 1879. [Google Scholar]
- 5.Brandis D. Indian trees. Archibald Constable & Co. Ltd.; London: 1906. [Google Scholar]
- 6.Kokwaro O. Medicinal Plant of East Africa. East African Literature Bureau Kampala; Nairobi, Dar es Salaam: 1976. [Google Scholar]
- 7.Johns S., Lumberton J., Sioumis A. Alkaloids of the Australian Leguminosae. Aust. J. Chem. 1966;19:893–896. doi: 10.1071/CH9660893. [DOI] [Google Scholar]
- 8.Imperato F. A chalcone glycoside from Acacia dealbata. Phytochemistry. 1982;21:480–481. doi: 10.1016/S0031-9422(00)95301-9. [DOI] [Google Scholar]
- 9.Foster P., Jefferies P. Labdane diterpenes from an Acacia species. Phytochemistry. 1985;24:2991–2993. doi: 10.1016/0031-9422(85)80042-X. [DOI] [Google Scholar]
- 10.Heerden F., Brandt E., Ferreira D., Roux D. Metabolites from the purple heartwoods of the Mimosoidae. Part 4. Acacia fasciculifera F. Muell ex. Benth: fasciculiferin, fasciculiferol, and the synthesis of 7-aryl-and 7-flavanyl-peltogynoids. J. Chem. Soc. Perkin Trans. I. 1981:2483–2490. [Google Scholar]
- 11.Anjaneyulu A., Bapuji M., Ramachandra L., Row Sree A. Structure of acacigenin-B, a novel triterpene ester isolated from Acacia concinna. Phytochemistry. 1979;18:463–465. doi: 10.1016/S0031-9422(00)81888-9. [DOI] [Google Scholar]
- 12.Pereira F.B.M., Domingues F.M.J., Silva A.M.S. Triterpenes from Acacia dealbata. Nat. Prod. Lett. 1996;8:97–103. doi: 10.1080/10575639608043247. [DOI] [Google Scholar]
- 13.Kulshreshtha D.K. Three new oxygenated triterpenoids of the lupane series from Gymnosporia wallichiana. Phytochemistry. 1977;16:1783–1785. doi: 10.1016/0031-9422(71)85089-6. [DOI] [Google Scholar]
- 14.Corbett R.E., Cong A.N.T., Holland P.T., Wilkins A.L. Extractives from Pseudocyphellaria rubella. Aust. J. Chem. 1987;40:461–468. doi: 10.1071/CH9870461. [DOI] [Google Scholar]
- 15.Christodoulopoulou L., Tsoukatou M., Tziveleka L.A., Vagias C., Petrakis P.V., Roussis V. Piperidinyl amides with insecticidal activity from the maritime plant Otanthus maritimus. J. Agr. Food Chem. 2005;53:1435–1439. doi: 10.1021/jf0481857. [DOI] [PubMed] [Google Scholar]
- 16.Kladi M., Xenaki H., Vagias C., Papazafiri P., Roussis V. New cytotoxic sesquiterpenes from the red algae Laurencia obtusa and Laurencia microcladia. Tetrahedron. 2006;62:182–189. [Google Scholar]
- 17.Mutai C., Abatis D., Vagias C., Moreau D., Roussakis C., Roussis V. Cytotoxic lupane-type triterpenoids from Acacia mellifera. Phytochemistry. 2004;65:1159–1164. doi: 10.1016/j.phytochem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Tinto W.F., Blair L.C., Alli A. Lupane triterpenoids of Salacia cordata. J. Nat. Prod. 1992;55:395–1992. doi: 10.1021/np50081a020. [DOI] [Google Scholar]
- 19.Abdel-Mogib M. A lupane triterpenoid from Maerua oblongifolia. Phytochemistry. 1999;51:445–448. [Google Scholar]
- 20.Burns D., Reynolds W.F., Buchanan G., Reese P.B., Enriquez R.G. Assignment of 1H and 13C spectra and investigation of hindered side-chain rotation in lupeol derivatives. Magn. Reson. Chem. 2000;38:488–493. doi: 10.1002/1097-458X(200007)38:7<488::AID-MRC704>3.0.CO;2-G. [DOI] [Google Scholar]
- 21.Park S.Y., Choi H.S., Yook C.S., Nohara T. A new lupane glycoside from the leaves of Acanthopanax koreanum. Chem. Pharm. Bull. 2005;53:97–99. doi: 10.1248/cpb.53.97. [DOI] [PubMed] [Google Scholar]
- 22.Chiang Y.M., Kuo Y.H. New peroxy triterpenes from the aerial roots of Ficus microcarpa. J. Nat. Prod. 2001;64:436–439. doi: 10.1021/np0004808. [DOI] [PubMed] [Google Scholar]
- 23.Corbett R.E., Cong A.N.T., Wilkins A.L., Thomson R.A. Lichens and fungi. Part 17. The synthesis and absolute configuration at C-20 of the (R) -and (S)- epimers of some 29-substituted lupane derivatives and of some 30-norlupan-20-ol derivatives and the crystal structure of (20R)-3β-acetoxylupan-29-ol. J. Chem. Soc. Perkin Trans. I. 1985:2051–2056. [Google Scholar]
- 24.Drewes S.E., Mashimbye M.J. Flavanoids and triterpenoids from Cassine papillosa and the absolute configuration of 11,11-dimethyl-1,3,8,10-tetra-hydroxy-9-methoxypeltogynan. Phytochemistry. 1993;32:1041–1044. doi: 10.1016/0031-9422(93)85252-M. [DOI] [Google Scholar]
- 25.Betancor C., Freire R., Gonzalez A.G., Salazar J.A., Pascard C., Prange T. Three triterpenes and other terpenoids from Catha cassinoides. Phytochemistry. 1980;19:1989–1993. doi: 10.1016/0031-9422(80)83019-6. [DOI] [Google Scholar]
- 26.Huneck S., Yoshimura I. Identification of Lichens Substances. Springer Verlag; Berlin: 1996. [Google Scholar]
- 27.De Carvalho M.G., De Carvalho G.J.A., Braz-Filho R. Chemical constituents from Ouratea floribunda: Complete 1H and 13C NMR. Assignments of atranorin and its new acetyl derivative. J. Braz. Chem. Soc. 2000;11:143–147. [Google Scholar]
- 28.Masan C., Woitke H.D., Hiller K., Franke P. Isolation of beta-sitosterol-O-D-glucoside from Astrantia major L. 31. The contents of some Saniculoideae. Pharmazie. 1978;33:382. [PubMed] [Google Scholar]
- 29.Grindley D.N. Investigation of the seed oils of some Sudan Mimosaceae. J. Soc. Chem. Ind. 1945;64:152. [Google Scholar]
- 30.Ogura M., Cordell G.A., Farnsworth N.R. Potential anticancer agents. IV. Constituents of Jacaranda causana Pittier (Bignoninaceae) Lloydia. 1977;40:157–168. [PubMed] [Google Scholar]
- 31.Sandberg F., Duchevska K., Khristov V., Spasov S. Spondianthus preussii var. grabber Engler. Pharmacological screening and occurrence of triterpenes. Acta Pharm. Suec. 1987;24:253–256. [PubMed] [Google Scholar]
- 32.Ryu S.Y., Choi S.U., Lee S.H., Lee C.O., Zaesung N., Ahn J. Antitumor triterpenes from medicinal plants. Arch. Pharmacal Res. 1994;17:375–377. doi: 10.1007/BF02974180. [DOI] [Google Scholar]
- 33.Hata K., Hori K., Ogasawara H., Takahashi S. Anti-leukemia activities of lup-28-al-20(29)-en-3-one, a lupane triterpene. Toxicol. Lett. 2003;143:1–7. doi: 10.1016/S0378-4274(03)00092-4. [DOI] [PubMed] [Google Scholar]
- 34.Roussakis C., Gratas C., Audoin A.F., Le Boterf J., Dabouis G., Verbist J.F. Study of in vitro drug sensitivity on a newly established cell line from a primary bronchial epidermoid carcinoma of human origin (NSCLC-N6) Anticancer Res. 1991;11:2239–2244. [PubMed] [Google Scholar]
- 35.Mossmann T. Rapid calorimetric assay cellular growth and survival: application to proliferation and cytotoxicity assay. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]