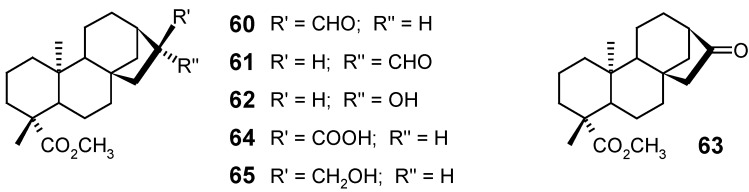Abstract
This paper presents a review on kaurane diterpenes and their glycoside derivatives, covering aspects of their occurrence, biological activities and the synthesis of these natural products and their analogues. First, it shows and classifies diterpenes, in accordance with the already established structural criteria in the literature. Then, kaurane diterpenes are presented, focusing on their chemical structures, occurrence in the plant kingdom and their main, recently described, biological activities. Moreover, the most significant works, published between 1964 and November 2006, which describe the total synthesis or structural transformations of some kaurane diterpenes, including either semisynthetic and/or microbiological methodologies, are consisely reviewed. At this point, some general considerations on glycosides are introduced, and kaurane glycosides are presented and discussed on the basis of their toxic importance and occurrence in the plant kingdom, having focused on related aspects of their biological activities and the relationships between these activities and the structural factors of their molecules. Finally, the principal methods of glycosidation by enzymatic and chemical processes are both presented, and a few papers on the synthesis of kaurane glycosides are succinctly discussed.
Keywords: Kaurane diterpenes, synthesis, kaurane glycosides, biological activities
Contents
Introduction: biosynthesis, occurrence and biological activities of kaurane diterpenes
- Synthesis and semisynthesis of kaurane derivatives
-
2.1Chemical routes
-
2.2Microbiological methods
-
2.1
- General considerations about glycosides
-
3.1Structures, occurrence and biological activities of naturally occurring kaurane glycosides
-
3.1
- Glycosidation methods
-
4.1Enzymatic glycosidation
-
4.2Chemical glycosidation
-
4.1
The synthesis of kaurane glycosides
Conclusions
1. Introduction: biosynthesis, occurrence and biological activities of kaurane diterpenes
Diterpenoids constitute a vast class of isoprenoid natural products, biosynthesized from mevalonic acid through 2E,6E,10E-geranylgeranyl pyrophosphate (GGPP) [1]. They are divided in acyclic (phytanes), bicyclic (labdanes, clerodanes), tricyclic (pimaranes, abietanes, cassanes, rosanes, vouacapanes, podocarpanes), tetracyclic (trachylobanes, kauranes, aphidicolanes, stemodanes, stemaranes, beyeranes, atisanes, gibberellanes), macrocyclic diterpenes (taxanes, cembranes, daphnanes, tiglianes, ingenanes) and mixed compounds, in accordance with the number and the cyclization patterns displayed by their skeleta [2]. They are found mainly in plants and fungi, although diterpenes have also been found in marine organisms and insects as well [1,3].
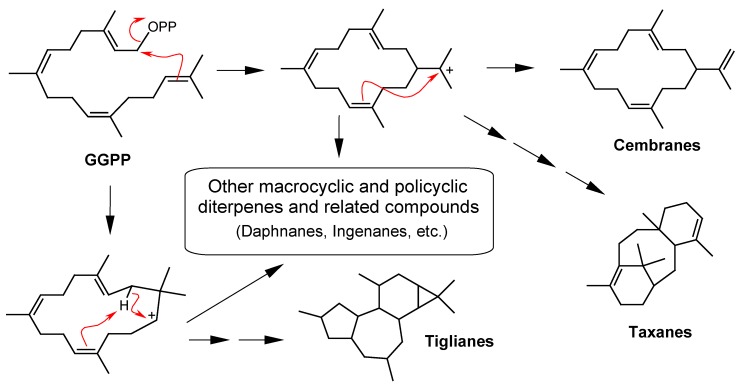
Cyclic diterpenes result from two different biosynthetic cyclization processes from GGPP (Figure 1 and Figure 2) [1,3]. In the first type of cyclization (Figure 1), the terminal pyrophosphate group (-OPP) acts as a nucleofuge (leaving group), generating an allylic carbocation that alkylates the double bond of the other extreme of the GGPP chain, creating, in the majority of the cases, one terminal isopropylidene cation that is very reactive and can be stabilized either by the elimination of the proton, leading to the formation of cembranes, or by subsequent intramolecular nucleophilic substitutions, leading to the related polycyclic diterpenes such as taxanes, tiglianes, daphnanes and ingenanes, among others.
Figure 1.
Cyclization of geranylgeranyl pyrophosphate (GGPP) with the pyrophosphate group (-OPP) acting as a nucleofuge (leaving group).
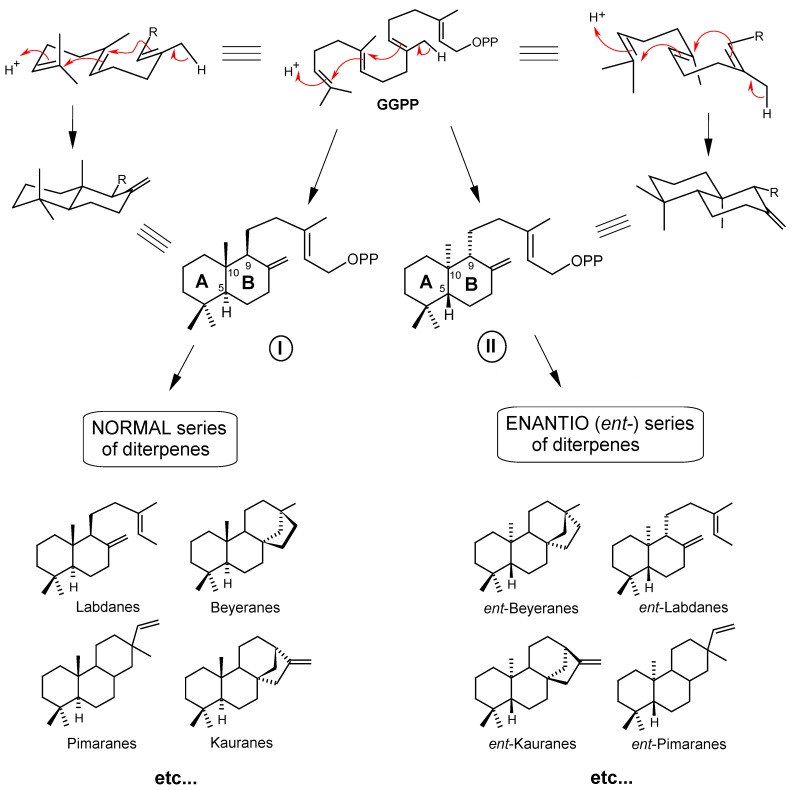
Figure 2.
Cyclization of geranylgeranyl pyrophosphate (GGPP) under acid catalysis, leading to the diterpenes of the “NORMAL” and “ENANTIO” (ent-) series.
The second and principal [3] type of cyclization (Figure 2) occurs under acid catalysis, in a very similar way to the cyclization that produces cyclic triterpenes. However, there is no previous epoxidation step as occurs in triterpene cyclization, but the double bound protonation on the initial isopropylidene unit of the GGPP chain leads to two perhydronaphtalene bicyclic intermediates (Figure 2, structures I and II), resulting in the two enantiomeric series that differ from each other in their inverted configurations of the carbons C-5, C-9 and C-10. Namely, the “normal" series are the structures whose fusion between A and B rings occurs in the same way as in steroids, while the structures of the “enantiomeric” series (denoted as “ent-”) are the corresponding specular images of the normal series.
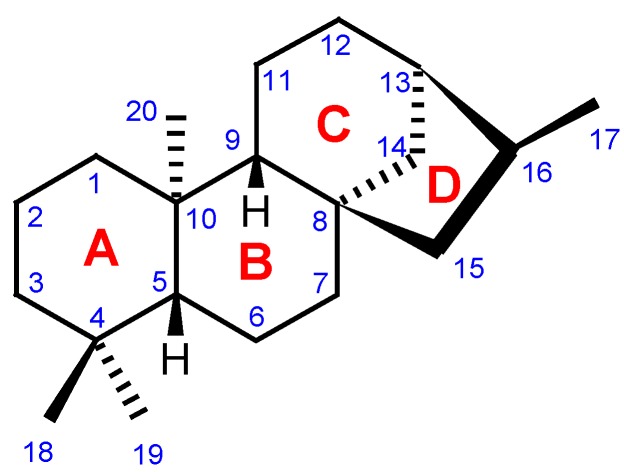
Kauranes represent an important group of tetracyclic diterpenes and their structures are constituted by a perhydrophenantrene unit (A, B and C rings) fused with a cyclopentane unit (D ring) formed by a bridge of two carbons between C-8 and C-13 (Figure 3) [4]. Different criteria are used for the nomenclature of the kaurane diterpenes, the most frequent being the inversion of the conventional description of stereochemistry when the name is preceded by the prefix "ent-" [1,5,6,7]. Furthermore, the nomenclature, numbering and stereochemistry of the ent-kaurane skeleton have already been established by IUPAC recommendations [7]. Except in the case of a few that present a double bond between C-9 and C-11, the majority of ent-kauranes are characterized for featuring negative values for the specific optical rotation, [α]D [6,8].
Figure 3.
Carbon-skeleton of an ent-kaurane diterpene [7].
Kaurane diterpenes are mostly found in different plant species belonging to several families such as Asteraceae [9,10,11,12,13,14,15,16,17] (Wedelia spp., Mikania spp., Oyedaea spp., Baccharis spp., Solidago spp., Vernonia spp., Xanthium spp., Eupatorium spp., Espeletia spp.), Annonnaceae [18,19,20] (Annonna spp., Xylopia spp., Mitrephora spp.), Euphorbiaceae [21,22,23,24] (Beyeria spp., Croton spp., Ricinocarpus spp., Suregada spp.), Celastraceae [25,26] (Tripterygium spp.), Apiaceae [27] (Alepidea spp.), Velloziaceae [28] (Vellozia spp.), Lamiaceae (= Labiatae) [29,30,31] (Rabdosia spp., Isodon spp., Sideritis spp.), Fabaceae [32] (Copaifera spp.), Rutaceae [33] (Phebalium spp.), Chrysobalanaceae [34] (Parinari spp.), Jungermanniaceae [35] (Jungermannia spp.), Erythroxylaceae [36] (Erythroxylum spp.) and Rhizophoraceae [37] (Bruguiera spp.), among others. The structures of 518 diterpenoid constituents isolated from Isodon species, especially those with an ent-kaurane skeleton, were the subject of a recent review by Sun and collaborators [38]. In addition, a general survey and some taxonomic implications of the occurrence of kaurane and other classes of diterpenes in the Asteraceae family are discussed by Alvarenga and collaborators [39].
Kaurenoic acid (1), an ent-kaurane diterpene that possesses a wide spectrum of bioactivities such as antiinflammatory, antibacterial, antifungal and moluscical properties, among others [6], is not commercially available, but it is relatively abundant in some species belonging to the Wedelia [40], Mikania [41], Annona [42] and Xylopia [43] genera, which has motivated its quantification in these plant species [42,43,44,45,46,47], enabling the use of all of them as natural sources of this diterpene.
Kaurenoic acid (1) is one of the intermediate compounds involved in the biosynthesis of diverse kaurane diterpenes, including gibberellins, a group of growth phytohormones. Therefore, it is not surprising that many kauranes and derivatives act as growth regulators in plants [48].
Diverse biological activities have been described for the kaurane diterpenes and many of them, including plant growth regulating, antimicrobial, antiparasitic, insect antifeedant, cytotoxic, antitumour, anti-HIV, steroidogenic, antifertility, hipotensive and antiinflamatory activities, among others, are referred to in the review published by Ghisalberti in 1997 [6]. Some of these kaurane biological effects have also been cited in recent literature [38,40,41,48,49,50,51,52,53,54,55,56,57]. For this reason, Table 1 enumerates other biological activities described for kaurane diterpenes and related compounds reported since 1997.
Table 1.
Some biological activities described for kaurane diterpenes, published since 1997.
| Biological Activity | References |
|---|---|
| Platelet antiaggregating | 58 |
| Antispasmodic | 59, 60 |
| Antidiabetic / antiobesity | 61 |
| Antinociceptive | 62 |
| Antiallergic | 63 |
| Embryotoxic | 64 |
| Genotoxic | 65 |
| Hypoglycemic | 45, 46 |
| Immunosuppressive | 25, 26, 66 |
| Inductor of apoptosis | 67 |
| Inhibitor of vascular smooth muscle contraction | 32, 33, 55, 68 |
| Stimulant of insect egg deposition | 69 |
| Toxic to insects (stored pests) | 70 |
2. Synthesis and semisynthesis of kaurane derivatives
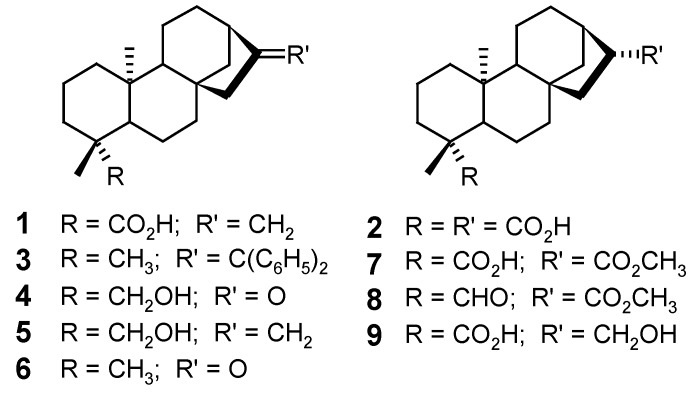
The broad spectrum of biological activities presented by the kaurane diterpenes has motivated countless studies of structural modifications of the kaurane skeleton aiming to obtain new potentially bioactive substances. These structural transformations have been achieved either chemically [54,71,72] or microbiologically, using microorganism cultures [73,74,75]. The chemical structures of some kaurane diterpenes and related non-glycosidic compounds are represented in Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15 and Figure 16.
Figure 4.
Figure 5.
Figure 4.
Figure 7.
Figure 8.
Figure 9.
Figure 10.
Figure 11.
Figure 12.
Figure 13.
Figure 14.
Figure 15.
Figure 16.
2.1. Chemical routes
Starting from the main constituent 2, isolated from the ethereal extract of Ricinocarpus stylosus (Euphorbiaceae), Henrick & Jefferies [76] carried out diverse esterification, oxidation, reduction and homologation (Wittig reaction) reactions to obtain the derivatives 3, 4, 5, 6, 7, 8 and 9, among others.
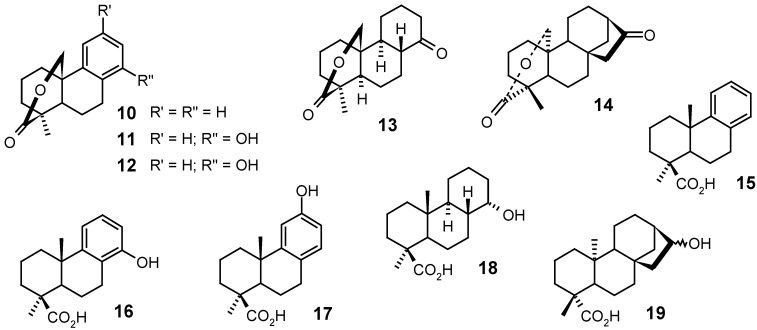
Mori and collaborators [77] synthesized several lactones, such as 10, 11, 12, 13 and 14, belonging to the series of podocarpanes and kauranes, using photolytic and non-photolytic methods for realizing chemical transformations on structures 15, 16, 17, 18 and 19, all of them presenting a carboxylic group at C-4 in the axial position.
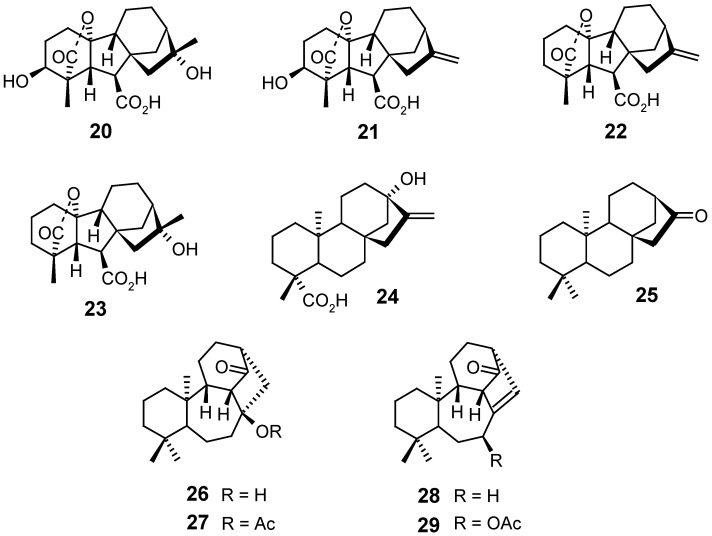
The total synthesis of kaurenoic acid (1) was carried out by Mori et al. [78], who had also synthesized the gibberellins A2 (20), A4 (21), A9 (22) and A10 (23), steviol (24) and several other kaurane diterpenes functionalized on the A ring.
The treatment of ent-17-norkauran-16-one (25) with thallium trinitrate in acetic acid caused an oxidative rearrangement supplying the derivatives 26, 27, 28 and 29 [79]. The formation of these products from 20 was attributed to the formation of a π complex between the C-15 atoms and thallium, with a subsequent rearrangement caused by the migration of the C-9/C-8 bond towards C-9/C-15 and the formation of the double bond between C-8 and C-14.
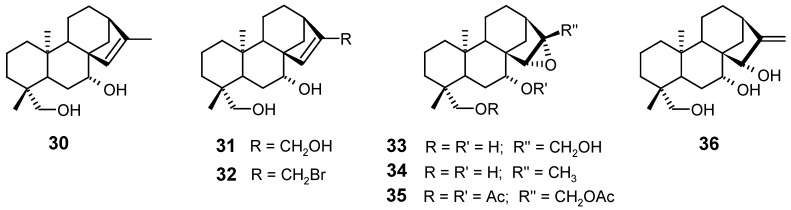
Using episideridiol (30), an abundant diterpene of Sideritis infernalis, as starting material, Bellino and Venturella carried out the synthesis of the derivatives 31, 32, 33, 34, 35 and 36, through several photooxidation, epoxidation, rearrangement, bromation, acetylation and hydrolysis reactions [80].
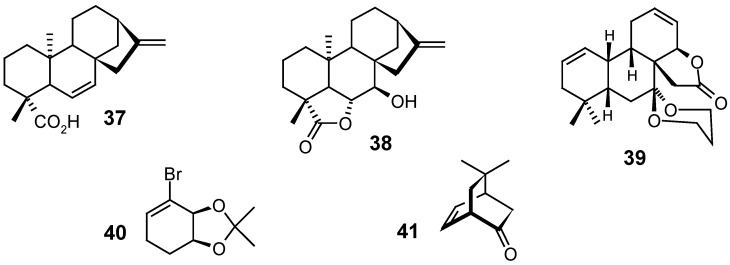
Interested in the stereochemistry elucidation of the enzymatic dehydrogenation of kaurenoic acid (1) by the fungus Gibberella fujikuroi, in order to produce the diene 37, an intermediate in the biosynthesis of the kaurane 38, Castellaro et al. synthesized diverse deuteroderivatives labelled at the C-6 and C-7 positions, employing several types of reactions such as esterifications, hydroborations, oxidations, reductions and nucleophilic substitutions [71].
The synthesis of the pentacyclic lactone 39, structurally related to the kaurane skeleton, was carried out by the coupling of compounds 40 and 41, followed by Cope rearrangement, Mitsunobu inversion, Claisen rearrangement, saponification and iodolactonization-elimination [81].
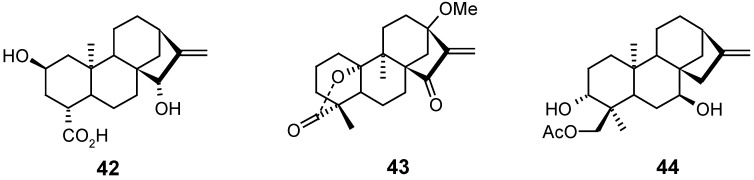
The Corey group [82] had previously achieved the total synthesis of (±)-atractyligenin 42 as a racemic mixture. However, 10 years later [83], they published an enantioselective synthetic route to the preparation of 42 with an enantiomeric excess over 87 %, which made possible the synthesis of this kaurane in the form which it is found in nature.
Diterpene lactone 43, a rearranged kaurane diterpenoid that presents some antitumoral and antimalarial activities, was obtained as a racemic mixture through a linear total synthesis consisting of 21 steps [72].
Some kaurane derivatives were semisynthesized from kaurenoic acid (1) and linearol (44), and their activities as trypanosomicidal [84] or antifeedant (appetite inhibitor of larvae of Spodoptera littoralis) [54] agents, respectively, were evaluated.
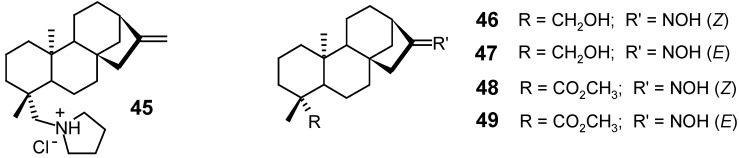
Aiming to reduce or eliminate the lytic effect of kaurenoic acid (1) on the erythrocytes of infected blood [40], which are usually used in the in vitro assays against trypomastigote forms of Trypanosoma cruzi, Vieira and collaborators [85] synthesized 19 kaurane derivatives containing a ketone or an oxime group at C-16, and alcohol, methyl ester, amine or amide functions at C-19. Of these, only the oxime 48 was more active than 1, presenting trypanosomicidal activity in all the concentrations tested (2.27 – 0.57 mM) along with a slight lysis of the red cells. Although hydrochloride 45 was the only product that did not produce haemolysis, its trypanosomicidal activity was comparable to that of the kaurenoic acid. Derivatives 46, 47 and 49 were as active as 1, presenting, however, a slight lysis of the erythrocytes. All the other synthesized compounds were inactive in the concentrations tested.
The relatively high natural abundance of kaurenoic acid (1) in some plant species such as Wedelia paludosa D.C. and Xylopia frutescens AUBL. [47], in addition to the lack of a general method for the synthesis of alkyl kaurenoates through the esterification of 1, motivated Boeck and collaborators [86] to carry out the chemical modification of this diterpene in order to synthesize new kaurane derivatives and to evaluate their respective potential pharmacological activities. As a result, a simple method was developed for preparing kauranic esters through the alkylation of the acids with alkyl halides, in a KOH-acetone system, avoiding the use of anhydrous conditions and establishing a reproducible method for the reaction. Moreover, it was observed that only kaurenoic acid (1) and derivatives containing a free carboxyl group showed moderate antifungal activity against the dermatophytes assayed, suggesting that the presence of hydrophilic groups could be necessary for the observed antifungal activity.
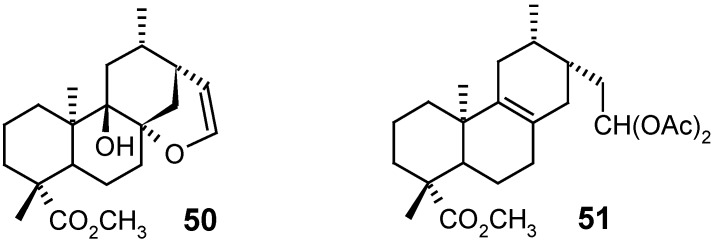
Alonso and collaborators [87] prepared diterpenes 50 and 51, among others, by an MCPBA epoxidation of grandiflorenic acid (9), isolated from species of the genus Espeletia (Asteraceae), followed by subsequent molecular rearrangement with borontrifluoride etherate or N-methyl-N-nitrosourea. Although these products had not presented, in general, any significant antimicrobial activity against Gram-positive or Gram-negative bacteria and yeasts (Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Bacillus cereus, Candida tropicalis and Aspergillus niger), the rearranged diterpenes 50 and 51 revealed considerable cytotoxic activity against diverse neoplastic human cell lines such as colon, lung, leukemia, melanoma, ovary, kidney and prostate, with IG50 values between 0.05 and 100 μM, indicating that these diterpenes (50 and 51) may be promising antitumoral substances.
Two kaurane-related compounds, ent-16α-hydroxy-15β-hydroxymethylbeyeran-19-oic acid (52) and its ethyl ester 53, were synthesized in good yield through an aldol-Cannizzaro reaction from isosteviol (54), which was obtained by hydrolysis of stevioside (78). Compound 53 displayed selective inhibition against β-glucosidases (61.3 %) and α-mannase (63.2 %), at the concentration of 0.1 mmol/L [88].
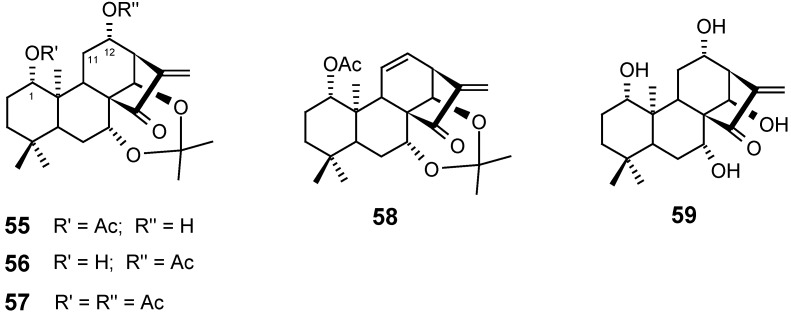
Many derivatives such as 1-O-monoacyl (55), 12-O-monoacyl (56), 1-,12-O-diacyl (57), and 11,12-dehydrated excisanin A 7,14-acetonide (58), among others, were synthesized from excisanin A (59) isolated from Rabdosia excisa. The structures and cytotoxic activity relationships (SAR) of these substances and analogues were studied using P388 murine leukemia cells [89].
New oxidized ent-kaurane (60 and 61) and ent-norkaurane (62) derivatives were synthesized by Batista et al. [90], starting from kaurenoic acid (1). The synthesis of compounds 60, 62, 63 and 64 was achieved by the oxidation of methyl ent-17-hydroxykauran-19-oate (65) under PDC conditions.
2.2. Microbiological methods
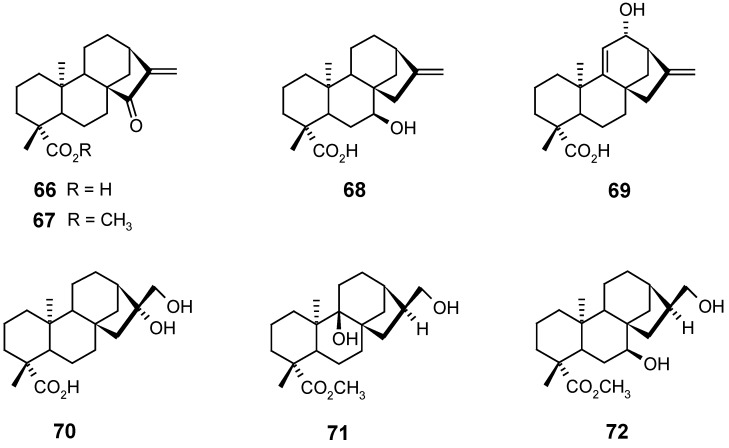
The acid 66 and its methyl ester 67 were biotransformed by the fungus Cephalosporium aphidicola, affording their respective ent-11α,16β-dihydroxy derivatives. These results indicated that the presence of a carboxylic group in the axial position at C-19 inhibited the ent-3β-hydroxylation by this microorganism, in contrast to what was observed in the biotransformation of kauranes possessing an axial hydroxymethyl group at C-19 [73,91].
Biotransformation of kaurenoic acid (1) and the methyl ester 65, through incubation with the fungus Rhizopus stolonifer, led to the formation of the alcohols 68, 69 and 70, respectively, in the first case [74], and of the alcohols 71 and 72, in the second one [92].
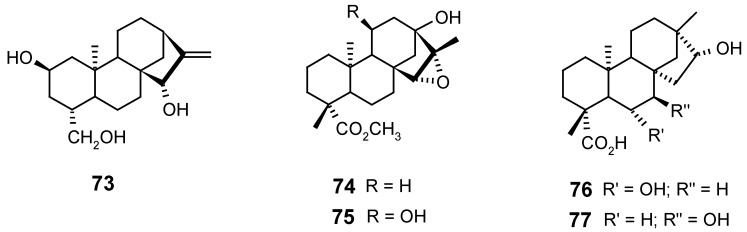
Fraga and co-workers [75,93] showed that the biotransformation of ent-15β,16β-dihydroxy- or ent-15β,16β-epoxy-kauranes by Gibberella fujikuroi produced selective hydroxylation at the ent-7β and ent-11α positions, and inhibited the oxidation of C-19. Similar results were also obtained in kauranes having the ent-16β,17-diol [94], ent-15α-hydroxyl or 15-oxo [95] groups. Besides, an ent-11α-hydroxylation seems to be directed by the presence of a 7-oxo group [96]. The non-oxidation of C-19 by this microorganism was also observed in kauranes having one ent-3β-hydroxyl or one carbonyl at C-3 [75,95].
Various mono- and diacetylated derivatives of atractyligenin (42), linearol (44) and atractylitriol (73) were regioselectively obtained through acetylation and deacetylation reactions catalyzed by lipases from Candida rugosa, C. antartica and Pseudomonas sp. [97].
Steviol derivatives, such as isosteviol (54) and the epoxykaurane 74, were submitted to biotransformations by the fungi Aspergillus niger and Fusarium moniliforme [98], leading stereo- and regioselectively to the hydroxylated compounds 75, 76 and 77.
3. General considerations about glycosides
"Glycoside" is the general chemical name given to any substance consisting of one aglycone linked to one or more glycosidic moieties, which can vary from monosaccharides to polysaccharides. Aglycones can be simple structures, such as short alkylic chain alcohols or fatty acids, as well as more complex ones, such as carotenoids, terpenoids, steroids, quinones, flavonoids, iridoids and alkaloids, among others [99]. Glycosides are widely distributed in nature and they can probably be found in all living organisms [100].
In plants, glycosides are responsible for functions related to accumulation, storage and transport of hydrophobic substances. In comparison with their free aglycones, they show a greater solubility in water and, generally, a lesser reactivity, facilitating their storage in the plant’s vacuole and protecting the organism from the toxicity of the aglycone [101]. A good example of storage of aglycones are the cyanogenic glycosides, that act as toxic substances in the defense mechanism of some plants against herbivorous animals [102]. Other specific functions are the protection against UV-B radiations [103] or the closing and opening of the leaves [104].
3.1. Structures, occurrence and biological activities of naturally occurring kaurane glycosides
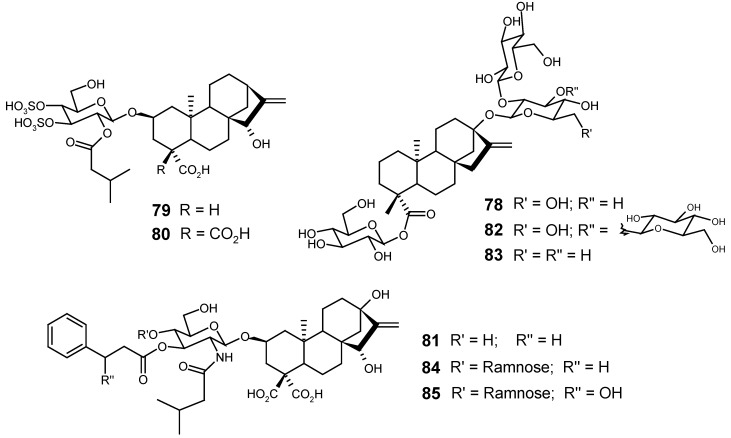
It can be said that kaurane glycosides have assumed more importance and have been studied in greater depth since their toxicity, therapeutic potential and economic value were demonstrated. The main examples of this class of compounds are stevioside (78), atractyloside (79), carboxyatractyloside (80) and wedeloside (81), the structures of which are shown in Figure 17, along with the structures of some respective analogues. The structures of other naturally occurring kaurane glycosides and related compounds are represented in Figure 18, Figure 19, Figure 20, Figure 21 and Figure 22.
Figure 17.
Figure 18.
Figure 19.
Figure 20.
Figure 21.
Figure 22.
Stevia rebaudiana (Bertoni) Bertoni (Asteraceae), a plant found in aboriginal regions of Paraguay and Brazil, and cultivated in Israel, South and North Korea, China and Japan, is used to sweeten drinks such as mate or tea. Its main component, stevioside (78), whose contents vary from 3 to 8 % in the dry leaf, sweetens between 250 and 300 times more than saccharose, besides not showing any cariogenic activity, and for these reasons the industrial use of this glycoside as a substitute for saccharose, in foods and dietary drinks is allowed in some countries around the world. From 110 species of Stevia spp., examined, stevioside was only found in two of them and, therefore, it is considered a natural product of very restricted distribution. Two other kaurane glycosides found in S. rebaudiana (Bertoni) Bertoni, with similar sweetening properties, are rebaudioside (82) and dulcoside (83). The study of some species of Stevia spp. has led to the isolation of other several kaurane glycosides, with or without sweetening properties [105].
The economic importance of stevioside (78), derived from its great world-wide consumption, has motivated diverse work aiming to characterize and establish the safe levels of this kaurane glycoside and its metabolites in the human organism [106,107]. Although some work has failed to demonstrate carcinogenic, teratogenic, mutagenic or toxic actions of stevioside (78), after oral administration to rats [108,109,110,111], at doses up to 1.2 % of the diet, and considering, therefore, a maximum ingestion recommendable of 7.94 mg·kg-1·d-1 for the human organism [112], others have demonstrated that steviol (24), an aglycone obtained from complete hydrolysis of stevioside, showed a high toxicity in pregnant females and embryos of hamsters, at doses of 0.75 and 1.00 mg·kg-1·d-1, administered from 6th to 10th day of gestation [112,113,114]. The animals treated with these doses showed acute renal failure, embryotoxic effects such as weight loss and retardation of the process of fetal ossification, and death. Among some in vitro evaluated effects, it was observed that stevioside (EC50 = 12 x 10-4 M) and steviol (EC50 = 0.4 x 10-4 M) affect some mitochondrial functions, inhibiting the oxidative phosphorylation, the exchange ADP/ATP, the transport of fatty acids and the enzymes NADH-oxidase and L-glutamate-dehydrogenase [115,116].
Atractyloside (79), initially isolated from rhizomes of Atractylis gummifera L. (Asteraceae) [117], and then from Wedelia glauca Ort. (Asteraceae) [118], is the main compound responsible for the lethality of these plants in bovine cattle from some regions of Argentina, Brazil and Uruguay, causing hypoglycemia, respiratory depression, nephrotoxicity, hypoxemia and cell injury. In humans, the poisoning with this glycoside can cause renal and hepatic failure, also followed by necrosis [119,120]. This toxic action is due, mainly, to the inhibition of mitochondrial oxidative phosphorylation [117]. Carboxyatractyloside (80), also isolated from Atractylis gummifera L., provoked a great inhibition of the nucleotide translation through the mitochondrial membrane, causing 10 times more toxicity than atractyloside in the in vivo assays. This greater toxicity of carboxyatractyloside is structurally due to the presence of a second carboxylic group in its structure (80) [117]. Other glycosides, which differ structurally from atractyloside in the glycosidic moiety, had been isolated from some species of coffee, such as Coffea arabica, C. robusta and C. arabusta (Rubiaceae) [121]. Carboxyatractyloside showed, among other activities, antitumoral action against melanoma cells (100 μM) and Erlich tumour (3 μM) [122], beyond the insecticidal and herbicidal activities [123]. These kaurane glycosides and/or some related compounds had also been isolated from species of Xanthium spp. (Asteraceae), Wedelia biflora (Asteraceae), Cestum paraqui (Solanaceae) and Iphiona aucheri (Asteraceae) [99,124,125].
Wedeloside (81), an aminoglycoside isolated from Wedelia asperrima Benth. (Asteraceae), represents the first example of the occurrence of one acylaminedeoxyhexose unit linked to a diterpene. This glycoside, considered the main toxic component of the plant, caused an inhibition of mitochondrial ADP/ATP transport, being as active as carboxyatractyloside. In addition to the amine group on the hexose portion, wedeloside differs structurally from both atractyloside (82) and carboxyatractyloside (80) by the presence of one hydroxyl at C-13 and by the absence of the two sulphate groups at the carbons C-3' and C-4' of the glycosidic unit [126]. Two other analogues of wedeloside, 84 and 85, have also been isolated from the same species [127].
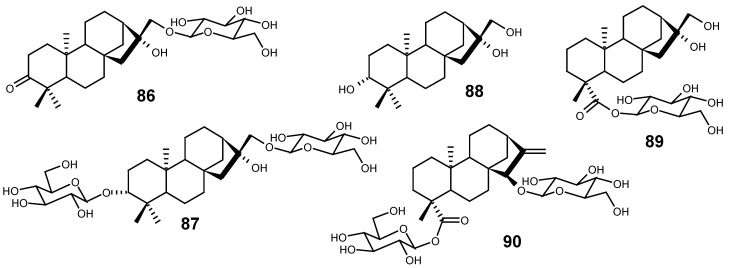
Artemisia sacrorum (Asteraceae) is traditionally used, in a northeast region of China, for the treatment of hepatitis. From the aqueous extract of the aerial parts of this plant, two kaurane glycosides, 86 and 87, have been isolated [128], in addition to the kauranetriol 88.
Francoeuria crispa Cass. (synon. Pulicaria sicula) (Asteraceae) supplied some sesquiterpene lactones, besides kaurane glycosides 89 and 90, in relatively high amounts [129].
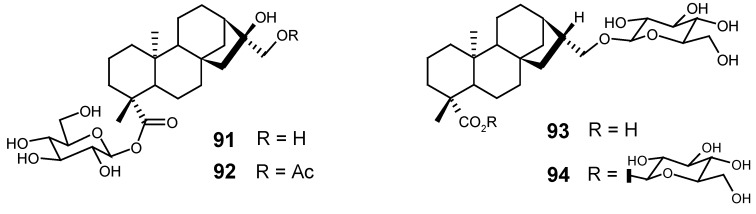
Considering that some species of the genus Aster (Asteraceae) are frequently used in Chinese and Tibetan traditional medicine for the treatment of fever and flu, the ether-methanol extract (1:1) of A. tongolensis was studied, yielding two kaurane glycosides, 91 and 92, together with their aglycones as free acids. The authors of this work had previously studied the species A. poliothamnus, and they were surprised by the high occurrence of kaurane compounds in A. tongolensis, since this class of diterpenes is rarely found in the genus Aster [130].
From the flowers of Inula britannica L. (var. chinensis) (Asteraceae), used in the traditional Chinese medicine for the treatment of bronchitis and inflammation, a n-butanolic fraction, obtained from the partition of its ethanolic extract with n-butanol and water, was subsequently chromatographed, leading to the isolation of two diterpene glycosides, 93 and 94 [131].
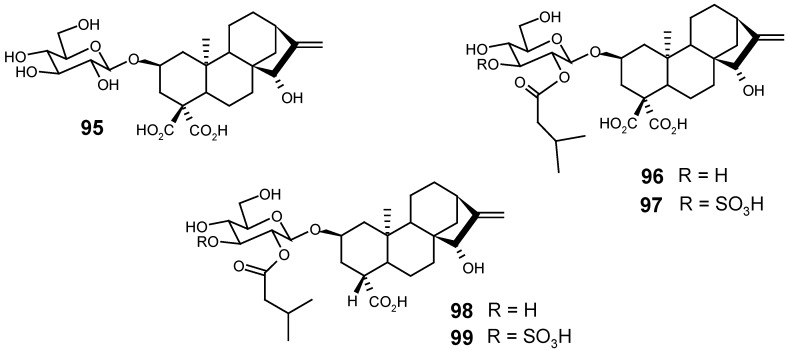
From the chloroform-methanol extract of the aerial parts of Xanthium spinosum (Asteraceae), the glycosides 95, 96, 97, 98 and 99, structurally related to atractyloside and carboxyatractyloside, were isolated [125].
The juice of the fresh leaves of Pieris formosa D. Don (Ericaceae), a very well known toxic species found extensively in some regions of south and southeast China, is used as an insecticide and as a lotion for the treatment of mycoses produced by Tinea spp. and scabies. The ethanolic extract of the leaves of this species was fractionated by partition between non-miscible solvents, affording the n-butanol fraction that, by successive chromatography on silica-gel, RP-18 and Sephadex (LH-20) columns, allowed the isolation of diterpene glycosides such as the seco-kaurane glycoside 100, in addition to other compounds of different structures, like guaianes [132].
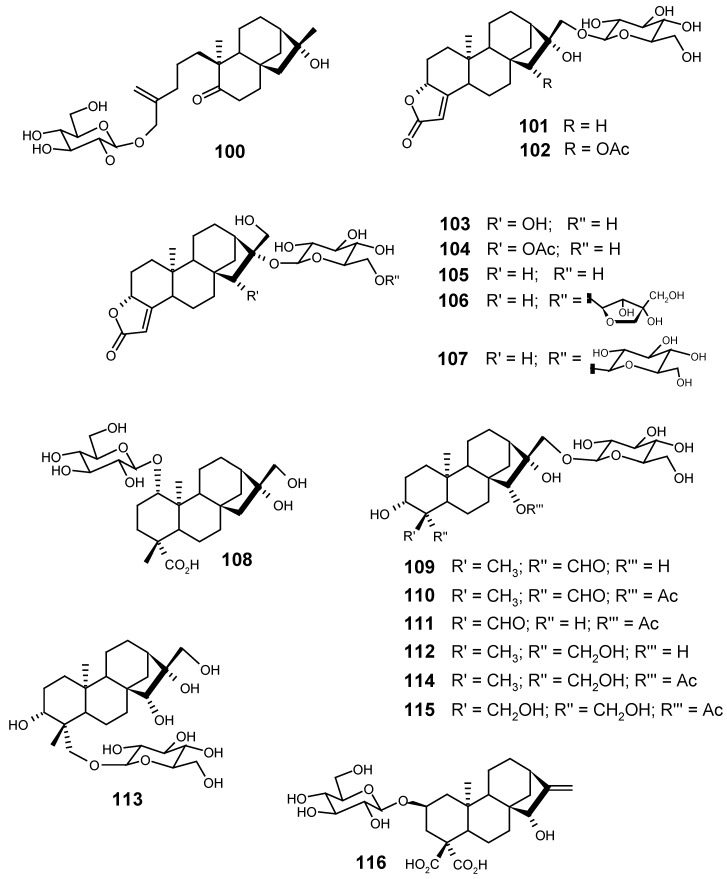
From Tricalysia dubia (Lindl) Ohwi. (synon. Canthium dubium Lindl), a Rubiaceae species found in southern China, Taiwan and southeast Japan, seven rearranged kaurane glycosides (tricalyosides A-G) were isolated by He and collaborators [133]: 101, 102, 103, 104, 105, 106 and 107. Three years later, they also described, from the same species, eight new ent-kaurane glucosides: 108, 109, 110, 111, 112, 113, 114 and 115, named respectively tricalyosides H-O [134].
From the aerial parts of Gnaphalium sylvaticum (Asteraceae), Konopleva and collaborators [135] isolated a new diterpene glucoside, structurally related to carboxyatractyloside, which they named sylviside (116).
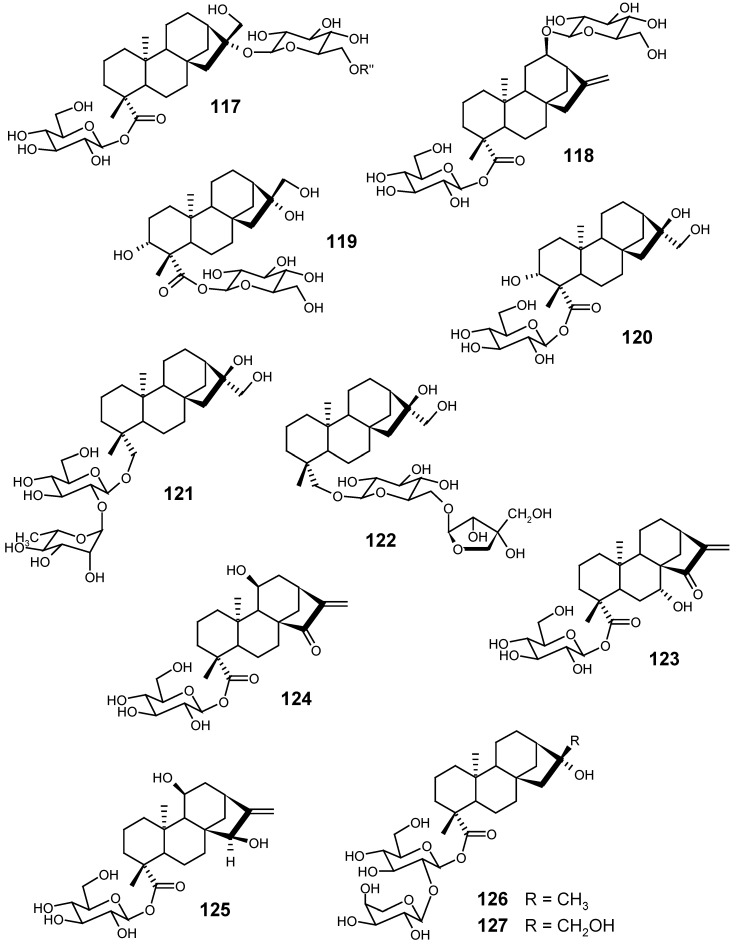
Species of the genus Cussonia (Araliaceae) have been investigated by Harinantenaina et al. The leaves of C. bojeri Seem. are used in the traditional medicine of Madagascar as a phytotherapeutic agent against acne, diarrhoea, stomach ulcers and syphilis. The methanolic extract from the leaves of this species afforded the glycosides 89, 91 and 117, besides their corresponding aglycones as free acids [136]. From the methanolic extract of the leaves of C. vantsilana Baker, the glycosides 118, 119, 120, 121 and 122 were isolated [137].
From the methanolic extract of the leaves of Hyalis argentea Don ex Hook. et Arn. var. argentea (Asteraceae), Ybarra and collaborators isolated [138], among other constituents, the kaurane glycosides 123, 124 and 125.
As several species of the genus Vernonia (Asteraceae) are used in popular medicine for the treatment of some illnesses, Kos and collaborators [14] investigated the aerial parts of V. triflosculosa Kunth, and they isolated, from its ether-methanol extract, three sesquiterpene lactones and three diterpenes, two of them being the new glycosides 126 and 127. This work described, for the first time, the isolation of kaurane diterpenes in species of the genus Vernonia.
4. Glycosidation methods
We report here some general methods to obtain glycosides, depending on whether they are synthesized by enzymatic or chemical routes.
4.1. Enzymatic glycosidation
In vivo, oligosaccharides and glycosides are synthesized by the action of glycosyltransferases, which selectively transfer a saccharide unit from an activated intermediate, such as a nucleotide, to an aglycone. However, on an industrial scale, the synthesis of glycosides in bioreactors that use these glycosyltransferases would only be viable if a system for the efficient recovery of the nucleotides was developed, since their cost is very high. Moreover, the high selectivity of transglycosidation catalyzed by glycosyltransferases makes the applications of these enzymes very limited and specific.
In industry, glycosidases are used for glycosidation reactions and these enzymes can be extracted from diverse natural sources, such as Prunus amygdalus (almond tree) and Pyrococcus furiosus (microorganism) [102,139]. The first and foremost natural function of these enzymes is to promote the hydrolysis of the glycosidic bonds. However, Bourquelot demonstrated that it was possible to use glycosidases inversely to synthesize glycosides, simply by adjusting the reaction conditions [140].
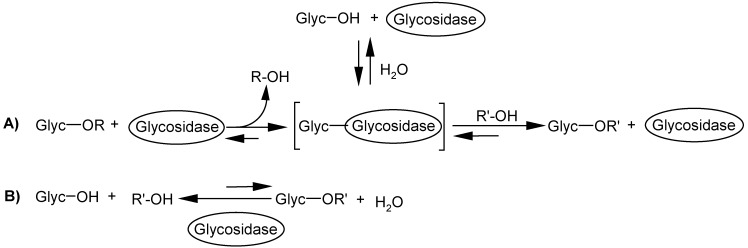
Scheme 1 represents two different glycosidation methods by glycosidases:
Scheme 1.
Different glycosidation methods with glycosidases: A) Transglycosidation; B) Direct glycosidation.
a) In a kinetically controlled transglycosidation, increased yields can be achieved in a short period of time, mainly due to the equilibrium of the system favourable to the formation of the intermediate carbohydrate-glycosidase complex. The main problem of this method is the use of special saccharide donors, such as cellobiose and fructose, which raise the cost of the process. Furthermore, by-product formation can harm the glycosidation process notably [101].
b) D irect glycosidation, a thermodynamically favoured process, is only preferable in the cases where a high yield is possible, achieved by the increase of the substrate concentration, the maintenance of a low water concentration in the reaction medium, and/or the selective removal of the product formed [141,142].
4.2. Chemical glycosidation
Chemical synthesis usually offers reasonable yields in the preparation of glycosides from the coupling between a donor, generally an aglycone bearing an alcohol or phenol function, and a glycoside unit.
There is no general method for promoting the coupling between a carbohydrate and an organic molecule (aglycone), that would allow the synthesis of the product with the desired stereo- and regioselectivity. The preparation of the two functionalized parts is necessary before carrying out the coupling:
A hydroxylated compound, called receptor, which is prepared from an organic molecule (aglycone), selectively protected;
The glycosidic part, called donor, which is activated at the anomeric position of the carbohydrate, before coupling.
For a successful coupling it is important to note the reactivity of the donor at the anomeric position and of the receptor hydroxyl group depends on the protecting groups, the conformation and the molecular size of each compound. Furthermore, the coupling must be stereoselective, leading preferentially to one of the two possible anomers. The two configurations to be reached by glycosidation are:
-
a)
1,2-trans, as in the cases of β-glucosides and β-galactosides;
-
b)
1,2-cis, as in the cases of α-glucosides and α-galactosides.
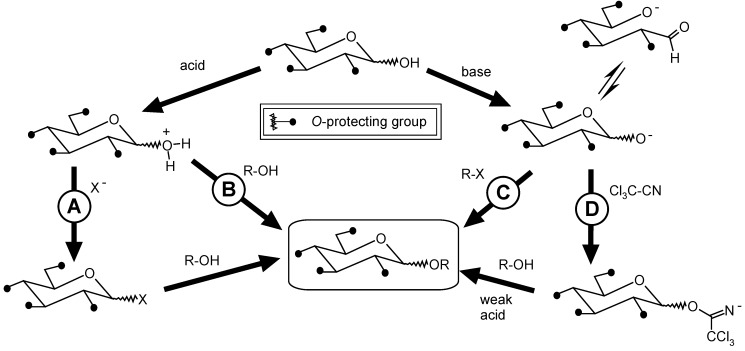
Scheme 2 presents the main chemical methods for glycosidation starting from an alcohol R-OH (receptor) and a glycoside unit (donor).
Scheme 2.
Classic glycosidation methods: A) Koenigs-Knorr; B) Fischer-Helferich; C) Schmidt or 1-O-alkylation; D) via trichloroacetimidates.
Glycosidation using the Koenigs-Knorr method (Scheme 2, A) consists of the reaction between a glycoside haloderivative and an alcohol, in the presence of promoters such as silver or mercury (II) salts, among others, in low or medium polarity solvents [143,144].
The Fischer-Helferich method (Scheme 2, B) involves the reaction between a hemiacetal glycoside and an alcohol, under acid catalysis. The main limitation of this method is that only relatively reactive alcohols can be used, due to the low reactivity of the glycoside moiety (hemiacetal) [145].
The preparation of glycosides by the 1-O-alkylation method, also known as “the Schmidt method” (Scheme 2, C), occurs by activation of the donor (glycoside moiety) by a base, reacting subsequently with the activated receptor, an alkyl halide. In this case, the yield, the regio- and the stereoselectivity of the method are governed by the stability of the generated anion, by the tautomeric balance between ring/open chain forms of the saccharide and by the relative reactivity of the three anions produced from the hemiacetal deprotonation [146].
Finally, the trichloroacetimidate method (Scheme 2, D) includes the reaction of an alcohol, in the presence of an acid catalyst, with a glycosidic trichloroacetimidate, produced from the treatment of a sugar with trichloroacetonitrile. This method does not involve the use of heavy metals as promoters, what makes it the preferred one in the synthesis of 1,2-trans-glycosides, in opposition to the Koenigs-Knorr method [147].
The synthesis of 2,3-dideoxyglycosides involves the mechanism known as "Ferrier rearrangement”, which takes place by the treatment of a glycal with a reactive alcohol, in the presence of a Lewis acid, such as iodo [148], indium chloride [149], cerium chloride [150] or scandium triflate [151], leading to glycosides with an unsaturation between the carbons 2-3 of the glycopyranose ring. This and other glycosidation methods, not commented here in greater depth, are satisfactorily dealt with in specific bibliographical references in the area of carbohydrates chemistry [145,152,153].
5. Synthesis of kaurane glycosides
Many biologically active compounds are glycosides. In fact, the glycosidic portion can be crucial for the biological activity of a drug, either for the improvement of its pharmacokinetic parameters, for the influence on the transport through the cellular membrane or through the interaction(s) with receptors [154].
Oligosaccharides and glycoconjugates promote better responses in immunochemical and immunological studies as a consequence of the great affinity between receptors and glycosides [155,156], which justifies the interest in the synthesis of glycosides [157]. The development of synthetic methods for the preparation of kaurane glycosides, as well as the spectrometric characterization of these compounds, are important for the definition of necessary structural requirements for their biological activities and for the understanding of the structure/activity relationships.
Few papers can be found in literature about the synthesis of kaurane glycosides, and most of them report to the synthesis of analogues of stevioside (78). Aiming to achieve an improvement in the organoleptic properties of stevioside (78) and other natural analogues, modifications on the glycosidic portion of these glycosides were carried out through glycosidations or partial hydrolysis by enzymatic way, using the cyclomaltodextrin-glucanotransferase system, which efficiently catalyzes the transglycosidation of one or more α-glycosyl units, proceeding from the starch, to the 4-OH position of the glycoside unit. This methodology provided the preparation of more pleasant products to the palate, mainly for increasing the sweetness of the resultant glycosides [158,159].
After previous observation that the glycosidic portions of stevioside (78) and rebaudioside (82), linked to the C-19 position of the kaurane skeleton, are not involved in essential interactions with the receptors of the gustative cells, DuBois and collaborators [160,161] proposed the synthesis of analogous glycosides by substitution of the glycosidic portions for other groups with similar polarities, without the loss of the sweetening properties. As result, these researchers observed that the increase of the hydrophilic character of the group at C-19 caused the reduction of the bitter taste, which is characteristic of 78 and 82, leading to the synthesis of products that did not produce any such bitterness.
The trypanosomicidal activity of kaurenoic acid (1) and derivatives motivated the synthesis of kaurane glycosides by Batista et al. [162], aiming to investigate a putative relationship between activity and water-solubility of these diterpenes. Thus, glycosidation of the alcohols 5 and 65, derived from kaurenoic acid (1), with α-D-(+)-glucose derivatives, under the Koenigs-Knorr reaction conditions was carried out [163,164,165]. In spite of the disadvantage of this method requiring heavy metals as promoters of the reaction, it usually gives the highest global yield in the chemical synthesis of the glycosides [101].
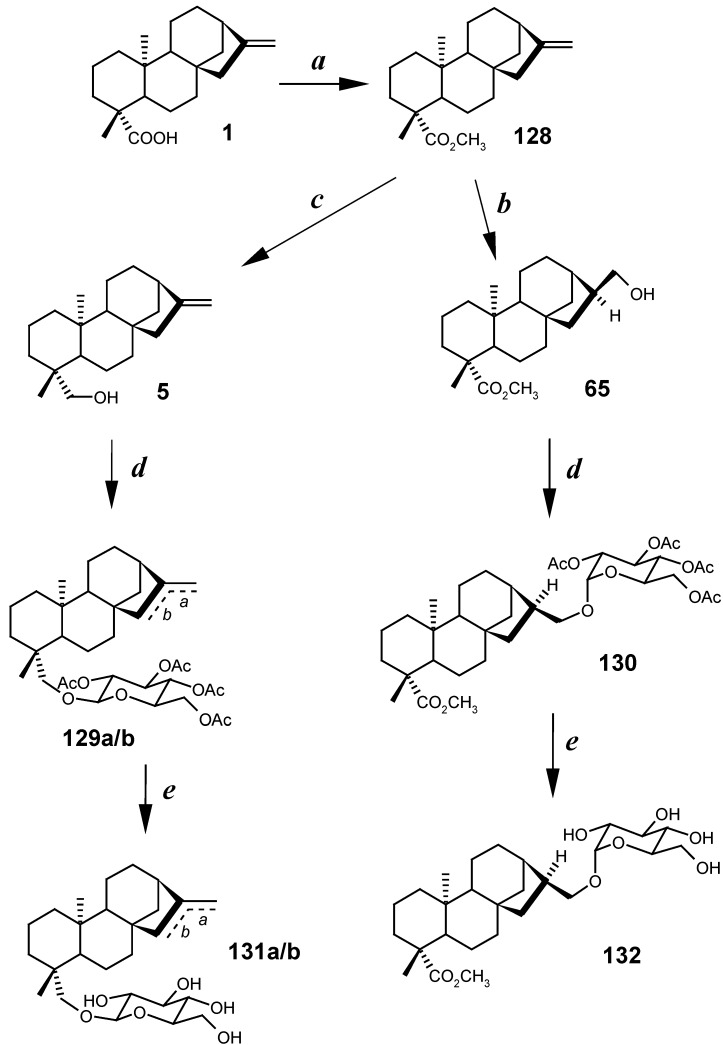
The synthetic route to the kaurane glycosides from kaurenoic acid (1) is depicted in Scheme 3. This acid 1 was esterified (giving 123) and converted into the alcohols 5 and 65 (steps a, b and c) that, in turn, were submitted to glycosidation with acetobromoglucose (step d), leading to the intermediates 129a/b and 130, which after subsequent hydrolysis of the acetoxyl groups (step e), yielded the glycosides 131a/b and 132 respectively.
Scheme 3.
Synthesis of kaurane glycosides from kaurenoic acid (1) [162].
In vitro assays against trypomastigote forms of Trypanosoma cruzi, the aetiological agent of Chagas disease, showed trypanosomicidal activity for the derivatives 65, 128 and 132 in all the assayed concentrations (0.31 – 5.00 mM), the glycoside 132 being the most active, while the glycosides 131a/b were inactive in the same concentrations. Methyl ester 128 and glycoside 132 were also trypanosomicidal on in vivo assays [162]. These results suggest that the carbonyl group at C-19 is an indispensable requirement for the trypanosomicidal activity, and that O-glycosidation at C-17 position of the kaurane skeleton intensifies the trypanosomicidal activity, probably due to the influence of the glycosidic portion in the transport of the kaurane molecule through the parasite cell membrane.
6. Conclusions
We have reviewed the occurrence and biological activities of kaurane diterpenes and their glycosides, as well as the synthetic and semisynthetic methods used to produce them. The accumulation of kaurenoic acid and other naturally occurring kaurane diterpenes in some plant species make them important sources of these compounds, which are thus available as starting materials in the synthesis of new derivatives for biomedical and industrial research. Indeed, in the last few years, a growing number of publications have reported the use of kaurane diterpenes for the synthesis of novel sweetening, antimicrobial, cytotoxic and trypanocidal agents, and this synthetic approach is still far from being fully exploited by the current interest in the chemistry of natural products.
In summary, the potential of these diterpenes as sweetners and as therapeutic agents invite us to continue exploring more and more this family of interesting and promising compounds.
Acknowledgments
The authors are grateful to Anna Quinault for linguistic assistance in the preparation of the paper. The synthesis of kaurane glycosides included in this review was carried out at the Universidade Federal de Minas Gerais – UFMG, Brazil, and was partly supported by the following Brazilian institutions: Universidade Estadual do Sudoeste da Bahia – UESB, CAPES and CNPq.
Footnotes
Sample Availability: Not applicable.
References
- 1.Bruneton J. Pharmacognosy, Phytochemistry, Medicinal Plants. Lavoisier; London: 1995. pp. 387, 511. part 3. [Google Scholar]
- 2.Hanson J.R. Nat. Prod. Rep. 2004;21:785. doi: 10.1039/b414026p. [DOI] [PubMed] [Google Scholar]
- 3.Hanson J.R. In: Diterpenoids. Methods in Plant Biochemistry. Dey P.M., Harborne J.B., (series eds.), Charlwood B.V., Banthorpe D.V., (vol. eds.). , editors. v.7. Academic Press; London: 1991. pp. 263–287. [Google Scholar]
- 4.Batista R., García P.A., Castro M.A., Del Corral J.M.M., Speziali N.L., Oliveira A.B. Acta Crystallogr., Sect. E: Struct. Rep. Online. 2005;E-61:o1525. [Google Scholar]
- 5.Velandia J.R., Carvalho M.G., Braz-Filho R. Quím. Nova. 1998;21:397. [Google Scholar]
- 6.Ghisalberti E.L. Fitoterapia. 1997;68:303. [Google Scholar]
- 7.Giles P. M., Jr. Pure Appl. Chem. 1999;71:587. [Google Scholar]
- 8.Reynolds W.F., Lough A.J., Sawyer J.F., Enriquez R.G., Ortiz B., Walls F. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1991;C-47:973. [Google Scholar]
- 9.Bohlmann F., Adler A., Schuster A., Gupta R.K., King R.M., Robinson H. Phytochemistry. 1981;20:1899. [Google Scholar]
- 10.Bohlmann F., Kramp W., Jakupovic J., Robinson H., King R.M. Phytochemistry. 1982;21:399. [Google Scholar]
- 11.Le Quesne P.W., Honkan V., Onan K.D., Morrow P.A., Tonkyn D. Phytochemistry. 1985;24:1785. [Google Scholar]
- 12.Roque N.F., Giannella T.L., Giesbrecht A.M., Barbosa R.C.S.B.C. Rev. Latinoam. Quím. 1987;18:110. [Google Scholar]
- 13.Metwally M.A., Dawidar A.M., Abou-Elzahab M.M. Pharmazie. 1985;40:736. [Google Scholar]
- 14.Kos O., Castro V., Murillo R., Poveda L., Merfort I. Phytochemistry. 2006;67:62. doi: 10.1016/j.phytochem.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Herz W., Sharma R.P. J. Org. Chem. 1976;41:1021. doi: 10.1021/jo00868a023. [DOI] [PubMed] [Google Scholar]
- 16.Przybylska M., Ahmed F.R. Acta Crystallogr., Sect. B: Struct. Sci. 1977;B33:366. [Google Scholar]
- 17.Klecakova-Karlickova J., Jahodar L. Ceska Slov. Farm. 2005;54:141. [PubMed] [Google Scholar]
- 18.Hasan C.M., Healey T.M., Waterman P.G. Phytochemistry. 1982;21:1365. [Google Scholar]
- 19.Chang F.R., Yang P.Y., Lin J.Y., Lee K.H., Wu Y.C. J. Nat. Prod. 1998;61:437. doi: 10.1021/np970497z. [DOI] [PubMed] [Google Scholar]
- 20.Zgoda-Pols J.R., Freyer A.J., Killmer L.B., Porter J.R. Fitoterapia. 2002;73:434. doi: 10.1016/s0367-326x(02)00124-7. [DOI] [PubMed] [Google Scholar]
- 21.Bandara B.M.R., Wimalasiri W.R., Macleod J.K. Phytochemistry. 1988;27:869. [Google Scholar]
- 22.Hogg R.W., Knox J.R. Aust. J. Chem. 1987;40:469. [Google Scholar]
- 23.Cannon J.R., Chow P.W., Jefferies P.R., Meehan G.V. Aust. J. Chem. 1966;19:861. [Google Scholar]
- 24.Jahan I.A., Nahar N., Mosihuzzaman M., Shaheen F., Atta-Ur-Rahman, Choudhary M.I. J. Nat. Prod. 2004;67:1789. doi: 10.1021/np020435v. [DOI] [PubMed] [Google Scholar]
- 25.Duan H., Takaishi Y., Momota H., Ohmoto Y., Taki T., Jia Y., Li Y. J. Nat. Prod. 1999;62:1522. doi: 10.1021/np9902183. [DOI] [PubMed] [Google Scholar]
- 26.Duan H., Takaishi Y., Momota H., Ohmoto Y., Taki T., Tori M., Takaoka S., Jia Y., Li Y. Tetrahedron. 2001;57:8413. [Google Scholar]
- 27.Somova L. I., Shode F. O., Moodley K., Govender Y. J. Ethnopharmacol. 2001;77:165. doi: 10.1016/s0378-8741(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 28.Pinto A.C., Pinchin R., Prado S.K. Phytochemistry. 1983;22:2017. [Google Scholar]
- 29.Kubo I., Ganjian I., Kubota T. Phytochemistry. 1982;21:81. [Google Scholar]
- 30.Li L.M., Li G.Y., Huang S.X., Li S.H., Zhou Y., Xiao W.L., Lou L.G., Ding L.S., Sun H.D. J. Nat. Prod. 2006;69:645. doi: 10.1021/np0600200. [DOI] [PubMed] [Google Scholar]
- 31.Ghoumari H., Benajiba M.H., Azmani A., Garcia-Granados A., Martinez A., Parra A., Rivas F., Socorro O. Phytochemistry. 2005;66:1492. doi: 10.1016/j.phytochem.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 32.Cunha K.M.A., Paiva L.A., Santos F.A., Gramosa N.V., Silveira E.R., Rao V.S. Phytother. Res. 2003;17:320. doi: 10.1002/ptr.1133. [DOI] [PubMed] [Google Scholar]
- 33.Ghisalberti E.L., Pennacchio M., Alexander E. Pharm. Biol. 1998;36:237. [Google Scholar]
- 34.Braca A., Abdel-Razik A.F., Mendez J., Morelli I. Fitoterapia. 2005;76:614. doi: 10.1016/j.fitote.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Kondoh M., Nagashima F., Suzuki I., Harada M., Fujii M., Asakawa Y., Watanabe Y. Planta Med. 2005;71:1005. doi: 10.1055/s-2005-873133. [DOI] [PubMed] [Google Scholar]
- 36.Santos C.C., Sousa-Lima M.A., Braz-Filho R., Simone C.A., Silveira E.R. Magn. Res. Chem. 2005;43:1012. doi: 10.1002/mrc.1700. [DOI] [PubMed] [Google Scholar]
- 37.Han L., Huang X., Sattler I., Dahse H.M., Fu H., Lin W., Grabley S. J. Nat. Prod. 2004;67:1620. doi: 10.1021/np040062t. [DOI] [PubMed] [Google Scholar]
- 38.Sun H.D., Huang S.X., Han Q.B. Nat. Prod. Rep. 2006;23:673. doi: 10.1039/b604174d. [DOI] [PubMed] [Google Scholar]
- 39.Alvarenga S.A.V., Ferreira M.J.P., Rodrigues G.V., Emerenciano V.P. Bot. J. Linnean Soc. 2005;147:291. doi: 10.1111/j.1095-8339.2005.00357.x. [DOI] [Google Scholar]
- 40.Batista R., Chiari E., Oliveira A.B. Planta Med. 1999;65:283. doi: 10.1055/s-2006-960781. [DOI] [PubMed] [Google Scholar]
- 41.Alves T.M.A., Chaves P.P.G., Santos L.M.S.T., Nagem T.J., Murta S.M.F., Ceravolo I.P., Romanha A.J., Zani C.L. Planta Med. 1995;61:85. doi: 10.1055/s-2006-958011. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira B.H., Sant'ana A.E., Bastos D.Z.L. Phytochem. Anal. 2002;13:368. doi: 10.1002/pca.670. [DOI] [PubMed] [Google Scholar]
- 43.Melo A.C., Cota B.B., Oliveira A.B., Braga F.C. Fitoterapia. 2001;72:40. doi: 10.1016/s0367-326x(00)00251-3. [DOI] [PubMed] [Google Scholar]
- 44.Vilegas J.H.Y., Marchi E., Lanças F.M. Phytochem. Anal. 1997;8:74. [Google Scholar]
- 45.Bresciani L.F.V., Cechinel-Filho V., Yunes R.A. Nat. Prod. Lett. 2000;14:247. doi: 10.1080/10575630008041239. [DOI] [Google Scholar]
- 46.Bresciani L.F.V., Yunes R.A., Burger C., Oliveira L.E., Bóf K.L., Cechinel-Filho V. Z. Naturforsch., C: J. Biosci. 2004;59C:229. doi: 10.1515/znc-2004-3-419. [DOI] [PubMed] [Google Scholar]
- 47.Batista R., Braga F.C., Oliveira A.B. Rev. Bras. Farmacogn. 2005;15:119. doi: 10.1590/S0102-695X2005000200009. [DOI] [Google Scholar]
- 48.Vieira H.S., Takahashi J.A., Pimenta L.P.S., Boaventura M.A.D. Z. Naturforsch., C: J. Biosci. 2005;60C:72. doi: 10.1515/znc-2005-1-214. [DOI] [PubMed] [Google Scholar]
- 49.Lee K.H., Morris-Natschke S.L. Pure Appl. Chem. 1999;71:1045. [Google Scholar]
- 50.Bremner P.D., Meyer J.J.M. South Afr. J. Bot. 2000;66:115. [Google Scholar]
- 51.Giang P.M., Son P.T., Matsunami K., Otsuka H. J. Nat. Med. 2006;60:93. [Google Scholar]
- 52.Killic T. Molecules. 2006;11:257. [Google Scholar]
- 53.Ito A., Chai H.B., Shin Y.G., García R., Mejía M., Gao Q., Fairchild C.R., Lane K.E., Menendez A.T., Farnsworth N.R., Cordell G.A., Pezzuto J.M., Kinghorn D. Tetrahedron. 2000;56:6401. [Google Scholar]
- 54.Bruno M., Rosselli S., Pibiri S., Piozzi F., Bondi M.L., Simmonds M.S.J. Phytochemistry. 2001;58:463. doi: 10.1016/s0031-9422(01)00252-7. [DOI] [PubMed] [Google Scholar]
- 55.Ambrosio S. R., Tirapelli C.R., da Costa F.B., de Oliveira A.M. Life Sci. 2006;79:925. doi: 10.1016/j.lfs.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Han Q.B., Lu Y., Wu L., He Z.D., Qiao C.F., Xu H.X., Zheng Q.T., Sun H.D. Tetrahedron Lett. 2005;46:5373. [Google Scholar]
- 57.Yeh S.H., Chang F.R., Wu Y.C., Yang Y.L., Zhuo S.K., Hwang T.L. Planta Med. 2005;71:904. doi: 10.1055/s-2005-871234. [DOI] [PubMed] [Google Scholar]
- 58.Yang Y.L., Chang F.R., Wu C.C., Wang W.Y., Wu Y.C. J. Nat. Prod. 2002;65:1462. doi: 10.1021/np020191e. [DOI] [PubMed] [Google Scholar]
- 59.Zamilpa A., Tortoriello J., Navarro V., Delgado G., Alvarez L. Planta Med. 2002;68:277. doi: 10.1055/s-2002-23146. [DOI] [PubMed] [Google Scholar]
- 60.Tirapelli C.R., Ambrosio S.R., Coutinho S.T., de Oliveira D.C.R., da Costa F.B., de Oliveira A.M. J. Pharm. Pharmacol. 2005;57:997. doi: 10.1211/0022357056578. [DOI] [PubMed] [Google Scholar]
- 61.Kim S., Na M.K., Oh H., Jang J.P., Sohn C.B., Kim B.Y., Oh W.K., Ahn J.S. J. Enzyme Inhib. Med. Chem. 2006;21:379. doi: 10.1080/14756360600741560. [DOI] [PubMed] [Google Scholar]
- 62.Block L.C., Santos A.R.S., Souza M.M., Scheidt C., Yunes R.A., Santos M.A., Monache F.D., Filho V.C. J. Ethnopharmacol. 1998;61:85. doi: 10.1016/s0378-8741(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 63.Cheenpracha S., Yodsaoue O., Karalai C., Ponglimanont C., Subhadhirasaku S., Tewtrakul S., Kanjana-opas A. Phytochemistry. 2006;67:2630. doi: 10.1016/j.phytochem.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 64.Costa-Lotufo L.V., Cunha G.M.A., Farias P.A.M., Viana G.S.B., Cunha K.M.A., Pessoa C., Moraes M.O., Silveira E.R., Gramosa N.V., Rao V.S.N. Toxicon. 2002;40:1231. doi: 10.1016/s0041-0101(02)00128-9. [DOI] [PubMed] [Google Scholar]
- 65.Cavalcanti B.C., Costa-Lotufo L.V., Moraes M.O., Burbano R.R., Silveira E.R., Cunha K.M.A., Rao V.S.N., Moura D.J., Rosa R.M., Henriques J.A.P., Pessoa C. Food Chem. Toxicol. 2006;44:388. doi: 10.1016/j.fct.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y., Liu J. W., Jia W., Zhao A. H., Li T. Int. Immunopharmacol. 2005;5:1957. doi: 10.1016/j.intimp.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Liu J.J., Huang R.W., Lin D.J., Peng J., Zhang M.H., Pan X.L., Hou M., Wu X.Y., Lin Q., Chen F. Cancer Invest. 2006;24:136. doi: 10.1080/07357900500524371. [DOI] [PubMed] [Google Scholar]
- 68.Müller S., Tirapelli C.R., Oliveira A.M., Murillo R., Castro V., Merfort I. Phytochemistry. 2003;63:391. doi: 10.1016/s0031-9422(03)00073-6. [DOI] [PubMed] [Google Scholar]
- 69.Morris B.D., Foster S.P., Grugel S.R., Charlet L.D. J. Chem. Ecol. 2005;31:89. doi: 10.1007/s10886-005-0976-2. [DOI] [PubMed] [Google Scholar]
- 70.Aslan I., Kilic T., Goren A.C., Topcu G. Ind. Crops Prod. 2006;23:171. doi: 10.1016/j.indcrop.2005.05.006. [DOI] [Google Scholar]
- 71.Castellaro S.J., Dolan S.C., Macmillan J., Willis C. Phytochemistry. 1990;29:1823. [Google Scholar]
- 72.Britton R.A., Piers E., Patrick B.O. J. Org. Chem. 2004;69:3068. doi: 10.1021/jo030389j. [DOI] [PubMed] [Google Scholar]
- 73.Oliveira A.B., Hanson J.R., Takahashi J.A. Phytochemistry. 1995;40:439. [Google Scholar]
- 74.Silva E.A., Takahashi J.A., Boaventura M.A.D., Oliveira A.B. Phytochemistry. 1999;52:397. doi: 10.1016/s0031-9422(99)00219-8. [DOI] [PubMed] [Google Scholar]
- 75.Fraga B.M., Guillermo R., Hernández M.G. J. Nat. Prod. 2004;67:64. doi: 10.1021/np030363n. [DOI] [PubMed] [Google Scholar]
- 76.Henrick C.A., Jefferies P.R. Austr. J. Chem. 1964;17:915. [Google Scholar]
- 77.Mori K., Matsui M., Fujisawa N. Tetrahedron. 1968;24:3113. [Google Scholar]
- 78.Shiozaki M., Mori K., Matsui M. Agric. Biol. Chem. 1972;36:2539. [Google Scholar]
- 79.Fujita E., Ochiai M. J. Chem. Soc. Perkin Trans. I. 1977:1182. doi: 10.1039/p19770001182. [DOI] [Google Scholar]
- 80.Bellino A., Venturella P. J. Nat. Prod. 1988;51:1246. [Google Scholar]
- 81.Paquete L.A., Tsui H.C. Synlett. 1996;2:129. [Google Scholar]
- 82.Singh A.K., Bakshi R.K., Corey E. J. J. Am. Chem. Soc. 1987;109:6187. [Google Scholar]
- 83.Corey E.J., Perez A.G., Lazerwith S. E. J. Am. Chem. Soc. 1997;119:11769. [Google Scholar]
- 84.Costa F.B., Albuquerque S., Vichnewski W. Planta Med. 1996;62:557. doi: 10.1055/s-2006-957971. [DOI] [PubMed] [Google Scholar]
- 85.Vieira H.S., Takahashi J.A., Oliveira A.B., Chiari E., Boaventura M.A.D. J. Braz. Chem. Soc. 2002;13:151. [Google Scholar]
- 86.Boeck P., Sá M.M., Souza B.S., Cercená R., Escalante A.M., Zacchino S.A., Filho V.C., Yunes R.A. J. Braz. Chem. Soc. 2005;16:1360. [Google Scholar]
- 87.Alonso R., Gomis H., Taddei A., Sajo C. Lett. Drug Des. Discov. 2005;2:255. doi: 10.2174/1570180054038323. [DOI] [Google Scholar]
- 88.Tao J.C., Tian G.Q., Zhang Y.B., Fu Y.Q., Dai G.F., Wu Y. Chin. Chem. Let. 2005;16:1441. [Google Scholar]
- 89.Aoyagi Y., Nishioka Y., Tobe F., Hasuda T., Takeya K., Gui M.Y., Jin Y.R., Li X.W. Bioorg. Med. Chem. 2006;14:5802. doi: 10.1016/j.bmc.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 90.Batista R., García P.A., Castro M.A., Miguel del Corral J.M., Feliciano A.S., Oliveira A.B. J. Braz. Chem. Soc. 2007 in press. [Google Scholar]
- 91.Hanson J.R., Hitchcock P.B., Takahashi J.A. Phytochemistry. 1995;40:797. [Google Scholar]
- 92.Vieira H.S., Takahashi J.A., Boaventura M.A.D. Appl. Microbiol. Biotechnol. 2000;53:601. doi: 10.1007/s002530051663. [DOI] [PubMed] [Google Scholar]
- 93.Fraga B.M., Hernandez M.G., Garcia-Tellado F., Gonzales P., Perales A. Phytochemistry. 1993;34:133. [Google Scholar]
- 94.Fraga B.M., Gonzalez P., Guillermo R., Hanson J.R., Hernandez M.G., Takahashi J.A. Phytochemistry. 1994;37:717. doi: 10.1016/s0031-9422(00)90345-5. [DOI] [PubMed] [Google Scholar]
- 95.Fraga B.M., Hernandez M.G., Guillermo R. J. Nat. Prod. 1996;59:952. [Google Scholar]
- 96.Fraga B.M., Gonzalez P., Hernandez M.G., Suárez S. Tetrahedron. 2005;61:5623. [Google Scholar]
- 97.Monsalve L.N., Rosselli S., Bruno M., Baldessari A. Eur. J. Org. Chem. 2005;10:2106. [Google Scholar]
- 98.Oliveira B.H., Filho J.D.S., Leal P.C. J. Braz. Chem. Soc. 2005;16:210. [Google Scholar]
- 99.Dembitsky V. Chem. Biodiv. 2004;1:673. doi: 10.1002/cbdv.200490060. [DOI] [PubMed] [Google Scholar]
- 100.Lis H., Sharon N. Eur. J. Biochem. 1993;218:1. doi: 10.1111/j.1432-1033.1993.tb18347.x. [DOI] [PubMed] [Google Scholar]; Winterhalter P., Skouroumounis G.K. Adv. Biochem. Eng. 1997;55:73. doi: 10.1007/BFb0102063. [DOI] [PubMed] [Google Scholar]
- 101.Roode B.M., Franssen M.C.R., Van Der Padt A., Boom R.M. Biotechnol. Prog. 2003;19:1391. doi: 10.1021/bp030038q. [DOI] [PubMed] [Google Scholar]
- 102.Vetter J. Toxicon. 2000;38:11. doi: 10.1016/s0041-0101(99)00128-2. [DOI] [PubMed] [Google Scholar]
- 103.Hofmann R.W., Swinny E.E., Bloor S.J., Markham K.R., Ryan K.G., Campbell B.D., Jordan B.R., Fountain D. W. Ann. Bot. (London) 2000;86:527. doi: 10.1006/anbo.2000.1216. [DOI] [Google Scholar]
- 104.Ueda M., Yamamura S. Angew. Chem. 2000;39:1400. doi: 10.1002/(sici)1521-3773(20000417)39:8<1400::aid-anie1400>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 105.Román L.U., Torres J.M., Reyes R., Hernández J.D., Cerda-García-Rojas C.M., Joseph-Nathan P. Phytochemistry. 1995;39:1133. [Google Scholar]
- 106.Chung M.S., Suh H.J., Yoo W., Choi S.H., Cho Y.J., Cho Y.H., Kim C.J. Food Add. Contam. 2005;22:1087. doi: 10.1080/02652030500202092. [DOI] [PubMed] [Google Scholar]
- 107.Geuns J.M.C. Phytochemistry. 2003;64:913. doi: 10.1016/s0031-9422(03)00426-6. [DOI] [PubMed] [Google Scholar]
- 108.Pezzuto J.M. New Trends Nat. Prod. Chem. 1986:371. [Google Scholar]
- 109.Yodyinguasd V., Bunyawong S. Hum. Reprod. 1991;6:158. doi: 10.1093/oxfordjournals.humrep.a137251. [DOI] [PubMed] [Google Scholar]
- 110.Suttajit M., Vinitketkaummuen U., Meevatee U., Buddahsukh D. Environ. Health Perspect. 1993;101(supl. 3):53. doi: 10.1289/ehp.93101s353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matsui M., Matsui K., Kawasaki Y., Oda Y., Noguchi T., Kitagawa Y., Sawada M., Hayashi M., Nohmi T., Yoshihira K., Ishidate M., Sofuni T. Mutagenesis. 1996;11:573. doi: 10.1093/mutage/11.6.573. [DOI] [PubMed] [Google Scholar]
- 112.Xili L., Chengjiany B., Eryi X., Reiming S., Yuengming W., Haodong S., Zhiyian H. Food Chem. Toxicol. 1992;30:957. doi: 10.1016/0278-6915(92)90181-j. [DOI] [PubMed] [Google Scholar]
- 113.Toskulkao C., Chaturat L., Temcharoen P., Glinsukon T. Drug Chem. Toxicol. 1997;20:31. doi: 10.3109/01480549709011077. [DOI] [PubMed] [Google Scholar]
- 114.Wasuntarawat C., Temcharoen P., Toskulkao C., Mungkornkarn P., Suttajit M., Glinsukon T. Drug Chem. Toxicol. 1998;21:207. doi: 10.3109/01480549809011648. [DOI] [PubMed] [Google Scholar]
- 115.Vignais P.V., Duce E.D., Vignais P.M., Huet J. Biochim. Biophys. Acta. 1966;118:465. doi: 10.1016/s0926-6593(66)80090-5. [DOI] [PubMed] [Google Scholar]
- 116.Constantin J., Ishii-Iwamoto E.L., Ferraresi-Filho O., Kelmer-Bracht A.M., Bracht A. Braz. J. Med. Biol. Res. 1991;24:767. [PubMed] [Google Scholar]
- 117.Danielli B., Bombardelli E., Bonati A., Gabetta B. Phytochemistry. 1972;11:3501. [Google Scholar]
- 118.Schteingart C.D., Pomilio A.B. J. Nat. Prod. 1984;47:1046. doi: 10.1021/np50036a029. [DOI] [PubMed] [Google Scholar]
- 119.Stewart M.J., Steenkamp V. Therap. Drug Monitor. 2000;22:641. doi: 10.1097/00007691-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 120.Obatomi D.K., Blackburn R.O., Bach P.H. Arch. Toxicol. 2001;75:487. doi: 10.1007/s002040100272. [DOI] [PubMed] [Google Scholar]
- 121.Obermann H., Spiteller G. Chem. Ber. 1976;109:3450. doi: 10.1002/cber.19761091024. [DOI] [PubMed] [Google Scholar]
- 122.D´Ancona S., Magon M., Milanesi C., Santi E. Fitoterapia. 1989;60:509. [Google Scholar]
- 123.Hedin P.A. Bioregulators for Pest Control. American Chemical Society; Washington D.C.: 1985. p. 463. [Google Scholar]
- 124.Roeder E., Bourauel T., Meier U., Wiedenfeld H. Phytochemistry. 1994;37:353. doi: 10.1016/0031-9422(94)85060-7. [DOI] [PubMed] [Google Scholar]
- 125.Piacente S., Pizza C., De Tommasi N., De Simone F. Phytochemistry. 1996;41:1357. [Google Scholar]
- 126.Eichholzer J.V., Lewis I.A.S., Macleod J.K., Oelrichs P.B. Tetrahedron. 1981;37:1881. [Google Scholar]
- 127.Macleod J.K., Lewis I.A.S., Moeller P.D.R., Oelrichs P.B. J. Nat. Prod. 1990;53:1256. doi: 10.1021/np50071a018. [DOI] [PubMed] [Google Scholar]
- 128.Li X., Zhang D., Onda M., Konda Y., Iguchi M., Harigaya Y. J. Nat. Prod. 1990;53:657. doi: 10.1021/np50069a018. [DOI] [PubMed] [Google Scholar]
- 129.Abdel-Mogib M., Jakupovic J., Dawidar A.M., Metwally M.A., Abou-Elzahab M. Phytochemistry. 1990;29:2581. [Google Scholar]
- 130.Tan R.X., Hu Y.H., Liu Z.L. J. Nat. Prod. 1993;56:1917. [Google Scholar]
- 131.Shao Y., Bai N.S., Zhou B.N. Phytochemistry. 1996;42:783. [Google Scholar]
- 132.Wang L.Q., Qin G.W., Chen S.N., Li C.J. Fitoterapia. 2001;72:779. doi: 10.1016/s0367-326x(01)00333-1. [DOI] [PubMed] [Google Scholar]
- 133.He D.H., Otsuka H., Hirata E., Shinzato T., Bando M., Takeda Y. J. Nat. Prod. 2002;65:685. doi: 10.1021/np010620t. [DOI] [PubMed] [Google Scholar]
- 134.He D.H., Matsunami K., Otsuka H., Shinzato T., Aramoto M., Bando M., Takeda Y. Phytochemistry. 2005;66:2857. doi: 10.1016/j.phytochem.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 135.Konopleva M.M., Matlawska I., Wojcinska M., Ahmed A.A., Rybczynska M., Paszel A., Ohta S., Hirata T., Bylka W., Mabry T.J., Cannon J.F. J. Nat. Prod. 2006;69:394. doi: 10.1021/np050144x. [DOI] [PubMed] [Google Scholar]
- 136.Harinantenaina L., Kasai R., Yamasaki K. Chem. Pharm. Bull. 2002;50:1122. doi: 10.1248/cpb.50.1122. [DOI] [PubMed] [Google Scholar]
- 137.Harinantenaina L., Kasai R., Yamasaki K. Phytochemistry. 2002;61:367. doi: 10.1016/s0031-9422(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 138.Ybarra M.I., Borkosky S.A., Catalán C.A.N., Cerdas-García-Rojas C.M., Nathan P.J. Phytochemistry. 1997;44:479. [Google Scholar]
- 139.Kengen S.W.M., Luesink E.J., Stams A.J.M., Zehnder A.J.B. Eur. J. Biochem. 1993;213:305. doi: 10.1111/j.1432-1033.1993.tb17763.x. [DOI] [PubMed] [Google Scholar]
- 140.Bourquelot E. J. Pharm. Chim. 1914;10:361,393. [Google Scholar]
- 141.Fujimoto H., Nishida H., Ajisaka K. Agric. Biol. Chem. 1988;52:1345. [Google Scholar]
- 142.Gelo-Pujic M., Guibé-Jampel E., Loupy A., Trincone A. J. Chem. Soc. Perkin Trans. I. 1997;7:1001. [Google Scholar]
- 143.Koenigs W., Knorr E. Chem. Ger. 1901;34:957. doi: 10.1002/cber.190103401162. [DOI] [Google Scholar]
- 144.Igarashi K. Adv. Carbohydr. Chem. Biochem. 1977;34:243. [Google Scholar]
- 145.Stick R.V. Carbohydrates: the Sweet Molecules of Life. Academic Press; London: 2001. [Google Scholar]
- 146.Schmidt R.R. Angew. Chem. Int. Edit. Engl. 1986;25:212. doi: 10.1002/anie.198602121. [DOI] [Google Scholar]
- 147.Schmidt R.R., Kinzy W. Adv. Carbohydr. Chem. Biochem. 1994;50:21. doi: 10.1016/s0065-2318(08)60150-x. [DOI] [PubMed] [Google Scholar]
- 148.Koreeda M., Houston T.A., Shull B.K., Klemke E., Tuinman R.J. Synlett. 1995;1:90. [Google Scholar]
- 149.Babu B.S., Balasubramanian K.K. Tetrahedron Lett. 2000;41:1271. doi: 10.1016/s0040-4039(00)00996-5. [DOI] [PubMed] [Google Scholar]
- 150.Yadav J.S., Reddy B.V.S., Reddy K.B., Satyanarayana M. Tetrahedron Lett. 2002;43:7009. [Google Scholar]
- 151.Yadav J.S., Reddy B.V.S., Murthy C.V.S.R., Kumar G.M. Synlett. 2000;10:1450. [Google Scholar]
- 152.El Khadem H.S. Carbohydrate Chemistry – Monosaccharides and Their Oligomers. Academic Press; London: 1988. [Google Scholar]
- 153.Collins P.M., Ferrier R.J. Monosaccharides: Their Chemistry and Their Roles in Natural Products. John Wiley & Sons; Chichester: 1995. [Google Scholar]
- 154.Kren V., Martinková L. Curr. Med. Chem. 2001;8:1303. doi: 10.2174/0929867013372193. [DOI] [PubMed] [Google Scholar]
- 155.Brandley B.K., Swiedler S.J., Robbins P.W. Cell. 1990;63:861. doi: 10.1016/0092-8674(90)90487-y. [DOI] [PubMed] [Google Scholar]
- 156.Yuen C.T., Lawson A.M., Chai W.G., Larkin M., Stoll M.S., Stuart A.C., Sullivan F.X., Ahern T.J., Feizi T. Biochemistry. 1992;31:9126. doi: 10.1021/bi00153a003. [DOI] [PubMed] [Google Scholar]
- 157.Grillon C., Monsigny M., Kieda C. Glycobiology. 1990;1:33. doi: 10.1093/glycob/1.1.33. [DOI] [PubMed] [Google Scholar]
- 158.Fukunaga Y., Miyata T., Nakayasu N., Mizutani K., Kasai R., Tanaka O. Agric. Biol. Chem. 1991;53:1603. [Google Scholar]
- 159.Ohtani K., Aikawa Y., Ishikawa H., Kasai R., Kitahata S., Mizutani K., Doi S., Nakamura M., Tanaka O. Agric. Biol. Chem. 1991;55:449. [PubMed] [Google Scholar]
- 160.DuBois G.E., Dietrich J.F.L., McGarraugh G.V., Stephenson R.A. J. Med. Chem. 1981;24:1271. doi: 10.1021/jm00143a001. [DOI] [PubMed] [Google Scholar]
- 161.DuBois G.E., Stephenson R.A. J. Med. Chem. 1985;28:93. doi: 10.1021/jm00379a017. [DOI] [PubMed] [Google Scholar]
- 162.Batista R., Humberto J.L., Chiari E., Oliveira A.B. Bioorg. Med. Chem. 2007;15:381. doi: 10.1016/j.bmc.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 163.Lemieux R.U., Bundle D.R., Baker D.A. J. Am. Chem. Soc. 1975;97:4076. doi: 10.1021/ja00847a035. [DOI] [PubMed] [Google Scholar]
- 164.Lubineau A., Gallic J., Lemoine R. J. Chem. Soc. Chem. Comm. 1993;111:1419. doi: 10.1039/c39930001419. [DOI] [Google Scholar]
- 165.Kovác P. J. Carbohyd. Chem. 1992;11:999. [Google Scholar]