Abstract
Bioguided fractionation of the ethanol extracts obtained from Platycodon grandiflorum roots led to isolation of two new triterpenoid saponins, characterized as 3-O-β-D-glucopyranosyl-2β,12α,16α,23,24-pentahydroxyoleanane-28(13)-lactone (1) and 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-2β,12α,16α,23α-tetrahydroxyoleanane-28(13)- lactone (2) by 1D- and 2D-NMR and MS techniques, as well as chemical means. Both compounds showed cytotoxic activity against human ECA-109 cells.
Keywords: Platycodon grandiflorum, Triterpenoid saponins, cytotoxic activity
Introduction
The roots of Platycodon grandiflorum have been used as a traditional Chinese medicine as an antiphlogistic, antitussive and expectorant [1]. This plant is well known to be abundant in triterpenoid saponins, and 55 such compounds have been isolated from the genus [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Platicodins have been found to exhibit varied biological activities, including anti-inflammatory, inhibition of prostaglandin E2 production, inhibitory effects on pancreatic lipase, antiobesity and hypolipidemic effects, apoptosis induction, inhibition of inducible nitric oxide synthase and cyclooxygenase II, antitumor and immuno-modulatory properties [19,20,21,22,23,24,25,26,27,28,29,30,31,32]. As part of our chemical studies on naturally occurring bioactive saponins, we report herein the isolation and characterization of two new saponins obtained from the ethanol extracts of the roots of this medicinal plant, as well as their cytotoxic activity.
Results and Discussion
Structure Elucidation
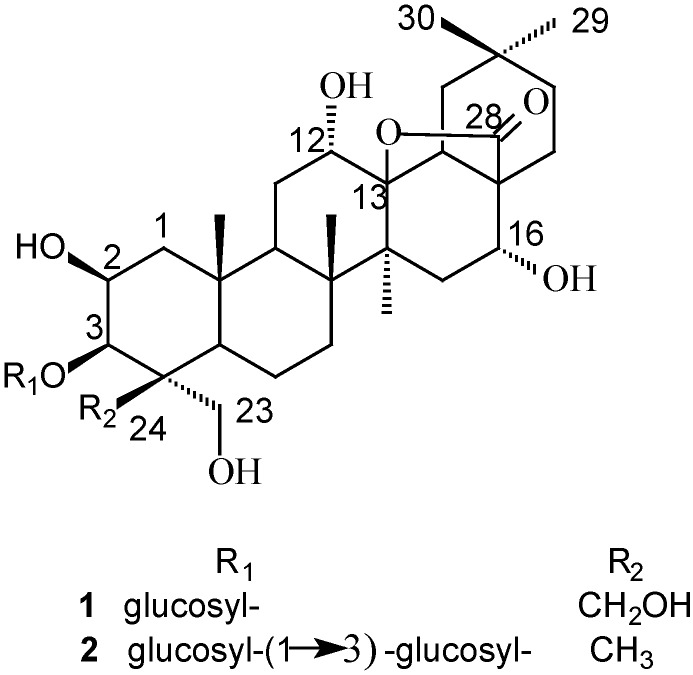
The combined 25% EtOH and 75% EtOH fractions eluted from a D101 macroporous resin column showed significant cytotoxic activity against human ECA-109 cells. Bioguided fractionation of the active fractions over silica gel eluting with a 95:5:0.5~50:50:5 CHCl3-CH3OH-H2O gradient led to the isolation of two triterpenoid saponins 1-2 (Figure 1).
Figure 1.
Structures of compounds 1 and 2.
Compound 1 was obtained as a white powder with mp 222~223°C (CH3OH) and : +11.76° (CH3OH; c 0.0170), that gave positive Libermann-Burchard and Molisch tests. The molecular formula was determined as C36H58O13, on the basis of its HRESIMS, which showed a [M+Na]+ quasi-molecular ion peak at m/z 721.3771 (calcd. for C36H58O13Na 721.3775). The spectral features and physicochemical properties suggested 1 to be a triterpenoid saponin. Five methyl groups (δ 1.09, 1.09, 1.34, 1.52 and 1.94) were observed in the 1H-NMR spectrum. The 13C-NMR spectrum of 1 (Table 1) showed 36 carbons, which were classified into five methyls, 11 methylenes, 12 methines and eight quaternary carbons by DEPT experiments. In addition, the 13C-NMR spectrum displayed five sp3 carbons at δ 18.4, 20.2, 21.0, 24.6 and 33.4, two oxygenated methylene carbons at δ 63.3 and 66.1, four oxygenated methine carbons at δ 69.2, 87.8, 66.1 and 72.1, and an oxygenated quaternary carbon at δ 92.8, attributable to the aglycone moiety. The NMR information thus indicated that 1 possessed a heptaoxygenated oleanane aglycone. D-glucose was detected by GC analysis after acidic hydrolysis and preparation of the corresponding thiazolidine derivative [33]. The presence of a β-glucose moiety was concluded based on a typical doublet peak of the anomeric proton of the glucose at δ 5.14 (1H, d, J=7.7 Hz) in the 1H-NMR, as well as the 13C-NMR signals at δ 106.5, 75.4, 78.9, 71.8, 78.9 and 62.7 of one glucose unit. In addition, the A-ring carbon signals of 1 were almost the same as those of a known compound, 3-O-β-D-glucopyranosylplatycodigenin methyl ester [10], indicating that the A-ring of 1 contained 2β,23,24-trihydroxyl and 3β-O-β-D-glucopyranosyl substituents. The 13C-NMR signals at δ 92.8 and 178.1 revealed the existence of a lactone moiety in its structure. Based upon the above findings, it was deduced that 1 was a 3-O-β-D-glucopyranosyl-2β,3β,23,24-tetrahydroxy oleanane with another three oxygenated carbons, one (δC 92.8) of which was due to the lactone moiety.
Table 1.
13C- (100 MHz) and 1H- (400 MHz) NMR Data of 1 and 2 (pyridine-d5) a.
| Position | 1 | 2 | ||
|---|---|---|---|---|
| C | H (J, Hz) | C | H (J Hz) | |
| 1 | 45.8 | 2.48, d, (7.0) | 44.6 | 2.41, d, (7.2) |
| 2.87, d, (7.9) | 2.85, d, (7.6) | |||
| 2 | 69.2 | 4.78, m | 70.8 | 4.78, m |
| 3 | 87.8 | 4.68, m | 82.8 | 4.32, m |
| 4 | 48.4 | 43.2 | ||
| 5 | 46.1 | 0.92, dd, (6.6, 7.0) | 47.9 | 0.83, dd, (6.4, 6.9) |
| 6 | 18.9 | 17.4 | ||
| 7 | 35.3 | 35.0 | ||
| 8 | 42.8 | 42.8 | ||
| 9 | 45.3 | 2.20, dd, (7.2, 7.8) | 45.1 | 2.22, dd, (7.3, 8.0) |
| 10 | 37.5 | 36.7 | ||
| 11 | 30.5 | 1.84, m | 30.2 | 1.87, m |
| 2.37, m | 2.40, m | |||
| 12 | 66.1 | 4.54, m | 66.2 | 4.46, m |
| 13 | 92.8 | 92.6 | ||
| 14 | 43.3 | 43.1 | ||
| 15 | 39.8 | 2.43, m | 39.8 | 2.37, m |
| 16 | 72.7 | 4.51, m | 72.7 | 4.43, m |
| 17 | 49.1 | 49.0 | ||
| 18 | 53.3 | 53.2 | ||
| 19 | 40.8 | 40.7 | ||
| 20 | 32.0 | 31.9 | ||
| 21 | 35.8 | 2.54, dd, (7.0, 7.6) | 35.7 | 2.48, dd, (7.0, 7.5) |
| 22 | 29.0 | 2.07, dd, (7.0, 15.5) | 29.0 | 2.02, dd, (7.0, 15.0) |
| 2.26, dd, (7.6, 15.5) | 2.30, dd, (7.5, 15.0) | |||
| 23 | 63.3 | 4.30, m | 65.0 | 4.10, m |
| 4.86, m | 4.52, m | |||
| 24 | 66.1 | 4.24, m | 14.9 | 1.31, s |
| 4.75, m | ||||
| 25 | 20.2 | 1.52, s | 18.8 | 1.51, s |
| 26 | 18.4 | 1.34, s | 18.5 | 1.30, s |
| 27 | 21.0 | 1.94, s | 21.1 | 1.89, s |
| 28 | 178.1 | 178.1 | ||
| 29 | 33.4 | 1.09, s | 33.4 | 1.01, s |
| 30 | 24.6 | 1.09, s | 24.5 | 1.01, s |
| 1′ | 106.5 | 5.14, d, (7.7) | 105.6 | 5.14, d, (7.0) |
| 2′ | 75.4 | 3.80 | 75.3 | 3.82 |
| 3′ | 78.9 | 4.05 | 88.8 | 3.93 |
| 4′ | 71.8 | 3.96 | 69.7 | 3.97 |
| 5′ | 78.9 | 3.87 | 77.9 | 3.86 |
| 6′ | 62.7 | 4.04, 4.18 | 62.6 | 4.05, 4.19 |
| 1″ | 106.0 | 5.22, d, (6.6) | ||
| 2″ | 75.6 | 3.77 | ||
| 3″ | 78.8 | 4.02 | ||
| 4″ | 71.7 | 3.94 | ||
| 5″ | 78.3 | 3.85 | ||
| 6″ | 62.3 | 3.99, 4.15 | ||
a Chemical shifts (δ) given in ppm
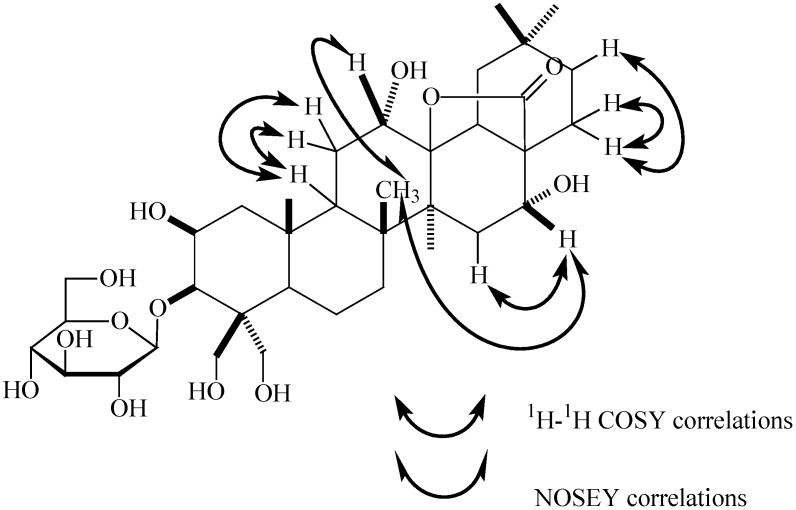
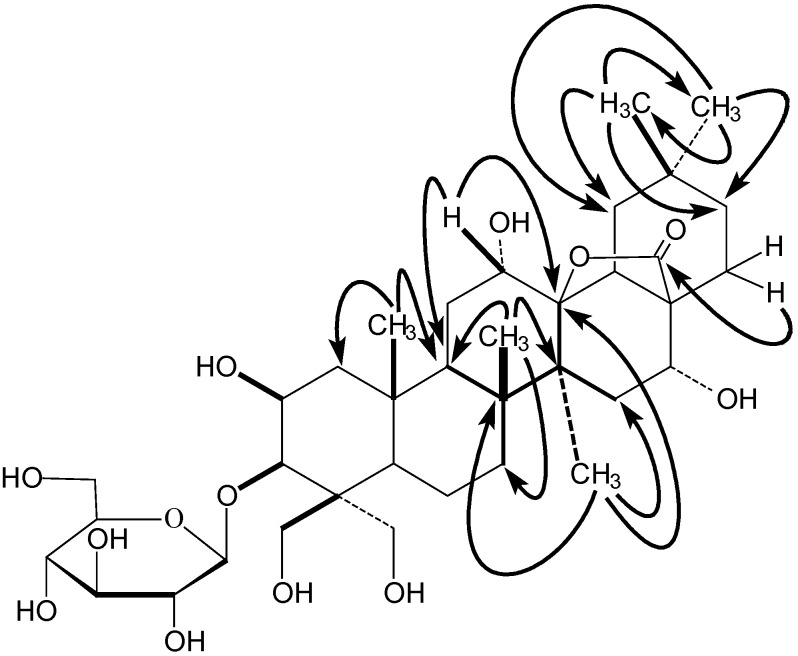
The positions of the other substituents were determined by its 1H-1H COSY (Figure 2) and HMBC (Figure 3) spectra and literature data. The correlations between 25-CH3 (δH 1.52) and δC 45.8 (C-1), 45.3 (C-9) could be observed in the HMBC spectrum of 1. Whilst δH 2.20 (1H, dd, J=7.2, 7.8 Hz, H-9) had correlations in the 1H-1H COSY spectrum with δH 2.37 and 1.84, corresponding to the same carbon signal at δC 30.5(C-11) on the basis of its HMQC spectrum. A second methyl signal at δH 1.34 (26-CH3) could also be seen in the HMBC spectrum to correlate with δC 45.3 (C-9), 35.3 (C-7), 42.8 (C-8) and 43.3 (C-14), which confirmed the 26-CH3 location. Thus another methyl signal correlating with C-8 (δC 42.8) at δH 1.94 in the HMBC spectrum must be located at C-27.
Figure 2.
Key correlations in the 1H-1H COSY and NOESY spectra of 1.
Figure 3.
Key correlations in the HMBC spectrum of 1.
The HMBC spectrum also displayed correlations of the 27-CH3 (δH 1.94) with δC 39.8 (C-15) and 92.8 (C-13), indicating that the oxygenated carbon signal due to the lactone moiety (δC 92.8) could be assigned to C-13. The HMBC correlations between the two methyl signals (δH 1.09) and δC 35.8 (C-21), 40.8 (C-19), 32.0 (C-20), 24.6 (C-30) and 33.4 (C-29), respectively, indicated that these two methyl groups were located at the same carbon with the signal at δC 32.0 (C-20). The proton signal at δH 2.54 (H-21) had correlations with δH 2.26 and 2.07 (both H-22) in the 1H-1H COSY spectrum. In addition, the correlations between δH 2.07(H-22) and δC 178.1(C-28) could also be observed in its HMBC spectrum, indicating that the carbonyl group of the lactone was located at C-28. Thus, it could be deduced that the lactone should be 28→13, a fact that was also be proved by comparison with the known oleanderolide-(lactone 28→13) [34]. The proton signal at δH 4.54, corresponding to an oxygenated methine carbon signal at δC 66.1 in the HMQC experiment, was observed in the HMBC to correlate with both δC 92.8 (C-13) and 45.3(C-9), showing that this proton should be located at C-12 and a hydroxyl group substitutent must exist at C-12. Correlations between H-15 (δH 2.43) and δH 4.51 (H-16) corresponding to the oxygenated methine carbon signal at δC 72.7 could be seen from the 1H-1H COSY data and were confirmed by the HMQC spectrum, indicating another hydroxyl group was located at C-16.
The configurations of the12,16-OH were determined by the NOESY spectrum (Figure 2). The presence of a NOESY effect between δH 4.54 (H-12) and δH 1.34 (26β-CH3), but the absence of any NOESY effect between δH 4.54 (H-12) and δH 1.94 (27α-CH3) indicated the α-configuration of 12-OH. In addition, the same result was also obtained in the NOESY spectrum for H-16 at δH 4.51. Therefore, the structure of 1 was identified as 3-O-β-D-glucopyranosyl-2β,12α,16α,23,24-pentahydroxy-oleanane- 28(13)-lactone, which was a new compound.
Compound 2 was isolated as white powder with mp 212~213°C (CH3OH) and : +31.71° (CH3OH; c 0.0410), giving positive Libermann-Burchard and Molish test results. The [M+Na]+ quasi-molecular ion peak at m/z 867.4352 (calcd. for C42H68O17Na 867.4354). The 1H-NMR spectrum gave six methyl groups (δ 1.01, 1.01, 1.30, 1.31, 1.51 and 1.89), corresponding to six sp3 carbons at δ 14.9, 18.5, 18.8, 21.1, 24.5 and 33.4, respectively in the 13C-NMR spectrum. In addition, all spectral features and physicochemical properties revealed that 2 might also be a triterpenoid saponin. The 13C-NMR spectrum of 2 showed aglycone signals that were broadly similar to those of 1, except those attributable to the A-ring carbons (see Table 1). Consequently, 2 was assumed to have an aglycone with some differences in the A-ring compared to 1. Among the A-ring carbon signals of 2, it could be observed that they were almost identical to those of methyl 3-O-β-laminaribiosylpolygalacate [10], suggesting that 2 had the same A-ring moiety as the latter. Thus, the aglycone of 2 was deduced to be 2β,3β,12α,16α,23α-pentahydroxyoleanane-28(13)-lactone. The sugar sequences of the disaccharide chain were determined by its spectrometric data. The negative mode ESI-MS, showing a quasi-molecular ion peak at m/z 843 [M-H]-, and the fragment ion peaks at m/z 681 [M-162(glucose)-H]-, 519 [681-162 (glucose)]-, indicated the presence of two glucose units. The 1H-NMR of 2 displayed two sugar anomeric protons at δH 5.14 (1H, d, J=7.0 Hz, H-1′) and δH 5.22 (1H, d, J=6.6 Hz, H-1″), respectively, coupling to two groups of sugar carbon signals in the 13C-NMR at δC 105.6 (C-1′), 75.3 (C-2′), 88.8 (C-3′), 69.7 (C-4′), 77.9 (C-5′), 62.6 (C-6′) and δC 106.0 (C-1″), 75.6 (C-2″), 78.8 (C-3″), 71.7 (C-4″), 78.3 (C-5″), 62.3 (C-6″) (Table 1). These findings confirmed the presence of two β-glucose units in this molecule. D-glucose was also detected by GC analysis after acid hydrolysis and preparation of the thiazolidine derivatives. The spin-systems associated with saccharides were identified by a HMQC-TOCSY experiment with the aid of a 1H-1HCOSY spectrum. All 1H- and 13C-NMR signals of the sugar moieties were assigned by HMQC experiment. The HMBC spectrum displayed correlations between the anomeric proton signal of the first D-glucose at δH 5.14 (H-1′) and C-3 of the aglycone at δC 82.8, suggesting the connection of this glucose at C-3. In addition, downfield chemical shifting of C-3′ by δC 88.8 together with the correlation between the anomeric proton signal of the second D-glucose at δH 5.22 (H-1″) and C-3′ (δC 88.8) were indicative of the existence of β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl disaccharide chain. Therefore, the structure of 2 was elucidated as 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-2β,12α,16α,23α-tetrahydroxyl- oleanane-28(13)-lactone, which was also a new compound.
Cytotoxic activity
The two new saponins identified in the present study were examined for their cytotoxic activity against the human Eca-109 cell line. Topotecan (IC50 0.032μg/mL) was used as a standard in the cytotoxic assay. The saponins exhibited cytotoxicity against human Eca-109 and gave IC50 values of 0.649μg/mL (1) and 0.503μg/mL (2), respectively.
Conclusions
Two new triterpenoid saponins, characterized as 3-O-β-D-glucopyranosyl-2β,12α,16α,23,24- pentahydroxy-oleanane-28(13)-lactone (1) and 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl- 2β,12α,16α,23α-tetrahydroxy-oleanane-28(13)-lactone (2). Each of them shows cytotoxic activity against human ECA-109 cells.
Experimental
General
All melting points were determined using a Fisher Johns apparatus and are uncorrected. Optical rotations were measured on a Perkin-Elmer 241 polarimeter. 1D- and 2D-NMR spectra were recorded on a Bruker 400 spectrometer. The ESIMS and HRESIMS were recorded in a LCQ DECA XP plus spectrometer. An Agilent 1100 series HPLC was used with a Zorbax SB-C18 preparative column.GC-MS was performed using a Shimadzu QP5050A instrument. Thin-layer chromatography employed precoated silica gel plates (Qingdao Haiyang). For column chromatography, silica gel (Qingdao Haiyang), D101 macroporous resin (Tianjin Nankai) and Sephadex LH-20 (Pharmacia) were used.
Plant Material
Roots of P. grandiflorum were collected from Hangzhou in the Zhejiang province of China and identified by Dr Lin Zhang (Institute of Modern Traditional Chinese Medicine, College of Pharmaceutical Sciences, Zhejiang University). A voucher specimen was deposited at the Institute of Modern Traditional Chinese Medicine, College of Pharmaceutical sciences, Zhejiang University, P.R. China.
Extraction and Isolation
Dried roots of P. grandiflorum (10 kg) were extracted twice with 95% EtOH and 50% EtOH, and the extracts were combined, then concentrated. The concentrates were submitted to D101 macroporous resin column chromatography eluting successively with water, 25% EtOH, 75% EtOH and 95% EtOH. The 25% EtOH and 75% EtOH fractions were combined and the solvent removed under reduced pressure to give a crude extract (197 g). The extract was chromatographed over silica gel, and eluted with a CHCl3-CH3OH-H2O gradient (95:5:0.5~50:50:5) to give 50 fractions. Fractions 24~26 (CHCl3-CH3OH-H2O, 80:20:2) were submitted to repeated column chromatography over silica gel with CHCl3-CH3OH-H2O and further purified on Sephadex LH-20 eluting with CH3OH to give compounds 1 (18 mg) and 2 (20 mg). For 1H- and 13C-NMR data see Table 1.
Acid Hydrolysis
Compound 1 and 2 (each 5 mg) were dissolved in water (100 mL) and 2M HCl (100 mL) and heated at 100 °C for 1 h. The water was passed through an Amberlite IRA-60E column (6×50 mm) and the eluates were concentrated. The residues were dissolved in pyridine (25 mL) and stirred with D-cysteine methyl ester (4.0 mg) for 1.5 h at 60 °C. To the reaction mixture, hexamethyldisilazane (10 mL) and trimethylsilyl chloride (10 mL) were added and the mixture was stirred for 30 min at 60 °C. The supernatants were then analyzed by GC [Column: DB-50, 25mm×30m, column temperature; 230 °C; carrier gas: N2, retention time D-Glc (16.4 min), L-Glc (16.0 min). From the new saponins, only D-glucose was detected.
Cytotoxicity Assays
Viability of ECA-109 cells in the presence or absence of experimental fractions or compounds was determined using the standard sulforhodamine B (SRB) assay as described previously [35]. Briefly, assays were carried out in 96-well plates. 4,000 cells/well were plated in media containing 5% FBS and were allowed to attach overnight. The cells were treated with 200 μL of media containing either 0.06% DMSO alone (control) or varying concentrations of test specimens dissolved in DMSO. The plates were incubated at 37 °C in a humidified incubator containing 5% CO2. The cells were fixed after 3 days by incubation in cold 50% TCA for 1 h at 4 °C in the dark. The media and TCA were removed and the plates were rinsed five times with water and then air-dried. The cells were stained by addition of 0.4% SRB (Sigma, St. Louis, MO) in 1% acetic acid (50 μL) for 5 min. The stain was removed and the cells were washed five times with 1% acetic acid, air-dried, and 150 μL of 10 mM unbuffered Tris was then added to each well to dissolve the dye. The plates were shaken for 5 min until the dye was uniformly distributed and then read on an Emax Precision Plate Reader (Molecular Devices, Sunnyvale, CA) at 570 nm. Media were used as the blank for these assays.
Acknowledgments
The authors expressed their gratitude to Mrs. Li-Ping Shi (Shanghai Institute of Organic Chemistry, China Academy of Sciences) for obtaining the 400 MHz NMR data. We were also grateful to Mr. Yu-Feng Zhang (Department of Chinese Medicine Science and Engineering, Zhejiang University) and Mr. Yuan-Bo Dai (Department of Chemistry, College of Science, Zhejiang University) for obtaining the ESI and HRESI data.
Footnotes
Sample availability: Available from the author.
References
- 1.Pharmacopoeia of the People’s Republic of China. Vol. I. Chemical Industry Press; Beijing: 2005. p. 196. [Google Scholar]
- 2.Akiyama T., Iitaka Y., Tanaka O. Structure of platicodigenin, a saogenin of Platycodon grandiflorum A. De Candolle. Tetrahedron Lett. 1968;53:5577–5580. doi: 10.1016/S0040-4039(00)75566-3. [DOI] [Google Scholar]
- 3.Fu W.W., Dou D.Q., Shimizu N., Takeda T., Fu R., Pei Y.H., Chen Y.J. Studies on the chemical constituents from the roots of Platycodon grandiflorum. J. Nat. Med. 2006;60:68–72. [Google Scholar]
- 4.Fu W.W., Hou W.B., Dou D.Q., Hua H.M., Gui M.H., Fu R., Chen Y.J., Pei Y.H. Saponins of polygalacic acid type from Platycodon grandiflorum. Acta Pharm. Sin. 2006;41:358–360. [PubMed] [Google Scholar]
- 5.Fu W.W., Shimizu N., Dou D.Q., Takeda T., Fu R., Pei Y.H., Chen Y.J. Five new triterpenoid saponins from the roots of Platycodon grandiflorum. Chem. Pharm. Bull. 2006;54:557–560. doi: 10.1248/cpb.54.557. [DOI] [PubMed] [Google Scholar]
- 6.Fu W.W., Shimizu N., Takeda T., Dou D.Q., Chen B.H., Pei Y.H., Chen Y.J. New A-ring lactone triterpenoid saponins from the roots of Platycodon grandiflorum. Chem. Pharm. Bull. 2006;54:1285–1287. doi: 10.1248/cpb.54.1285. [DOI] [PubMed] [Google Scholar]
- 7.He Z.D., Qiao C.F., Han Q.B., Wang Y., Ye W.C., Xu H.X. New triterpenoid saponins from the roots of Platycodon grandiflorum. Tetrahedron. 2005;61:2211–2215. doi: 10.1016/j.tet.2004.12.032. [DOI] [Google Scholar]
- 8.Ishii H., Tori K., Tozyo T., Yoshimura Y. Structures of Polygalacin-D and -D2, and Their Monoacetates, Saponins isolated from Platycodon grandiflorum A. DC., determined by Carbon-13 Nuclear Magnetic Resonance Spectroscopy. Chem. Pharm. Bull. 1978;26:674–677. [Google Scholar]
- 9.Ishii H., Tori K., Tozyo T., Yoshimura Y. Structures of platycodin-D3, platyconic acid -A, and their derivatives, saponins isolated from roots of Platycodon grandiflorum A. De Candolle, determined by carbon-13 NMR spectroscopy. Chem. Lett. 1978:719–722. [Google Scholar]
- 10.Ishii H., Tori K., Tozyo T., Yoshimura Y. Saponins from roots of Platycodon grandiflorum. Part 1. Structure of prosapogenins. J. Chem. Soc. Perkin Trans. 1. 1981:1928–1933. doi: 10.1039/p19810001928. [DOI] [Google Scholar]
- 11.Ishii H., Tori K., Tozyo T., Yoshimura Y. Saponins from roots of Platycodon grandiflorum. Part 2. Isolation and structure of new triterpene glycosides. J. Chem. Soc. Perkin Trans. 1984:661–668. [Google Scholar]
- 12.Kim Y.-S., Kim J.-S., Choi S.-U., Kim J.-S., Lee H.-S., Roh S.-H., Jeong Y.-C., Kim Y.-K., Ryu S.-Y. Isolation of a new saponin and cytotoxic effect of saponins from the root of Platycodon grandiflorum on human tumor cell lines. Planta Med. 2005;71:566–568. doi: 10.1055/s-2005-864161. [DOI] [PubMed] [Google Scholar]
- 13.Konishi T., Rada A., Shoji J., Kasai R., Tanaka O. The Structures of Platcodin A and C, Monoacetylated Saponins of the Roots of Platycodon grandiflorum A. DC. Chem. Pharm. Bull. 1978;26:668–670. doi: 10.1248/cpb.26.668. [DOI] [Google Scholar]
- 14.Kubota T., Kitatani H., Hinoh H. Structure of platycogenic acids A, B and C Further Trierpenoid Constituents of Platycodon grandiflorum. J. Chem. Soc. D. 1969;22:1313–1314. doi: 10.1039/c29690001313. [DOI] [Google Scholar]
- 15.Nikaido T., Koike K., Mitsunaga K., Saeki T. Tirterpenoid saponins from root of Platycodon grandiflorum. Nat. Med. 1998;52:54–59. [Google Scholar]
- 16.Nikaido T., Koike K., Mitsunaga K., Saeki T. Two New Triterpenoid Saponins from Platycodon grandflorum. Chem. Pharm. Bull. 1999;47:903–904. doi: 10.1248/cpb.47.903. [DOI] [PubMed] [Google Scholar]
- 17.Tada A., Kaneiwa Y., Shoji J., Shibata S. Studies on the saponins of the Roots of Platycodon grandiflorum A. De Candolle. I. Isolation and the Stricture of Platycodin-D. Chem. Pharm. Bull. 1975;23:2965–2972. doi: 10.1248/cpb.23.2965. [DOI] [PubMed] [Google Scholar]
- 18.Fu W.W., Dou D.Q., Zhao C.J., Shimizu N., Pei Y.P., Pei Y.H., Takeda T., Chen Y.J., Takeda T. Triterpenoid saponins from Platycodon grandiflurum. J. Asia Nat. Prod. Res. 2007;9:35–40. doi: 10.1080/10286020500289600. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y.-P., Lee E.-B., Kim S.-Y., Li D.-W., Ban H.-S., Lim S.-S., Shin K.-H., Ohuchi K. Inhibition of Prostaglandin E2 Production by Platycodin D Isolated from the Root of Platycodon grandiflorum. Planta Med. 2001;67:362–364. doi: 10.1055/s-2001-14317. [DOI] [PubMed] [Google Scholar]
- 20.Kim J.-Y., Hwang Y.-P., Kim D.-H., Han E.-H., Chung Y.-C., Roh S.-H., Jeong H.-G. Inhibitory effect of the saponins derived from roots of Platycodon grandiflorum on carrageenan-induced inflammation. Biosci. Biotech. Biochem. 2006;70:858–864. doi: 10.1271/bbb.70.858. [DOI] [PubMed] [Google Scholar]
- 21.Kim J.-Y., Kim D.-H., Kim H.-G., Song G.-Y., Chung Y.-C., Roh S.-H., Jeong H.-G. Inhibition of tumor necrosis factor-alpha-induced expression of adhesion molecules in human endothelial cells by the saponins derived from roots of Platycodon grandiflorum. Toxicol. Appl. Pharmacol. 2006;210:150–156. doi: 10.1016/j.taap.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Kubo A., Sasada M., Yamamoto K., Nishiyama H., Nishimura T., Nakamura T., Uchino H. Immune Pharmacological Studies on Platycodi Radii (I). Effect on the Phagocytosis in Mouse. Shoyakugaku Zasshi. 1986;40:367–374. [Google Scholar]
- 23.Lee K.-J., Kim J.-Y., Choi J.-H., Kim H.-G., Chung Y.-C., Roh S.-H., Jeong H.-G. Inhibition of tumor invasion and metastasis by aqueous extract of the radix of Platycodon grandiflorum. Food Chem. Toxico. 2006;44:1890–1896. doi: 10.1016/j.fct.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Shin C.-Y., Lee W.-J., Lee E.-B., Choi E.-Y., Ko K.-H. Platycodin D and D3 Increase Airway Mucin Release in vivo and in vitro in Rats and Hamsters. Planta Med. 2002;68:221–225. doi: 10.1055/s-2002-23130. [DOI] [PubMed] [Google Scholar]
- 25.Takagi K., Lee E.-B. Pharmacological Studies on Platycodon grandiflorum A.DC. I. Acute Toxicity and Central Depressant Activity of Crude Platycodin. Yakugaku Zasshi. 1972;92:951–960. doi: 10.1248/yakushi1947.92.8_951. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama H., Hiai S., Oura H. Rat Plasma Corticosterone Secretion- inducing Activities of Total Saponin and Prosapogenin Methyl Esters from the Roots of Platycodon grandiflorum A.DC. Yakugaku Zasshi. 1982;102:1191–1194. doi: 10.1248/yakushi1947.102.12_1191. [DOI] [PubMed] [Google Scholar]
- 27.Xu B.J., Han L.K., Zheng Y.N., Lee J.-H., Sung C.-K. In vitro inhibitory of triterpenoidal saponins from platycodi radix on pancreatic lipase. Arch. Pharm. Res. 2005;28:180–185. doi: 10.1007/BF02977712. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H.L., Kim Y.-S. Determination of kinetic properties of platycodin D for the inhibition of pancreatic lipase using a 1,2-diglyceride-based colorimetric assay. Arch. Pharm. Res. 2004;27:1048–1052. doi: 10.1007/BF02975430. [DOI] [PubMed] [Google Scholar]
- 29.Zhao H.L., Sim J.-S., Shim S.H., Ha Y.W., Kang S.S., Kim Y.S. Antiobese and hypolipidemic effects of platicodin saponins in diet-induced obese rats: evidences for lipase inhibition and calorie intake restriction. Int. J. Obesity. 2005;29:983–990. doi: 10.1038/sj.ijo.0802948. [DOI] [PubMed] [Google Scholar]
- 30.Zhao H.L., Cho K.-H., Ha Y.W., Jeong T.-S., Lee W.S., Kim Y.S. Cholesterol-lowering effect of platycodin D in hypercholesterolemic ICR mice. Eur. J. Pharmacol. 2006;537:166–173. doi: 10.1016/j.ejphar.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 31.Ahn K.S., Hahn B.-S., Kwack K.B., Lee E.B., Kim Y.S. Platycodin D-induced apoptosis through nuclear factor-κB activation in immortalized keratinocytes. Eur. J. Pharmacol. 2006;537:1–11. doi: 10.1016/j.ejphar.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Ahn K.S., Noh E. J., Zhao H.L., Jung S.H., Kang S.S., Kim Y.S. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-κB activation in RAW 264.7. Life Sci. 2005;76:2315–2328. doi: 10.1016/j.lfs.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 33.Hara S., Okabe H., Mihashi K. Separation of aldose enantiomers by gas-liquid chromatography. Chem. Pharm. Bull. 1986;34:1843–1844. doi: 10.1248/cpb.34.1843. [DOI] [Google Scholar]
- 34.Li W.-F., Zhang S.-J., Li N., Wang M.-Z., Sakai J., Hasegawa T., Mitsui T., Kataoka T., Oka S., Kiuchi M., Hirose K., Ando M. Three new triterpenes from Nerium oleander and biological activity of the isolated compounds. J. Nat. Prod. 2005;68:198–206. doi: 10.1021/np040072u. [DOI] [PubMed] [Google Scholar]
- 35.Guido F., Pauli The cardenolide of Speirantha convallarioides. Planta Med. 1995;61:162–166. doi: 10.1055/s-2006-958039. [DOI] [PubMed] [Google Scholar]