Abstract
Dried leaves of Catharanthus roseus were extracted with aqueous acidic 0.1 M solution of HCl. Alkaloid-embonate complexes were obtained as precipitates by treating the extract with an alkaline (NaOH) solution of embonic acid (4,4’-methylene-bis-3-hydroxynaphtalenecarboxylic acid). The precipitate mainly consisted of catharanthine and vindoline embonates and it was directly used as the starting material for a semi-synthesis of the anti-cancer bisindole alkaloid vinblastine. The coupling reaction involved oxidation of catharanthine in aqueous acidic medium by singlet oxygen (1O2), continuously produced in situ by the reaction between H2O2 with NaClO. An excess of NaBH4 was used for the reduction step. Analysis of the reaction mixture indicated a maximum yield of 20% for vinblastine at pH 8.3, based on the initial amount of catharanthine concentration. Direct-injection electrospray ionization mass spectrometry in positive ion mode was used for the identification of vinblastine. The mass spectra of vinblastine were dominated by the corresponding protonated molecular ion [M+H]+ at m/z 811 and the characteristic fragment ions matched with those of the standard compound.
Keywords: Indole alkaloids, vinblastine, semi-synthesis, mass spectrometry, electrospray ionization
Introduction
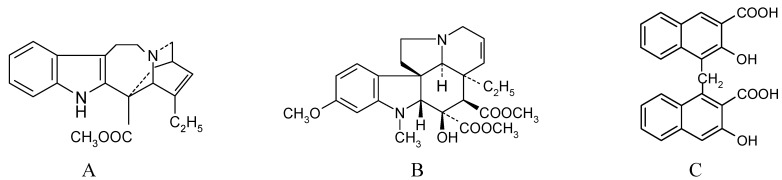
Catharanthus roseus (L.) G. Don is regarded as a rich source of pharmaceutically important terpenoid indole alkaloids. Vindoline and catharanthine are the major monomer alkaloids as well as biosynthetic precursors for the "dimeric" alkaloids, vinblastine and vincristine, two well known anti-cancer drugs used in the treatment of acute leukemia and Hodgkin’s disease [1]. Low "dimeric" alkaloid contents in the plant have encouraged intense research for alternative production methods involving cell cultures [2,3], metabolic engineering [4], semi-synthesis [5,6] or even total chemical synthesis [7]. Total synthesis has proved difficult due to structural complexity of the molecules and complicated reaction steps involving stereochemical constraints. Various semi-synthetic procedures have been developed for these alkaloids on the basis of chemical [5,6] or enzymatic [8] coupling of commercially available catharanthine and vindoline (Figure 1, A, B). As a means of simpler and economically feasible semi-synthesis of vinblastine and vincristine, a photochemical one pot synthesis has been proposed [9].
Figure 1.
Structures of A. catharanthine, B. vindoline and C. embonic acid, 4,4'-methylene-bis-3-hydroxynaphtalene carboxylic acid.
The present synthetic procedure and the reaction mechanism is partly based on the approach that has been described in detail in the photochemical method [9]. The two monomers are separated as stable complexes from the plant extract and directly used for the coupling reaction. High performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI-MS) provides a highly selective and sensitive means for identification of the alkaloids [10,11]. Direct-injection ESI-MS has also been used for rapid identification of Catharanthus alkaloids [12]. In this work compounds from the reaction mixture were analyzed and identified using HPLC-UV and the identification was confirmed by direct-injection ESI-MS/MS in positive ion mode. The aims of this work were i) to extract catharanthine and vindoline from a registered variety of C. roseus, ii) to precipitate catharanthine and vindoline as insoluble embonates from crude plant extract using alkaline solutions of embonic acid (4,4’-methylene-bis-3-hydroxynaphtalenecarboxylic aci, Figure 1, C) and iii) to carry out the synthesis of vinblastine and related dimeric alkaloids by directly using the above precipitate as starting material.
Results and discussion
The plant extract from C. roseus is a complex mixture of alkaloids with a wide range of polarities. The traditional solid-liquid extraction procedure for Catharanthus alkaloids from an aqueous acidic medium is based on their general basic properties. The alkaloids form salts in aqueous acidic media, showing improved solubility and enhanced stability at low pH values [13]. In addition, protons in the aqueous acidic media assist in breaking the sample matrix to release the analytes more easily. Water insoluble embonic acid complexes of catharanthine and vindoline were prepared by adding an aqueous alkaline (pH 10.5) solution of embonic acid to the aqueous acidic solution (pH 1.5) of the plant extract containing the alkaloids as their soluble hydrochloride salts. The alkaloids were exhaustively precipitated when pH 5 was reached during this process [14,15]. The precipitate most probably consists of complexes, since embonates from nitrogen compounds seem to lack clear crystalline structures [16]. The rather stable embonate complexes of catharanthine and vindoline were useful starting materials for vinblastine synthesis.
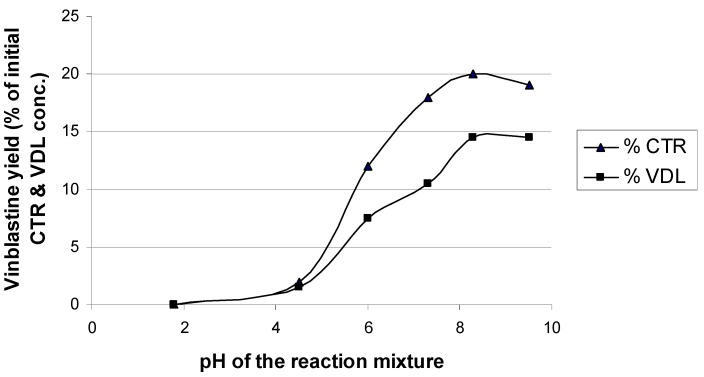
During the reaction process samples were collected at pH 4.5, 6.0, 7.3 8.3 and 9.5. A plot of vinblastine yield as a function of pH is shown in Figure 2. As the pH of the reaction mixture exceeds 5 the dissolution of embonic acid slowly increases. As a result, small amounts of catharanthine and vindoline are released for the coupling reaction, which presumably proceed mainly on solid surfaces of the finely powdered complex particles. The yield of vinblastine is usually expressed as a percentage of the amount of catharanthine and vindoline [17] or vindoline [18] used in the reaction. Based on the initial percentage of the amount of catharanthine and vindoline used, moderate yields of 20 % and 14.5 %, respectively, were obtained when the pH of the reaction mixture was 8.3 (Table 1).
Figure 2.
Vinblastine yield as a function of pH in the reaction mixture. Each point represents the mean of three values (n = 3). CTR = catharanthine; VDL = vindoline.
Table 1.
Reaction conditions and yields of vinblastine in synthesis. (VLB = vinblastine; CTR = catharanthine; VDL = vindoline).
| Starting materials and reagents for synthesis reaction | Chemicals and reagents added during synthesis | Reaction temp (°C) | Reaction time (h) | Final pH | Yield of VLB based on % of initial concentrations of CTR VDL |
|
|---|---|---|---|---|---|---|
| 100 mg embonic acid precipitate + 10 mL 0.1 M HCL + 10 mL 0.1 M citric acid solution + 10 mL dichloromethane |
10 mL of 30% H2O2
+ 10 mL of 10% NaOCl + 10-20 mL of 0.1% solution of NaBH4 in methanol |
0 - (-5) | 0 | 1.8 | 0 | 0 |
| 3 | 4.5 | 2 | 1.5 | |||
| 3.5 | 6.0 | 12 | 7.5 | |||
| 4 | 7.3 | 18 | 10 | |||
| 4.5 | 8.3 | 20 | 14.5 | |||
| 5 | 9.5 | 19 | 14.5 | |||
In the present synthesis, the precipitation procedure is simple and economically feasible. Instead of commercial pure monomers, the process can be started with fresh plant material using aqueous extraction. The monomer alkaloids are exhaustively precipitated by simple complex formation and the wet precipitate is applicable for synthesis as well. In these preliminary experiments, relatively low yield could be expected. However, it can be improved by further optimization of the present reaction conditions using computer-assisted methods and the handling of dry compounds can be left at the final step of vinblastine purification. Such features are also valuable when considering the highly strict GMP and GLP requirements for pharmaceutical production of anti-cancer compounds.
The present semi-synthetic process is partially based on an earlier method involving photoactivation of catharanthine, as well as photolytic singlet oxygen production in an aqueous reaction solution [9]. In that method the high energy needed for singlet oxygen production from water, however, results in excessive catharanthine consumption by many side reactions.
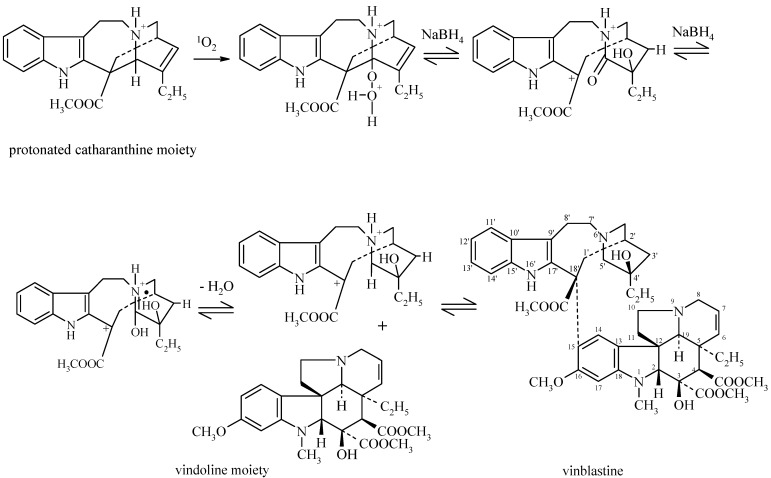
As an improvement to the above photochemical method, singlet oxygen (1O2) is here produced in situ from hydrogen peroxide and sodium hypochlorite [19]. Moreover, a sufficient degree of activation seems to be derived into the structure of catharanthine from complexation with embonic acid. Strong and specific hydrogen bonds between free carboxyl groups of embonic acid and pertinent structural positions in the catharanthine molecule possibly contribute to an electron arrangement in the latter structure to enable "dimerization" with vindoline in a concerted multi-centre reaction.
An allylic hydrogen in the protonated catharanthine moiety is presumably attacked by singlet oxygen to yield a hydroperoxide of catharanthine which then possibly undergoes reduction in the presence of sodium borohydride. The further reaction most probably proceeds through intermediate radical formation as depicted in Figure 3. Here a final aromatic substitution reaction yields vinblastine through the crucial C18'-C15 bond linking the top indole half of the catharanthine moiety (C18') and the bottom dihydroindole half of the vindoline moiety (C15). Vindoline is a relatively strong nucleophile in view of the electron density at C15 and / or C17 due to the methoxy group (-OCH3) present at C16. Of the two possible sites of reaction, C15 is clearly preferred in view of steric factors which should disfavour C17 [20,21].
Figure 3.
Proposed mechanism for the coupling reaction involved in vinblastine synthesis.
In the present process, further reaction enhancement might be provided by phase transfer catalysis in the interfacial layer between aqueous and organic solvents. Free embonic acid is insoluble in dichloromethane, as are the protonated monomer alkaloids from early stages of this process, but covalent bonding between catharanthine and vindoline presumably confer increased lipophilicity to the nascent "dimer" intermediate, compared to the monomeric species. This should result in better positioning of the reaction center towards the interface layer between the aqueous and organic phases. When acidity in the reaction medium was slowly reduced in this synthetic process, alkaloid amines were gradually transferred to organic phase, while embonic acid is released from complexation and precipitated, or dissolved in the aqueous phase as sodium embonate at higher pH-values.
Direct-injection electrospray ionization mass spectrometry in positive ion mode was used to confirm the identification of alkaloids in the reaction mixture. Under the conditions described in the experimental section, the positive ion mode ESI-MS spectrum showed the characteristic molecular ions [M+H]+ at m/z 337, 457, 793, 811, and 825 related to the molecular masses of catharanthine, vindoline, anhydrovinblastine, vinblastine and vincristine respectively. The mass spectra of vinblastine also showed diprotonated ion [M+2H]+2 at m/z 406. Multiple mass spectrometry (MS/MS) studies were performed by isolating [M+H]+ and applying a supplementary RF voltage (tickle voltage) of appropriate amplitude. The fragmentation pattern obtained for the molecular ions of vinblastine and vindoline matched exactly with those obtained for the standard compounds. However, the MS/MS fragment ions for catharanthine showed additional ions (320, 308, 293), which were not observed in the standard compound (Table 2). These ions were probably produced by other isomeric compounds of catharanthine like vindolinine and tabersonine, both having the same molecular mass of 336. Zhou et al. [12] have also shown that direct-injection ESI-MS/MS not only gives important information on the molecular masses of catharanthus alkaloids but also provides characteristic structural information that is useful in identifying the compounds.
Table 2.
Direct-injection ESI-MS/MS data for catharanthine, vindoline and vinblastine obtained by a Perkin-Elmer Sciex API 300 triple quadrupole instrument equipped with an ESI ion source.
| Alkaloid | Precursor ion [M+H]+ m/z | Major ions in MS/MS spectra of standard compounds | Major ions in MS/MS spectra of the compounds in reaction mixture |
|---|---|---|---|
| Catharanthine | 337 | 337, 173, 144 | 337, 320, 308, 293, 173, 144 |
| Vindoline | 457 | 457, 439, 397, 188 | 457, 439, 397, 188 |
| Vinblastine | 811 | 811, 793, 751, 733, 649, 542, 355, 337 | 811, 793, 751, 733, 680, 649, 542, 355, 337 |
Experimental
General
Embonic acid (4,4'-methylene-bis-3-hydroxynaphtalene carboxylic acid) was purchased from Merck-Schuchardt & Co (München, FRG). Acetonitrile, methanol (both HPLC-grade) and methyl t-butyl ether were from Rathburn (Walkerburn, Scotland); disodium hydrogen phosphate from Merck (Dramstadt, Germany); ammonia, hydrochloric acid and petroleum ether (b.p. 40-60 oC) from Reidel-de Haen Ag (Seelze, Germany). Vindoline and catharanthine were purchased from LKT Laboratories, Inc. (St. Paul, USA) and vinblastine sulphate from Sigma Chemical Co. (St. Louis, MO, USA). Water was obtained from the Milli-Q plus purification system (Millipore, Molsheim, France).
Extraction and precipitation of alkaloid-embonates
Homogenous dried leaves of a registered Finnish variety of C. roseus (1.0 g) were extracted for 30 minutes with 0.1 M hydrochloric acid solution (100 mL) in an ultrasonic bath (USF Finnsonic W 181, Ultra Sonic Finland). The mixture was then centrifuged at 2000 rpm for 10 min and the sediment was re-extracted with additional HCl (100 mL) for another 30 minutes. The combined supernatant from two repeated extractions was filtered and extracted with petroleum ether (200 mL) to eliminate chlorophyll and other lipophilic compounds. The acidic fraction was separated and an alkaline solution (pH 10.5) of 10 % embonic acid was slowly added for the precipitation of alkaloids as their embonate complexes. The pH of the resultant solution was increased to 5.0. The precipitate was separated simply by decantation and it was used as starting material for the semi-synthesis.
Semi-synthesis of vinblastine
The precipitate (100 mg) was mixed with 0.1 M hydrochloric acid (10 mL) and 0.1 M citric acid buffer (pH 2.2, 10 mL) was added. This mixture was cooled to 0 - (-5) oC using ice bath and dichloromethane (10 mL) was added. The oxidation-reduction reaction was initiated by stirring the mixture and slowly dropping in 30% aqueous hydrogen peroxide (10 mL), 10 % aqueous sodium hypochlorite (10 mL) and 0.1 % solution of sodium borohydride in methanol simultaneously over 3-5 h. The pH of the reaction mixture (measured by a pH meter) slowly increased from 1.8 to 9.5. As the pH increased, samples (1 mL) were stepwise collected from the organic layer and evaporated to dryness. The dry residue was dissolved in methanol (500 µL), filtered (0.45 µm filter) and 10 µL was used for HPLC analysis.
HPLC and MS analysis
The analysis was performed using a Waters (Milford, MA, USA) HPLC system comprising a Waters 2996 programmable photodiode array detector, Waters 717 autosampler and WatersTM 600 pump. The chromatographic data were recorded and processed using Waters Millenium32 software. The separations were carried out using a Hypersil BDS-C18 (Agilent) column (150 x 4.6 mm i.d, 5 µm particle size). The mobile phase, partially modified from Miura et al. [22], contained 5 mM Na2HPO4 x 2H2O buffer (pH adjusted to 7.3 by H3PO4)-acetonitrile-methanol in proportions of 60:23:17 for eluent A and 30:40:30 for eluent B, respectively. Eluent B was increased from 0 to 100% in 20 min linear gradient at the flow rate of 1.0 mL/min, maintained throughout the run. All injections were of 10 µL and the detection was performed at 214 nm. Vinblastine was identified by co-injecting the sample with the reference standard compound and comparing their retention times and UV spectra.
For mass spectrometric identification the samples were dissolved in freshly prepared solution of 5 mM ammonium acetate and analysed by a Perkin-Elmer Sciex API 300 (Sciex, Concord, Canada) triple quadupole instrument equipped with ESI ion source. Samples were introduced by direct injection with a Rheodyne (Cotati, CA, USA) 7725 injector with 10-µL sample loop. A microsyringe pump (Harvard Apparatus) was used to deliver the solvent to the ESI source with a flow rate of 8 µL/min. The capillary voltage was 5.0 kV in ESI positive ion mode measurements. Synthetic air (80% N2 and 20% O2, Oy AGA Ab, Finland) was used as nebulizing gas with a flow rate of 1.2 L/min. The mass range in ESI-MS experiments was m/z 100-900. Multiple mass spectrometry (MS/MS) studies were performed by isolating [M+H]+ and applying the supplementary RF voltage (tickle voltage) of appropriate amplitude. The fragmentation pattern was obtained for the molecular ions of vinblastine, catharanthine and vindoline by means of collisionally induced dissociation (CID) of the protonated molecule and compared with the similar data produced for the standard alkaloids under the same conditions.
Conclusions
Indole alkaloids from Catharahthus roseus were extracted into an aqueous acidic medium and precipitated as water insoluble embonate complexes using an alkaline solution of embonic acid (4,4’-methylene-bis-3-hydroxynaphtalenecarboxylic acid). Such complexes of catharanthine and vindoline seem to be useful as starting materials for the semi-synthesis of vinblastine. Under the given conditions the highest yield of vinblastine in the above procedure was 20 %, based on the initial amount of catharanthine, when the pH of the reaction mixture was increased to 8.3. However, parameters such as time of the reaction, temperature, pH, and the concentration of the reagents added during the reaction need further optimization for higher yields of vinblastine and related dimeric alkaloids.
Acknowledgments
The authors thank Tiia Kuuranne, PhD, Division of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Helsinki, for carrying out the LC-MS analysis of alkaloids.
Footnotes
Sample Availability: Contact the authors.
References
- 1.Noble R. L. The discovery of the vinca alkaloids-chemotherapeutic agents against cancer. Biochem. Cell Biol. 1990;68:1344–1351. [PubMed] [Google Scholar]
- 2.Verpoorte R., Contin A., Memelink J. Biotechnology for the production of plant secondary metabolites. Phytochem. Rev. 2002;1:13–25. doi: 10.1023/A:1015871916833. [DOI] [Google Scholar]
- 3.De Luca V., Cutler A. J. Subcellular localization of enzymes involved in indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 1987;85:1099–1102. doi: 10.1104/pp.85.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Luca V., St. Pierre B. The cell and developmental biology of alkaloid biosynthesis. Trends Plant Sci. 2000;5:168–173. doi: 10.1016/s1360-1385(00)01575-2. [DOI] [PubMed] [Google Scholar]
- 5.Goodbody A. E., Vukovic J. Production of alkaloid dimers using ferric ion. WO88/02002. PCT Pat. 1988
- 6.Kutney J. P., Choi L. S. L., Nakano J., Tsukamoto H., Boulet C. A., McHugh M. Process of synthesis of vinblastine and vincristine. 5047528. U.S. Pat. 1991
- 7.Kuehne M. E., Matson P. A., Bornmann W. G. Enantioselective synthesis of Vinblastine, Leurosidine, Vincovaline and 20′ -epi-Vincovaline. J. Org. Chem. 1991;56:513–528. doi: 10.1021/jo00002a008. [DOI] [Google Scholar]
- 8.Moreno P. R. H., Van der Heijden R., Verpoorte R. Cell and tissue cultures of Catharanthus roseus: A literature survey II. Updating from 1998 to 1993. Plant Cell Tissue Organ Cult. 1995;42:1–25. [Google Scholar]
- 9.Pennanen S., Huhtikangas A. Photochemical one pot synthesis of vinblastine and vincristine. Photochem. Photobiol. 1990;51:515–518. doi: 10.1111/j.1751-1097.1990.tb01959.x. [DOI] [PubMed] [Google Scholar]
- 10.Chu I-H., Bodnar J. A., Bowman R. N., White E. L. Determination of vincristine and vinblastine in Catharanthus roseus plants by high performance liquid chromatography/ electrospray ionization mass spectrometry. J. Liq. Chrom. Rel. Technol. 1997;20:1159–1174. [Google Scholar]
- 11.Favretto D., Piovan A., Filippini R., Caniato R. Monitoring the production yields of vincristine and vinblastine in Catharanthus roseus from somatic embryogenesis. Semiquantitative determination by flow-injection electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2001;15:364–369. doi: 10.1002/rcm.239. [DOI] [PubMed] [Google Scholar]
- 12.Zhou H., Tai Y., Sun C., Pan Y. Rapid determination of vinca alkaloids by direct-injection electrospray ionization tandem mass spectrometry and confirmed by high-performance liquid chromatography. Phytochem. Anal. 2005;16:328–333. doi: 10.1002/pca.852. [DOI] [PubMed] [Google Scholar]
- 13.Goodbody A. E., Watson C. D., Chapple C. C. S., Vukovic J., Misawa M. Extraction of 3’,4’-Anhydrovinblastine from Catharanthus roseus. Phytochemistry. 1988;27:1713–1717. doi: 10.1016/0031-9422(88)80430-8. [DOI] [Google Scholar]
- 14.Merrell Toraude. Vincamine salt and its use as a pharmaceutical composition. 1361779. French Pat. 1974
- 15.Saesmaa T., Tötterman A. M. Dissolution studies of ampicillin embonate and amoxicillin embonate. J. Pharm. Biomed. Anal. 1990;8:61–65. doi: 10.1016/0731-7085(90)80007-C. [DOI] [PubMed] [Google Scholar]
- 16.Saesmaa T., Mäkelä T., Tanninen V.P. Physical studies of benzathine and embonate salts of some β-lactam antibiotics. Part I. X-ray powder diffractometric study. Acta Pharm. Fenn. 1990;99:157–162. [Google Scholar]
- 17.Goodbody A. E., Endo T., Vukovic J., Kutney J. P., Choi L.S.L., Misawa M. Enzymic coupling of catharanthine and vindoline to form 3’,4’-anhydrovinblastine by Horseradish Peroxidase. Planta Med. 1988;54:136–140. doi: 10.1055/s-2006-962371. [DOI] [PubMed] [Google Scholar]
- 18.Hirata K., Duangteraprecha S., Morihara E., Honda M., Akagi T., Nakae M., Katayama H., Miyamoto K. Biomimetic one-pot synthesis of vinblastine: NAD(P)H-mediated vinblastine synthesis from the product of FMN-mediated vindoline–catharanthine coupling under near-ultraviolet light. Biotechnol. Lett. 1997;19:53–57. [Google Scholar]
- 19.Aubry J. M. Search for singlet oxygen in the decomposition of hydrogen peroxide by compounds in aqueous solutions. J. Am. Chem. Soc. 1985;107:5844–5849. doi: 10.1021/ja00307a002. [DOI] [Google Scholar]
- 20.Kutney J. P., Beck J., Bylsma F., Cook J., Cretney W. J., Fuji K., Imhof R., Treasurywala A. M. Total syntheis of indole and dihydroindole alkaloids. Studies on the synthesis of bisindole alkaloids in the vinblastine- vincristine series. The chloroindolenine approach. Helv. Chim. Acta. 1975;58:1690–1719. doi: 10.1002/hlca.19750580622. [DOI] [PubMed] [Google Scholar]
- 21.Magnus P., Stamford A., Ladlow M. Synthesis of antitumor bisindole alkaloid vinblastine: Distereoselectivity of the crucial C-15 – C-18’ bond. J. Am. Chem. Soc. 1990;112:8210–8212. doi: 10.1021/ja00178a079. [DOI] [Google Scholar]
- 22.Miura Y., Hirata K., Kurano N. Isolation of vinblastine in callus culture with differentiated roots of Catharanthus roseus. Agric. Biol. Chem. 1987;51:611–614. doi: 10.1271/bbb1961.51.611. [DOI] [Google Scholar]