Abstract
A new acetylated flavanol, 3,7-O-diacetyl (–)-epicatechin (3), and seven known flavanol derivatives, (–)-epicatechin (1), 3-O-acetyl (–)-epicatechin (2), 3,3′,4′,5,7-O-pentaacetyl (–)-epicatechin (4), (+)-afzelechin (5), (+)-catechin (6), cinchonain Ib (7), and proanthocyanidin B2 (8), were isolated from the stems and twigs of the mangrove plant Rhizophora stylosa and identified. The crude extract, the different fractions and all of the purified compounds were evaluated for DPPH radical scavenging activity.
Keywords: Rhizophoraceae, Rhizophora stylosa, flavanol, radical scavenging activity
Introduction
Mangrove plants are well-known as a rich source of tannins. The bark of true mangrove plants contains about 1%~30% tannins [1]. The vegetable tannins or plant polyphenols group is comprised of two classes of compounds, the “hydrolysable” and the “condensed” tannins. Flavan-3-ols constitute the latter group [2]. Some species of the mangrove plant genus Rhizophora are used by the local people as folk medicines for many diseases. For example, the bark of R. mucronata is used for the treatment of diabetes in India [3], and when boiled in water it is used as an astringent for diarrhea, nausea, and vomiting and as an antiseptic in Thailand [4]. The extract of some species of this genus has been reported to have antifungal activity [5], antibacterial activity [6], anti-inflammatory activity [7], gastric antiulcer properties [8], efficacy in wound healing [9], and a protective role in naphthalene-induced mitochondrial dysfunction [10]. Furthermore, the major active principles of these pharmacological and biomedicinal properties are polyphenols that behave as antioxidants.
During our phytochemical investigation of Rhizophora stylosa Griff (Rhizophoraceae), one new acetylated flavanol, 3,7-O-diacetyl (–)-epicatechin (3), and seven known flavanol derivatives 1, 2, 4–8 were isolated from the stems and twigs of this plant and identified. The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of the crude extract, the different fractions, and all of the purified compounds were evaluated.
Results and Discussion
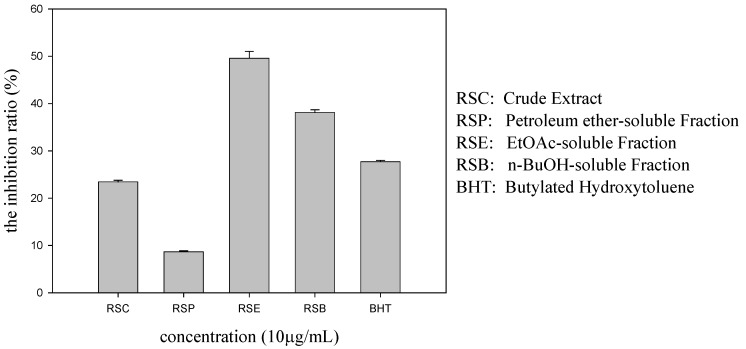
The antioxidant activity of the crude extract (RSC), the petroleum ether-soluble fraction (RSP), the EtOAc-soluble fraction (RSE), and the n-BuOH-soluble fraction (RSB) were determined using the DPPH radical assay, as described in our previous report [11]. As shown in Figure 1, RSE and RSB were demonstrated to be the most active fractions. Further phytochemical investigation of these two fractions resulted in the isolation and characterization of compounds 1–8. Each of these compounds displayed various levels of DPPH radical scavenging activity.
Figure 1.
The inhibition ratio (percentage) of extract and different fractions derived from R. stylosa (at 10 μg/mL) in DPPH radical scavenging assay. The results are given as means ± SD (n = 3).
Compound 3 was obtained as a yellow oil and its molecular formula was determined to be C19H18O8 by HR-ESI-MS ([M + Na]+ m/z 397.0893, calcd. for C19H18O8Na, 397.0899) which was in agreement with the 1H- and 13C-NMR spectral data. Its IR spectrum exhibited absorption bands at 3362 cm-1 for hydroxyl, at 1738 cm-1 for ester carbonyl, and at 1604 cm-1, 1519 cm-1 and 1443 cm-1 for the phenyl functionalities in the molecule. The 1H-NMR spectrum of 3 was characteristic of a flavanol, revealing the presence of five aromatic protons at δ 7.01 (1H, br s, H-2′), 6.81 (2H, br s, H-5′ and H-6′), 6.32 (1H, d, J = 2.4 Hz, H-8) and 6.25 (1H, d, J = 2.4 Hz, H-6); two oxymethine protons at δ 5.37 (1H, m, H-3) and 5.08 (1H, d, J = 6.2 Hz, H-2) and two methylene group protons at δ 2.97 (1H, d, J = 4.7, 17.4 Hz, H-4a) and 2.79 (1H, d, J = 2.2, 17.4 Hz, H-4b). Detailed inspection of the NMR spectral data revealed that the chemical structure of 3 was very similar to that of (–)-epicatechin (1) [12], the difference being the presence of two methyl signals at δ 2.25 (3H, s, OCOCH3) and 1.86 (3H, s, OCOCH3), which showed direct correlation with carbonyl carbon signals at δ 169.0 and 170.3, respectively, in the HMBC spectrum. These signals clearly indicated the presence of two acetoxyl groups in 3. The proton at δ 5.37 (H-3) showed a long-range correlation with the carbonyl carbon signal at δ 170.3 in the HMBC spectrum, indicating that one of the acetoxyl groups was attached to C-3. The second acetoxyl group was deduced to be attached at C-7, rather than at C-5, based on the fact that the 13C-NMR signal of C-7 was upfield shifted (to δ 151.6) in 3, compared to that of (–)-epicatechin (1) (δ 156.9). On the other hand, the chemical shift for the methylene carbon C-4 at δ 26.5 in 3 was similar to that of 3-O-acetyl (–)-epicatechin (2) (δ 26.4), indicating that the 5-OH group was not acetylated, otherwise, this signal would shift to a remarkably upfield position, i.e., δ 23.9, for a C-5 acetoxylated compound such as 3,3′,4′,5,7-O-pentaacetyl (–)-epicatechin (4). From the above evidence, the structure of compound 3 was determined to be 3,7-O-diacetyl (–)-epicatechin.
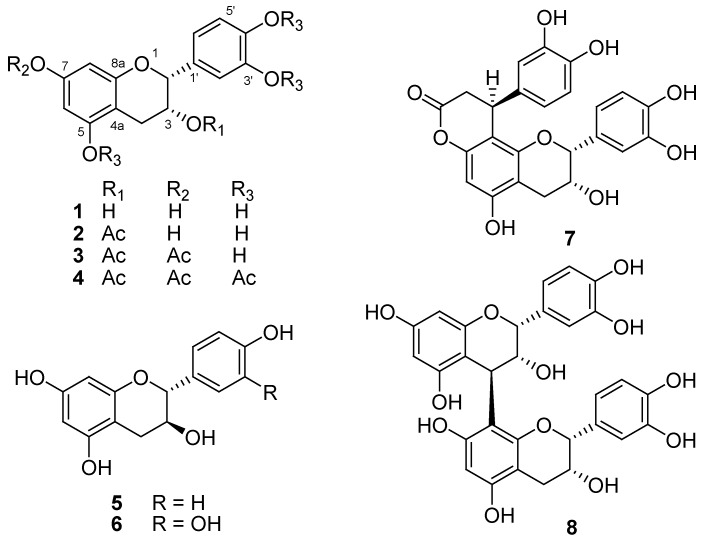
Figure 2.
The chemical structure of compounds 1-8.
The known compounds 1, 2, 4–8 were characterized as (–)-epicatechin (1) [12], 3-O-acetyl (–)-epicatechin (2) [13], 3,3′,4′,5,7-O-pentaacetyl (–)-epicatechin (4) [14], (+)-afzelechin (5) [15], (+)-catechin (6) [12], cinchonain Ib (7) [16], and proanthocyanidin B2 (8) [12], respectively, based on the detailed comparison of the 1H- and 13C-NMR spectral data with those of the literature reports. To the best of our knowledge, this is the first report of the isolation of compound 2 as a natural substance, as previously 2 had been obtained after hydrolysis of compound 4. This strategy was used to protect phenolic hydroxyl groups in modifications and synthesis of various bioactive natural products [13,17]. The 13C-NMR spectral data of compounds 2 and 4 are reported here for the first time (see Experimental).
DPPH Radical Scavenging Activity of isolated compounds
The DPPH radical scavenging activity of compounds 1-8 was presented in Table 1.
Table 1.
The IC50 (μg/mL) of compounds 1–8 in DPPH radical scavenging assay.
| Compound | Chemical name | IC50 (μg/mL) |
|---|---|---|
| 1 and 6 | (–)-epicatechin and (+)-catechin | 6.5 |
| 2 | 3-O-acetyl (–)-epicatechin | 15.0 |
| 3 | 3,7-O-diacetyl (–)-epicatechin | 25.4 |
| 4 | 3,3′,4′,5,7-O-pentaacetyl (–)-epicatechin | >1000 |
| 5 | (+)-afzelechin | 36.7 |
| 7 | cinchonain Ib | 7.8 |
| 8 | proanthocyanidin B2 | 4.3 |
| BHT | butylated hydroxytoluene | 18.0 |
The results revealed that except for compound 4, compounds 1–8 isolated from R. stylosa displayed DPPH radical scavenging activity comparable to that of the positive control butylated hydroxytoluene (BHT). Compound 8 showed the strongest activity, with IC50 4.3 μg/mL, four times more active than the positive control, BHT (IC50 18.0 μg/mL). The DPPH radical scavenging activities of these compounds seemed to be related to the number of phenol groups. Data analysis revealed that compounds having adjacent hydroxyl groups on the ring-B possessed higher activity, a fact confirmed by the literature [18]. From the above data, it can be deduced that the main components responsible for the antioxidant activities of R. stylosa extracts were the flavanol derivatives. Further work is in progress to assess whether this species could be used as a source of natural antioxidant for pharmaceutical and food applications.
Experimental
General
Optical rotations were obtained on JASCO P-1020 digital polarimeter. UV spectra were recorded on a PuXi TU-1810 UV-visible spectrophotometer. IR spectra were taken on a Nicolet 510P FT-IR spectrometer. NMR spectra were recorded on a Bruker Avance 500 MHz NMR spectrometer using TMS as internal standard and chemical shifts were recorded as δ values (500 MHz for 1H and 125 MHz for 13C). ESI-MS were measured on a VG AutoSpec 3000 mass spectrometer. Silica gel (200–300 mesh, Qingdao Haiyang Chemical Factory, Qingdao, China) and reversed-phase silica gel C18 (40-75 μm, Fuji Silysia Chemical Ltd) were used for open column chromatography (CC). Sephadex LH-20 (18-110 μm) was obtained from Pharmacia. Thin-layer chromatography (TLC) was performed on precoated 0.2 mm thick silica gel 60 GF254 plates (Qingdao Haiyang Chemical Factory, Qingdao, China); spots were detected by spraying with anisaldehyde–H2SO4 reagent, followed by heating.
Plant Material
The stems and twigs of Rhizophora stylosa Griff. were collected on Hainan Island on the South China coastline in July 2004 and authenticated by Prof. H. Peng at Kunming Institute of Botany, Chinese Academy of Sciences. A voucher specimen (0407A) was deposited at the Key Laboratory of Experimental Marine Biology of the Institute of Oceanology, Chinese Academy of Sciences.
Extraction and Isolation
The pulverized stems and twigs of R. stylosa (6.1 kg) were macerated three times with a mixture of CHCl3/MeOH (1:1) at room temperature for 5 days for each time. The combined extracts were concentrated and yielded 827 g of a crude extract (RSC). This extract was suspended in water and then was further extracted with petroleum ether, EtOAc, and n-BuOH to yield 41 g of petroleum ether-soluble fraction (RSP), 109 g of EtOAc-soluble fraction (RSE), and 244 g of n-BuOH-soluble fraction (RSB), respectively. The RSE fraction was subjected to a column chromatography (CC) over silica gel eluted with different solvents of increasing polarity (petroleum ether/acetone, 3:1 and 1:1; then CHCl3/MeOH, from 10:1 to 1:1) to yield 10 fractions (E-1~E-10) on the basis of TLC analysis. Fraction E-6 (1.0 g) was subjected to CC using petroleum ether-acetone (2:1) as an eluent to afford seven subfractions (E-6-1~E-6-7). E-6-6 (87.5 mg) was subjected to CC on Sephadex LH-20 using CHCl3/MeOH (1:1) as an eluent to afford compound 5 (34.3 mg). E-7 (6.8 g) was subjected to CC on silica gel using CHCl3/MeOH (12:1) as an eluent to afford four subfractions (E-7-1~E-7-9). E-7-7 (387.6 mg) was subjected to CC on Sephadex LH-20 using CHCl3/MeOH (1:1) as an eluent to afford four subfractions (E-7-7-1~E-7-7-4). E-7-7-2 (67.9 mg) was subjected to CC on silica gel using petroleum ether/acetone (4:1) as an eluent to afford compounds 2 (6.2 mg), 3 (3.2 mg), and 4 (25.7 mg). E-8 (9.6 g) was subjected to VLC on silica gel using CHCl3/MeOH (18:1, 10:1, 5:1, 4:1) as an eluent to afford four subfractions (E-8-1~E-8-4). E-8-3 was subjected to CC on Sephadex LH-20 using CHCl3/MeOH (1:1) as an eluent, followed by reversed-phase silica gel C18 chromatography using MeOH as an eluent to afford a mixture of compounds 1 and 6 (81.6 mg) and 7 (15.3 mg). The n-BuOH-soluble fraction was subjected to a column chromatography over silica gel eluted with CHCl3/ MeOH (5:1). The eluents were combined into 10 fractions (B-1–B-10). Fraction B-4 was subjected to Sephadex LH-20 chromatography using MeOH as an eluent to yield compound 8 (140.0 mg).
3-O-acetyl (–)-epicatechin (2): 13C-NMR (acetone-d6) δ: 170.3 (OCOCH3), 157.8 (C-7), 157.4 (C-8a), 157.0 (C-5), 145.6 (C-3′), 145.6 (C-4′), 131.3 (C-1′), 119.2 (C-6′), 115.6 (C-5′), 114.8 (C-2′), 98.8 (C-4a), 96.4 (C-6), 95.8 (C-8), 77.7 (C-2), 69.0 (C-3), 26.4 (C-4), 20.9 (OCOCH3).
3,7-O-diacetyl (–)-epicatechin (3): 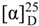 : – 6.85° (c 0.045, acetone); IR (KBr) cm–1: 3,362, 2,925, 1,738, 1,626, 1,604, 1,519, 1,443, 1,372, 1,234, 1,046; UV λmax (acetone) nm (log ε): 212 (2.79), 327 (2.20); 1H-NMR (acetone-d6) δ 7.01 (1H, br s, H-2′), 6.81 (2H, br s, H-5′ and H-6′), 6.32 (1H, d, J = 2.4 Hz, H-8), 6.25 (1H, d, J = 2.4 Hz, H-6), 5.37 (1H, m, H-3), 5.08 (1H, d, J = 6.2 Hz, H-2), 2.97 (1H, d, J = 4.7, 17.4 Hz, H-4a), 2.79 (1H, d, J = 2.2, 17.4 Hz, H-4b), 2.25 (3H, s, OCOCH3), and 1.86 (3H, s, OCOCH3); 13C-NMR (acetone-d6) δ: 170.3 (OCOCH3), 169.0 (OCOCH3), 157.7 (C-5), 156.9 (C-8a), 151.6 (C-7), 145.8 (C-3′),145.7 (C-4′), 130.8 (C-1′), 119.2 (C-6′), 115.7 (C-5′), 114.9 (C-2′), 104.6 (C-4a), 103.6 (C-8), 101.7 (C-6), 78.1 (C-2), 68.3 (C-3), 26.5 (C-4), 20.9 (OCOCH3), 20.8 (OCOCH3); ESI-MS: 413 [M + K]+, 397 [M + Na]+, 375 [M + H]+, 360, 316, 301, 274, 156, 141,116; HR-ESI-MS: m/z 397.0893 (calcd for C19H18O8Na, [M + Na]+, 397.0899).
: – 6.85° (c 0.045, acetone); IR (KBr) cm–1: 3,362, 2,925, 1,738, 1,626, 1,604, 1,519, 1,443, 1,372, 1,234, 1,046; UV λmax (acetone) nm (log ε): 212 (2.79), 327 (2.20); 1H-NMR (acetone-d6) δ 7.01 (1H, br s, H-2′), 6.81 (2H, br s, H-5′ and H-6′), 6.32 (1H, d, J = 2.4 Hz, H-8), 6.25 (1H, d, J = 2.4 Hz, H-6), 5.37 (1H, m, H-3), 5.08 (1H, d, J = 6.2 Hz, H-2), 2.97 (1H, d, J = 4.7, 17.4 Hz, H-4a), 2.79 (1H, d, J = 2.2, 17.4 Hz, H-4b), 2.25 (3H, s, OCOCH3), and 1.86 (3H, s, OCOCH3); 13C-NMR (acetone-d6) δ: 170.3 (OCOCH3), 169.0 (OCOCH3), 157.7 (C-5), 156.9 (C-8a), 151.6 (C-7), 145.8 (C-3′),145.7 (C-4′), 130.8 (C-1′), 119.2 (C-6′), 115.7 (C-5′), 114.9 (C-2′), 104.6 (C-4a), 103.6 (C-8), 101.7 (C-6), 78.1 (C-2), 68.3 (C-3), 26.5 (C-4), 20.9 (OCOCH3), 20.8 (OCOCH3); ESI-MS: 413 [M + K]+, 397 [M + Na]+, 375 [M + H]+, 360, 316, 301, 274, 156, 141,116; HR-ESI-MS: m/z 397.0893 (calcd for C19H18O8Na, [M + Na]+, 397.0899).
3,3′,4′,5,7-O-pentaacetyl (–)-epicatechin (4): 13C-NMR (CDCl3) δ: 170.1, 169.0, 168.3, 168.0, 168.0 (OCOCH3), 154.4 (C-8a), 149.8 (C-7), 149.4 (C-5), 142.1, 142.1 (C-3′, 4′), 136.1 (C-1′), 124.4 (C-6′), 123.7 (C-5′), 121.7 (C-2′), 110.2 (C-4a), 108.7 (C-6), 107.7 (C-8), 77.6 (C-2), 68.3 (C-3), 23.9 (C-4), 21.1, 20.9, 20.7, 20.6, 20.6 (OCOCH3).
DPPH Radical Scavenging Activity
The scavenging effects of samples on DPPH radicals were determined according to the method of literature [11]. Synthetic antioxidant BHT was used as positive control.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 30530080) and by the Department of Science and Technology of Shandong Province (No. 2006GG2205023). We wish to thank Prof. H. Peng at Kunming Institute of Botany of the Chinese Academy of Sciences for identifying the plant material. The Bairen Jihua program from the Chinese Academy of Sciences (awarded to B.-G. Wang) is also gratefully acknowledged.
Footnotes
Sample Availability: Samples of the compounds 1–8 are available from the authors.
References and Notes
- 1.Lin Y. M., Xiang P., Lin P. Studies on Tannins of Mangroves-A Review. Mar. Sci. 2005;29:59–63. [Google Scholar]
- 2.de Bruyne T., Pieters L., Deelstra H., Vlietinck A. Condensed Vegetable Tannins: Biodiversity in Structure and Biological Activities. Biochem. Syst. Ecol. 1999;27:445–459. [Google Scholar]
- 3.Anjaneyulu A. S. R., Anjaneyulu V., Rao V. L. New Beyerane and Isopimarane Diterpenoids from Rhizophora mucronata. J. Asian Nat. Prod. Res. 2002;4:53–61. doi: 10.1080/10286020290019703. [DOI] [PubMed] [Google Scholar]
- 4.Laphookhieo S., Karalai C., Ponglimanont C. New Sesquiterpenoid and Triterpenoids from the Fruits of Rhizophora mucronata. Chem. Pharm. Bull. 2004;52:883–885. doi: 10.1248/cpb.52.883. [DOI] [PubMed] [Google Scholar]
- 5.Cacéres A., López B. R., Juarez X., del Aguila J., García S. Plants Used in Guatemala for the Treatment of Dermatophytic Infections. 2. Evaluation of Antifungal Activity of Seven American Plants. J. Ethnopharmacol. 1993;40:207–213. doi: 10.1016/0378-8741(93)90070-l. [DOI] [PubMed] [Google Scholar]
- 6.Melchor G., Armenteros M., Fernández O., Linares E., Fragas I. Antibacterial Activity of Rhizophora mangle bark. Fitoterapia. 2001;17:689–691. doi: 10.1016/S0367-326X(01)00294-5. [DOI] [PubMed] [Google Scholar]
- 7.Marrero E., Sánchez J., de Armas E., Escobar A., Melchor G., Abad M. J., Bermejo P., Villar A. M., Megías J., Alcaraz M. J. COX-2 and sPLA2 Inhibitory Activity of Aqueous Extract and Polyphenols of Rhizophora mangle (Red Mangrove) Fitoterapia. 2006;77:313–315. doi: 10.1016/j.fitote.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Berenguer B., Sánchez L. M., Quílez A., López-Barreiro M., de Haro O., Gálvez J., Martín M. J. Protective and Antioxidant Effects of Rhizophora mangle. L. Against NSAID-induced Gastric Ulcers. J. Ethnopharmacol. 2006;103:194–200. doi: 10.1016/j.jep.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Fernández O., Capdevila J. Z., Dalla G., Melchor G. Efficacy of Rhizophora mangle Aqueous Bark Extract in the Healing of Open Surgical Wounds. Fitoterapia. 2002;73:564–568. doi: 10.1016/s0367-326x(02)00229-0. [DOI] [PubMed] [Google Scholar]
- 10.Vijayavel K., Anbuselvam C., Balasubramanian M. P. Free Radical Scavenging Activity of the Marine Mangrove Rhizophora apiculata Bark Extract with Reference to Naphthalene Induced Mitochondrial Dysfunction. Chem-Biol. Interact. 2006;163:170–175. doi: 10.1016/j.cbi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Duan X. J., Zhang W. W., Li X. M., Wang B. G. Evaluation of Antioxidant Property of Extract and Fractions Obtained from a Red Alga, Polysiphonia urceolata. Food Chem. 2006;95:37–43. doi: 10.1016/j.foodchem.2004.12.015. [DOI] [Google Scholar]
- 12.Foo L. Y., Lu Y., Mcnabb W. C., Waghorn G., Ulyatt M. J. Proanthocyanidins from Lotus pedunculatus. Phytochemisty. 1997;45:1689–1696. doi: 10.1016/S0031-9422(97)00198-2. [DOI] [Google Scholar]
- 13.Ramesh C., Mahender G., Ravindranath N., Das B. A Mild, Highly Selective and Remarkably Easy Procedure for Deprotection of Aromatic Acetates Using Ammonium Acetate as a Neutral Catalyst in Aqueous Medium. Tetrahedron. 2003;59:1049–1054. [Google Scholar]
- 14.Hergert H. L., Kurth E. F. The Isolation and Properties of Catechol from White Fir Bark. J. Org. Chem. 1953;18:521–529. doi: 10.1021/jo01133a008. [DOI] [Google Scholar]
- 15.Wan S. B., Chan T. H. Enantioselective Synthesis of Afzelechin and Epiafzelechin. Tetrahedron. 2004;60:8207–8211. doi: 10.1016/j.tet.2004.06.113. [DOI] [Google Scholar]
- 16.Chen H. F., Tanaka T., Nonaka G. I., Fujioka T., Mihashi K. Phenylpropanoid-substituted Catechins from Castanopsis hystrix and Structure Revision of Cinchonains. Phytochemisty. 1993;33:183–187. doi: 10.1016/0031-9422(93)85419-R. [DOI] [Google Scholar]
- 17.Basak A., Mandal S., Bandyopadhyay S. Regioselective Hydrolysis of Pentaacetyl Catechin and Epicatechin by Porcine Liver Esterase. Bioorg. Med. Chem. Lett. 2003;13:1083–1085. doi: 10.1016/S0960-894X(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 18.Xia K. H., Zhou L., Zuo Z. L. The Quantitative Structure-activity Relationship on Antioxidative Activities of Flavanol Compounds. West China J. Pharma. Sci. 2003;18:321–323. [Google Scholar]