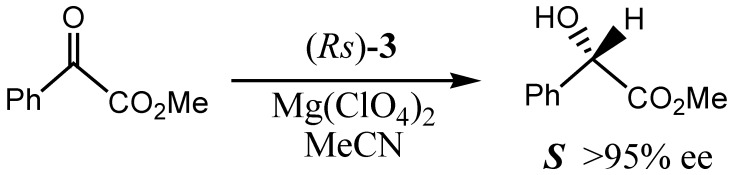Abstract
We have synthesized a novel chiral NADH model compound, N-methyl-(R)-3-(tert-butyl)-sulphinyl-1,4-dihydropyridine with high enantioselectivity and used it in the reduction of methyl benzoylformate, producing (S)-methyl mandelate in 95% ee. The absolute structure of its precursor, 3-(tert-butyl)sulfinyl pyridine, was determined by X-ray analysis.
Keywords: NADH model compound, chiral, sulfoxide, dihydropyridine, synthesis
Introduction
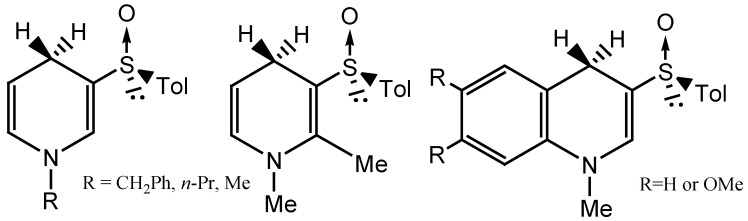
Since Ohno and coworkers reported the first example of asymmetric reduction of ketones with a coenzyme NADH model compound in 1975 [1], numerous efforts have been made to construct other models mimicking such asymmetric reduction [2]. Most of the model compounds reported consist of 3-carbonylated (e.g. amide or ester) 1,4-dihydropyridines with a chiral group attached far from the C(4) reaction center, which often result in products of relatively low optical purity [3]. Model compounds possessing a sulfinyl group as a chiral inducer, on the other hand, have proved to be highly efficient, allowing for the asymmetric reduction of various prochiral substrates with a high level of stereospecificity [4,5,6]. To the best of our knowledge, thus far only three such chiral sulfoxides have been reported as coenzyme NADH models (Figure 1).
Figure 1.
The structures of three chiral sulfoxide coenzyme NADH model compouds.
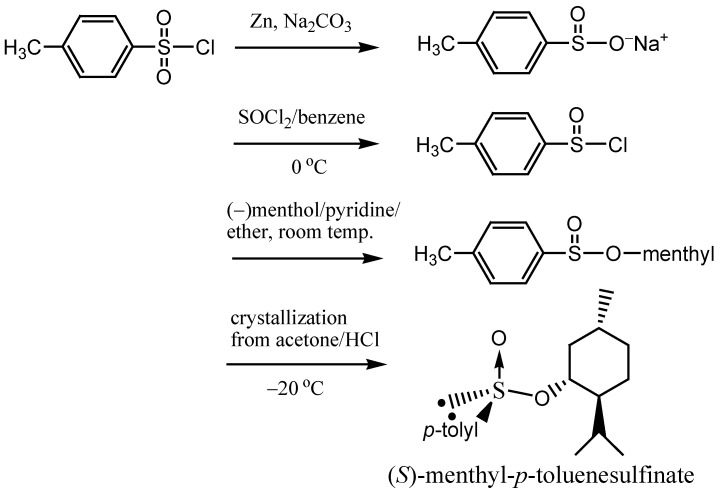
The precursor used to make these three chiral sulfoxides is (S)-menthyl-p-toluenesulfinate, synthesized from p-toluenesufonyl chloride and (−)-menthol in 4 steps (Scheme 1) [7].
Scheme 1.
Preparation of (S)-menthyl-p-toluenesulfinate.
The total yield of the reaction sequence was rather low. Besides, the chiral auxiliary is fixed to be the p-tolylsufinyl group. In the present investigation, we have designed a novel chiral NADH model, which possesses a tert-butylsulfinyl group at the C(3) position in place of the traditional p-tolylsufinyl group. The tert-butylsulfinyl moiety has proven to be a good chiral auxiliary, which has found extensive use both in academics and industry [8]. It is well known that the tert-butyl group has a large steric hindrance and consequently the C(4) prochiral hydrogen atoms in the dihydropyridine nucleus are expected to be diastereomerically distinguished by the neighboring chiral tert-butylsulfinyl group and the formal hydride transfer from the model compound to the carbonyl substrate should be highly enantioselective. Herein we wish to report the synthesis of the novel chiral NADH model compound: N-methyl-(R)-3-(tert-butyl)-sulfinyl-1,4-dihydropyridine and its use in the asymmetric reduction of methyl benzoylformate.
Results and Discussion
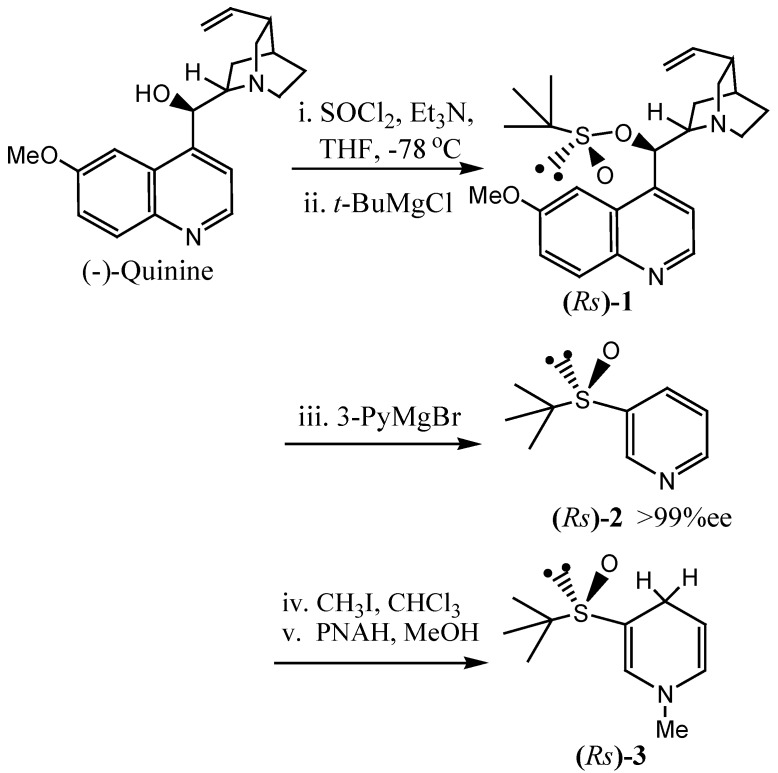
The synthesis of N-methyl-(R)-3-(tert-butyl)-sulfinyl-1,4-dihydropyridine can be represented by the following scheme (Scheme 2).
Scheme 2.
Asymmetric synthesis of N-methyl-(R)-3-(tert-butyl)-sulphinyl-1,4-dihydropyridine.
The first goal of the synthesis was to find a good sulfinyl transfer reagent for preparing optical pure R-tert-butanesulfinate. After reviewing the literature, we adopted a reported method [9] for the preparation of a quinine derivative of tert-butanesulfinate. The experiment must be carried out in the complete absence of oxygen and water, and the tert-butyl magnesium chloride (1.0M in THF) must be freshly prepared.
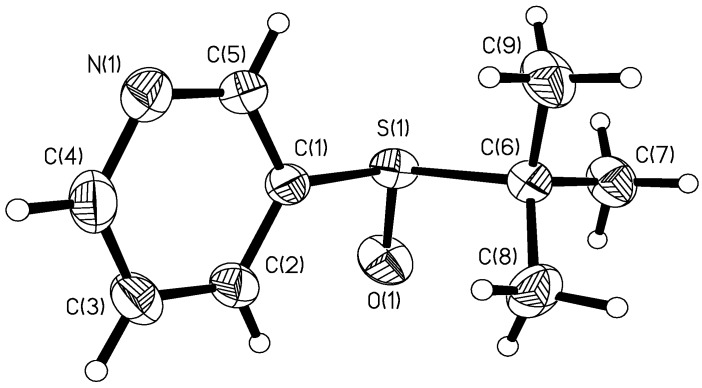
Secondly, the displacement of the quinine auxiliary with the 3-pyridyl group was examined. Following the literature [10], 3-pyridylmagnesium bromide was prepared. In order to maintain the enantiomeric purity of the sulfinyl group, we cooled the 3-pyridylmagnesium bromide to -78°C and tert-butylsulfinate added to it. To our surprise, the anticipated product was not obtained. However, when the reaction was carried out at room temperature, 3-(tert-butyl)sulfinyl pyridine (2) was obtained. By decreasing the concentration of reactants in the reaction mixture, the stereoselectivity of the reaction could be increased from 88% ee to >99% ee. The stereochemistry of 2 was fully determined by X-ray crystallographic analysis, and the absolute configuration of the molecular skeleton was unambiguously established to be R (Figure 2) with a Fleck parameter of 0.09(8).
Figure 2.
ORTEP atoms labeled and drawn as 30% thermal ellipsoids for compound 2.
In the following step, the quaternization of compound 2 met with some difficulties. When the reaction of 2 with benzyl bromide was carried out at 60 °C overnight according to the literature [4b], the conversion was only about 50%. After continued heating for 2 days, the result was still unsatisfactory. When compound 2 was heated in a toluene bath at 60 °C for two days for comparison, the enantiomeric excess of compound 2 decreased from >99% to 90% and some byproducts were obtained. We then tried to react compound 2 with methyl triflate according to the known method [6], and the reaction was monitored by TLC analysis. After reaction for 30 min, nearly 100% conversion was achieved, but the product was not the expected one, as its 1H-NMR spectrum showed no signal for the tert-butyl group. Finally, we succeeded in quaternization of compound 2 by reaction with excess methyl iodide at room temperature for 48 h. The product was used for further reaction after evaporation under reduced pressure without additional purification.
Initial attempts to reduce the quarternary pyridinium salt by the usual method with Na2S2O4/Na2CO3 [11] failed. The reaction mixture obtained was quite complex and it was difficult to separate the product. We then tried to use N-benzyl-1,4-dihydronicotinamide (BNAH) and N-propyl-1,4-dihydronicotinamide (PNAH) as the reducing reagent and obtained the desired product in moderate yields. The latter gave a more satisfactory yield (80%) of N-methyl-(Rs)-3-(tert-butyl)-sulfinyl-1,4-dihydropyridine (3) than the former route (yield 50%).
The enantioselectivity of compound 3 was assessed by the reduction of methyl benzoylformate (Scheme 3). For comparison with the known models bearing a chiral sulfinyl group at C(3), model compound 3 was tested under standard conditions, i.e. in the presence of magnesium perchlorate in acetonitrile [4a, 6 ]. Under these conditions, (S)-methyl mandelate was obtained in 80% yield and >95% ee, indicating that compound 3 was an efficient model of coenzyme NADH.
Scheme 3.
Asymmetric reduction of methyl benzoylformate with model (Rs)-3.
Experimental
General
Unless otherwise specified, all reagents were obtained from commercial sources and used without further purification. THF was distilled from benzophenone/Na. Reactions were carried out under dry Ar. tert-Butyl magnesium chloride (1M in THF) and iso-propyl magnesium chloride (2M in THF) were purchased from Aldrich. Anhydrous quinine and 3-bromopyridine were supplied by ABCR. 1H-NMR and 13C-NMR were recorded on Bruker AC-300 FT (1H: 300 MHz, 13C: 75.46 MHz) or Bruker AV-400FT (1H: 400 MHz, 13C: 100 MHz) instruments using TMS as internal reference. The chemical shifts (δ) and coupling constants (J) were expressed in ppm and Hz respectively. IR spectra were recorded on a Perkin-Elmer, 2000FTIR, and [α]D on WZZ-2 polarimeter. Chiral HPLC was performed on Waters 600E series instrument equipped with a 2487 dual absorbance UV detector. Chiralcel OD-H and Chiralcel OB-H columns were purchased from Daicel Chemical Industries. High-resolution mass spectra (HRMS) were determined on a Micromass GCT-MS mass spectrometer. Melting points were measured on X-4 digital microscope melting point apparatus of Beijing Tech Instrument Co., Ltd.
3-(tert-Butyl) sulfinyl pyridine (2)
The quinine derivative of tert-butanesulfinate (Rs)-1 was prepared in 80% yield according to the literature [9]. Bromine-magnesium exchange of 3-bromopyridine was achieved by a reported method [10], except that the volume of the solvent (THF) was increased five-fold. iPrMgCl (10 mmol) was added to 3-bromopyridine (0.96 mL, 10 mmol) in THF (50 mL) at room temperature. After stirring for 1 h, the solution of 1 (1M, 5 mmol) was added. The reaction mixture was stirred at ambient temperature for 18 hours and then quenched with saturated aqueous NH4Cl solution. Extraction with EtOAc (3 x 30 mL), drying over Na2SO4 and column chromatography on silica gel (eluent: EtOAc-petrolum ether = 4:1) gave (Rs)-2 as a light yellow solid in 75% yield (>99% ee), which was recrystallized from EtOAc-petroleum ether to give colorless needles, mp 92 °C; [α]22D +109.7o (c= 0.012, CHCl3); IR (KBr): 3038, 1571, 1039 cm-1; 1H-NMR (CDCl3, 300 MHz) δΗ (ppm): 1.19 (9H, s), 7.47 (dd, J = 4.9 and 7.9 Hz, 1H), 7.99 (d, J = 8.4 Hz, 1H,), 8.71 (2H, m); 13C-NMR (CDCl3, 75 MHz) δC(ppm): 22.71 (CH3), 56.38 (C), 123.738 (CH), 134.063 (CH), 136.971 (C-S), 147.618 (CH), 152.124 (CH); Anal. Calcd. for C9H13NOS: C.58.98, H. 7.149, N. 7.642. Found: C. 59.01, H. 7.066, N. 7.30.
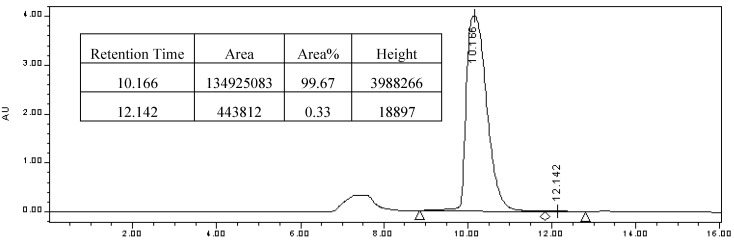
Enantiomeric excess was determined by HPLC analysis (Figure 3) using a Chiracel OB-H column (0.46 cm Φ x 25 cm; 10 μm particle size). Chromatographic conditions: injection: 10 μL; eluent: hexane-2-propanol=70: 30; flow rate: 0.5 mLmin-1; temperature: 25 °C; UV detection: λ = 254 nm; tR = 10.166 min [(R)-enantiomer] and 12.142 min [(S)-enantiomer].
Figure 3.
Resolution of compound 2 by HPLC with OB-H chiral stationary phase.
X-ray crystal structure determination of compound 2
A colorless platelet crystal of 2 with approximate dimensions of 0.40 x 0.19 x 0.11 mm, mounted on a fiberglass, was used for the X-ray study. All measurements were made on a Bruker Apex II CCD area diffractometer with graphite monochromated Mo-Kα radiation. The structure was solved by direct methods (SHELXS-97) and expanded using Fourier techniques (SHELXL-97). The nonhydrogen atoms were refined anisotropically. H atoms were treated as riding, with C-H = 0.93 Å and with Uiso(H) = 1.2Ueq(C,). The final cycle of full-matrix least-squares refinement was based on 1863 observed reflections and 113 variable parameters and converged with unweighted and weighted agreement factors of R = 0.0292 and Rw = 0.0631. Crystal data: C9H13NOS, M = 183.26, crystal system: orthorhombic, space group: P212121 (#19). Lattice parameters: a = 6.2116(9) Å, b = 6.2116(9) Å, c = 16.310(2) Å, V = 1007.0(2) Å3, Z = 4, Dcalc = 1.209 Mg/m3. CCDC-630911 contains the supplementary crystallographic data for this complex. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk).
(Rs)-1-Methyl-3-(tert-butyl)-sulfinyl-1,4-dihydropyridine (3)
A solution of 2 (100 mg, 0.55 mmol) and methyl iodide (0.1 mL, 1.65 mmol) in CHCl3 (4 mL) was stirred in the dark at room temperature for 48 h. Removal of solvent and unreacted CH3I using a rotary evaporator gave crude (Rs)-1-methyl-3-(tert-butylsulfinyl)pyridinium iodide (180 mg). It was treated with PNAH (125 mg, 0.825 mmol) in methanol (8 mL), and the mixture was stirred under an Ar atmosphere at room temperature in the dark for 6 h. Removal of solvent and purification by column chromatography (eluent: EtOAc-petrolum ether-triethylamine = 10:15:2) afforded (Rs)-3 as a light yellow oil which turned into a white solid after standing at ambient temperature (88 mg, 80% yield). [α]18D +89.29o (c= 0.0014, CHCl3); 1H-NMR (CDCl3, 400 MHz) δΗ (ppm): 1.24 (s, 9H), 2.92 (s, 3H), 3.06, 3.15 (dm, J = 20 Hz, 2H), 4.58 (m, 1H), 5.69 (dq, J = 7.84 Hz, 1H), 6.32 (d, J = 0.64 Hz, 1H); 13C-NMR (CDCl3, 100 MHz) δC(ppm): 21.177 (CH2), 23.981 (CH3), 40.648 (CH3), 55.978 (C), 99.859 (CH), 105.346 (C), 130.650 (CH), 138.639 (CH); HRMS: 199.1031 (calcd. for C10H17NOS: 199.1031); Anal. Calcd. for C10H17NOS: C. 60.26; H. 8.597; N. 7.028. Found: C. 60.08; H. 8.561; N. 6.786.
Reduction of methyl benzoylformate with (Rs)-3
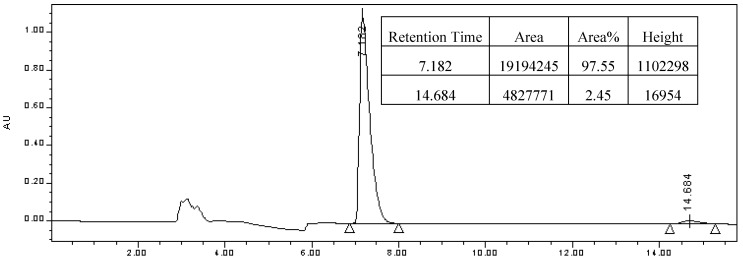
Under Ar atmosphere, compound 3 (20 mg, 0.1 mmol), dry MeCN (2 mL), methyl benzoylformate(14 μL, 0.1 mmol) and Mg(ClO4)2 (22.4 mg, 0.1 mmol) were successively introduced into the flask. The resulting mixture was stirred at r.t. for 24h in the dark. After evaporation of the solvent, the residue was purified by chromatography on silica gel (eluent: EtOAc-petrolum ether = 1:5) to give (S)-methyl mandelate, yield 70%. Enantiomeric excess was determined by HPLC analysis (Figure 4) using a Chiracel OD-H column (0.46 cm Φ x 25 cm; 10 μm particle size). Chromatographic conditions: injection: 10 μL; eluent: hexane-2-propanol = 90:10; flow rate: 1 mL min-1; temperature: 25 °C; UV detection: λ= 235 nm; tR = 7.182 min [(S)-enantiomer] and 14.684 min [(R)-enantiomer].
Figure 4.
Resolution of methyl mandelate with OD-H chiral stationary phase.
Acknowledgments
Support from National Natural Science Foundation of China (Grant No. 20072036) and Specialized Research Fund for the Doctoral Program of Higher Education of China is gratefully acknowledged.
Supplementary Material
Supplementary material file: http://www.mdpi.org/molecules/papers/12030415sm.pdf
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Ohnishi Y., Kagami M., Ohno A. Reduction by a model of NAD(P)H. Effect of metal ion and stereochemistry on the reduction of alpha.-keto esters by 1,4-dihydronicotinamide derivatives. J. Am. Chem. Soc. 1975;97:4766–4768. doi: 10.1021/ja00849a055. [DOI] [PubMed] [Google Scholar]
- 2. Inouye Y., Oda J., Baba N. In: Asymmetric Synthesis. Morrison J.D., editor. Vol. 2. Academic Press; New York: 1983. pp. 91–124. Burgess V. A., Davies S. G., Skerlj R. T. NADH mimics for the stereoselective reduction of benzoylformates to the corresponding mandelates. Tetrahedron: Asymmetry. 1991;2:299–328. and references cited therein.
- 3.Ohno A., Ushida S., Oka S. NAD(P)+-NAD(P)H model. 39. Asymmetric reduction by 1,4-dihydronicotinamide derivative bound to protein. Bull. Chem. Soc. Jpn. 1983;56:564–567. doi: 10.1246/bcsj.56.564. [DOI] [Google Scholar]
- 4. Imanishi T., Hamano Y., Yoshikawa H., Iwata C. J. 1-Substituted (S)-3-(p-tolyl)sulphinyl-1,4-dihydropyridines: Novel NADH model compounds. Chem. Commun. 1988:473–475. Imanishi T., Obika T., Nishiyama T., Nishimoto M., Hamano Y., Miyashita K., Iwata C. Studies on novel and chiral 1,4-dihydropyridines.I. Synthesis and conformational analysis of novel NADH model compounds, N-substituted (S)-3-(P-tolylsulfinyl)-1,4-dihydropyridines. Chem. Pharm. Bull. 1996;44:267–272. Obika S., Nishiyama T., Tatematsu S., Miyashita K., Imanishi T. Stereospecific 4-hydrogen transfer in the asymmetric reduction using (SS)-3-(P-tolylsufinyl)-1,4-dihydropyridines, NADH model compounds. Chem. Lett. 1996:853–854. Obika S., Nishiyama T., Tatematsu S., Miyashita K., Iwata C., Imanishi T. Studies on novel and chiral 1,4-dihydropyridines. III. Asymmetric reduction of some ketones with novel NADH model compounds, (SS)-3-(P-Tolylsulfinyl)-1,4-dihydropyridines. Tetrahedron. 1997;53:593–602. Miyashita K., Nishimoto M., Murafuji H., Murakami A., Obika S., In Y., Ishida T., Imanishi T. Effect of the neighbouring oxygenated substituent on asymmetric reduction with Hantzsch-type 1,4-dihydropyridines having a chiral sulfinyl group. Chem. Commun. 1996:2535–2536.
- 5.Li J., Liu Y. C., Deng J. G. Asymmetric reduction of 2-bromo-1-phenylethylidenemalononitrile with chiral NAD(P)H models. Tetrahedron: Asymmetry. 1999;10:4343–4347. [Google Scholar]
- 6.Stephane G., Cyril P., Francis M., Georges D., Vincent L. Preparation of 1,4-dihydroquinolines bearing a chiral sulfoxide group: new highly enantioselective recyclable NADH mimics. Synlett. 2005;3:441–444. [Google Scholar]
- 7.Solladie G., Hutt J., Girardin A. Improved preparation of optically active methyl p-tolyl sulfoxide. Synthesis. 1987:173. [Google Scholar]
- 8. Ellman J. A., Owens T. D., Tang T. P. N-tert-Butanesulfinyl imines: versatile intermediates for the asymmetric synthesis of amines. Acc. Chem. Res. 2002;35:984–995. doi: 10.1021/ar020066u. and references cited therein. Ellma J. A. Applications of tert-butanesulfinamide in the asymmetric synthesis of amine. Pure. Appl. Chem. 2003;75:39–46. Zhou P., Chen B. C., Davis F. A. Recent advances in asymmetric reactions using sulfinimines (N-sulfinyl imines) Tetrahedron. 2004;60:8003–8030.
- 9.Lu B. Z., Jin F., Zhang Y., Wu X., Wald S. A., Senanayake C. H. New general sulfinylating process for asymmetric synthesis of enantiopure sulfinates and sulfoxides. Org. Lett. 2005;7:1465–1468. doi: 10.1021/ol0501020. [DOI] [PubMed] [Google Scholar]
- 10. Trécourt F., Breton G., Bonnet V., Mongin F., Marsais F., Quéguiner G. Pyridylmagnesium chlorides from bromo and dibromopyridines by bromine-magnesium exchange: a convenient access to functionalized pyridines. Tetrahedron Lett. 1999;40:4339–4342. Trécourt F., Breton G., Bonnet V., Mongin F., Marsais F., Quéguiner G. New synthesis of substituted pyridines via bromine-magnesium exchange. Tetrahedron. 2000;56:1349–1360.
- 11.Cauhhey W. S., Schellenberg K. A. Characterization of an Intermediate in the Dithionite Reduction of a Diphosphopyridine Nucleotide Model as a 1,4-Addition Product by Nuclear Magnetic Resonance Spectroscopy. J. Org. Chem. 1966;31:1978–1982. doi: 10.1021/jo01344a512. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.