Abstract
Four new diterpenoid alkaloids: tiantaishansine (1), tiantaishannine (2), tiantaishanmine (3), and tiantaishandine (4) have been isolated from the roots of Delphinium tiantaishan. Their structures were elucidated by chemical evidence and spectral analyses, including ESI-MS, HR-EI-MS, 1D- and 2D-NMR.
Keywords: Dephinium tiantaishanense, tiantaishansine, tiantaishannine, tiantaishanmine, tiantaishandine
Introduction
The plant Dephinium tiantaishanense W. J. Zhang et G. H. Chen is a new species plant of Dephinium L., mainly distributed around Tiantai Mountain in Pengzhou County, Sichuan Province, China [1]. To our knowledge, no phytochemical investigation of this plant has been undertaken. In the course of our comparative studies of diterpenoid alkaloids from Aconitum and Delphinium species, four new diterpenoid alkaloids: tiantaishansine (1), tiantaishannine (2), tiantaishanmine (3), and tiantaishandine (4), were isolated from the roots of D. tiantaishanense. This paper describes the isolation and structure elucidation of these new alkaloids.
Results and Discussion
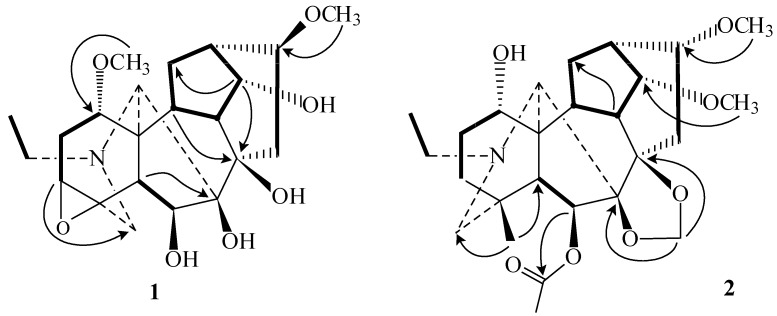
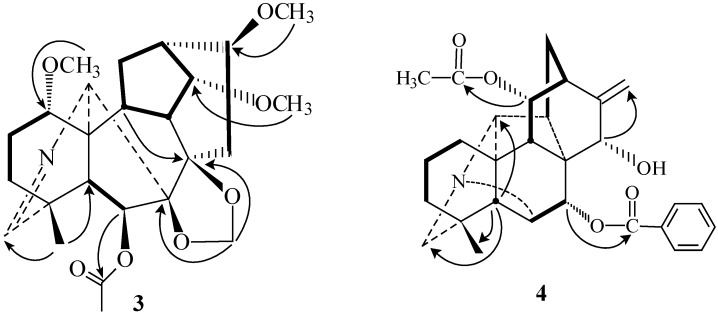
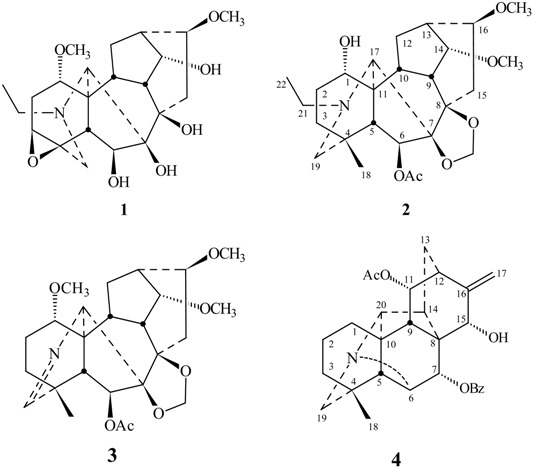
Tiantaishansine (1) was obtained as an amorphous powder. Its molecular formula C22H33NO7 was inferred from its HR-ESI-MS and 13C-NMR. Its NMR spectra displayed an N-ethyl group (δH 1.07, 3H, t, J = 7.2 Hz; δC 49.9 t, 13.9 q) and two methoxyl groups (δH 3.51, 3.41, s, each 3H; δC see Table 1). These two methoxyl groups could be assigned at C-1 and C-16, respectively, based on the long-range correlations between 1- OCH3 (δH 3.51)/C-1 (δC 77.7) and 16- OCH3 (δH 3.41)/C-16 (δC 82.3) in HMBC spectrum (Figure 1). The 13C-NMR spectrum showed the 3,4-epoxy group based on the downfield signal at C-3 (δC 57.8, d) and C-4 (δC 58.3 s) [2]. The one-proton triplet (J = 4.4 Hz) at δH 4.03 in the 1H-NMR spectrum of 1 was assigned to H-14β based on the multiplicity and the coupling constant, resulting in location of the hydroxy group at C-14 [2]. The IR (3425 cm-1) and 13C-NMR (δC 91.3 s, 84.4 s, 79.8 d, 74.3 d) spectra showed that there were two tertiary hydroxyl groups and two secondary hydroxyl groups. Except for the secondary hydroxyl group at C-14, the other two tertiary hydroxyl groups could be attributed to C-7 and C-8 based on the HMBC correlations between 5-H/C-7 and 9-H/C-8, respectively. The remaining hydroxyl group could be located at C-6 due to the chemical shift of H-6 (δH 4.82 s) and the long-range correlations between H-6/C-5, C-7 in the HMBC spectrum (Figure 1). Thus, on the basis of these observations, the structure of tiantaishansine was established as 1, which corresponds to a C18-diterpenoid alkaloid.
Table 1.
NMR data of compounds 1 and 2 in CDCl3 (400 MHz for 1H, 100 MHz for 13C).
| NO. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH Mult (J = Hz) | δC | δH Mult (J = Hz) | |
| 1 | 77.7 d | 3.90 s | 71.6 d | 3.81 br s |
| 2 | 31.6 t | 1.26 m (α) | 29.2 t | 1.26 br s (α) |
| 2.18 m (β) | 1.56 br s (β) | |||
| 3 | 57.8 d | 3.05 m | 30.9 t | 1.54 m (β) |
| - | 1.58 m (α) | |||
| 4 | 58.3 s | - | 32.8 s | - |
| 5 | 52.2 d | 1.56 s | 51.1 d | 1.39 m |
| 6 | 79.8 d | 4.82 s | 78.2 d | 5.44 s |
| 7 | 91.3 s | - | 90.9 s | - |
| 8 | 84.4 s | - | 84.4 s | - |
| 9 | 43.0 d | 3.12 t (5.6) | 39.5 d | 3.44 m |
| 10 | 43.5 d | 2.12 m | 45.2 d | 2.14 m |
| 11 | 54.0 s | - | 51.3 s | - |
| 12 | 29.5 t | 1.58 m (β) | 30.3 t | 1.87 m (β) |
| 2.12 m (α) | 2.18 m (α) | |||
| 13 | 40.4 d | 2.26 m | 37.2 d | 2.44 m |
| 14 | 74.3 d | 4.03 t (4.4) | 83.5 d | 3.73 t (4.0) |
| 15 | 26.7 t | 1.97 m (α) | 34.2 t | 1.88 m (α) |
| 2.59 m (β) | 2.51 m (β) | |||
| 16 | 82.3 d | 3.39 s | 82.2 d | 3.28 m |
| 17 | 67.2 d | 2.91 d (1.5) | 65.3 d | 3.02 s |
| 18 | - | - | 26.7 q | 0.99 s |
| 19 | 54.3 t | 2.49 ABq | 61.1 t | 2.44 m |
| - | 3.36 ABq hidden | 2.51 m | ||
| 21 | 49.9 t | 2.98 m | 49.6 t | 2.74 m |
| 3.39 m | 2.87 m | |||
| 22 | 13.9 q | 1.07 t (7.2) | 13.3 q | 1.11 t (7.2) |
| 1-OCH3 | 51.7 q | 3.51 s | - | - |
| 14-OCH3 | - | 57.4 q | 3.43 s | |
| 16-OCH3 | 56.4 q | 3.41 s | 56.1 q | 3.36 s |
| -OCH2O- | 93.9 t | 4.92 s | ||
| 4.96 s | ||||
| OAc | 169.7 s | |||
| 21.5 q | 2.07 s | |||
Figure 1.
key 1H-1H COSY ( ) and HMBC (
) and HMBC ( ) correlations of 1 and 2.
) correlations of 1 and 2.
Tiantaishannine (2) was isolated as an amorphous powder, mp 206-208 °C. Its pseudo-molecular formula, C26H40NO7, was derived from HR-ESI-MS (m/z 478.2801 [M+H]+, calcd. 478.2799) and 13C-NMR. The NMR data of compound 2 gave distinctive signals at δH 0.99 (3H, s), δC 26.7 q, for an angular methyl group, δH 1.11 (3H, t, J = 7.2 Hz), δC 49.6 t, and 13.3 q for the N-ethyl group, δH 3.36, 3.43 (each 3H, s) for two methoxyl groups, δH 2.07 (3H, s), δC 21.5 q and 169.7 s for an acetyl group. The signals at δH 4.92 and 4.96 (each 1H, s) in 1H- NMR along with signals at δC 84.4 s, 90.9 s, and 93.9 t in 13C-NMR showed the presence of 7,8-methylenedioxy group, indicating a lycoctonine-type C19-diterpenoid alkaloid [2,3]. Two methoxyl groups could be located at C-14 and C-16 due to the long-range correlations between 14-OCH3 (δH 3.43 s) and C-14 (δC 83.5 d), 16-OCH3 (δH 3.36, s) and C-16 (δC 82.2 d) in the HMBC spectrum of 2 (Figure 1), as well as the acetyl group could be located at C-6 based on the HMBC correlations between H-6 (δH 5.44, s)/6-OCOCH3 (δC 169.7). The broadened singlet (δH 3.81), which could be assigned to H-1β, in combination with the C-1 chemical shift at δC 71.6 d, suggested a hydroxyl substitution at C-1 [2]. Therefore, the structure of taintaishannine was determined as 2.
The molecular formula (C25H35NO7) of compound 3 was determined by HR-ESI-MS (m/z 462.2473, [M+H]+). The NMR spectra strongly suggested that it was a lycoctonine-type diterpenoid alkaloid [2,3], with an angular methyl group (δH 1.17, 3H, s; δC 22.1 q), three methoxyl groups (δH 3.22, 3.34, 3.45, each 3H, s; δC see Table 2), an acetyl group (δH 2.06, 3H, s; δC 21.5 q, 169.5 s), and a methylenedioxy group (δH 4.93, 4.97, each 1H, s; δC 93.9 t). The significant absence of N-methyl, N-ethyl showed that there are no substitutions on the nitrogen atom of 3 besides the imine moiety (δH 7.46 br s; δC 169.9 d). The downfield signal at δC 44.4 s can be assigned to C-4 adjacent to a double bond (N=C-19) [4]. Three methoxyl groups could be located at C-1, C-14, and C-16 based on the HMBC correlations of 1- OCH3 (δH 3.22) /C-1 (δC 80.4), 14-OCH3 (δH 3.45)/C-14 (δC 83.4), 16- OCH3 (δH 3.34)/C-16 (δC 81.3) (Figure 2). The one-proton singlet at δH 5.25 s was assigned to H-6α suggesting an acetyl substitution at C-6 due to the HMBC correlation of H-6/COCH3. All these evidence supported the structure of compound 3 and named tiantaishanmine.
Table 2.
NMR data of compounds 3 (600 MHz for 1H, 150 MHz for 13C) and 4 (400 MHz for 1H, 100 MHz for 13C) in CDCl3.
| NO. | 3 | 4 | ||
|---|---|---|---|---|
| δC | δH Mult (J = Hz) | δC | δH Mult (J = Hz) | |
| 1 | 80.4 d | 3.23 m | 29.0 t | 1.55 m (β) |
| - | 1.81 m (α) | |||
| 2 | 25.6 t | 1.53 m (α) | 19.9 t | 0.87 br s (β) |
| 1.92 m (β) | 1.61 m (α) | |||
| 3 | 31.9 t | 1.31 m (β) | 33.1 t | 1.25 m |
| 1.72 m (α) | 1.47 m | |||
| 4 | 44.4 s | - | 37.5 s | - |
| 5 | 52.5 d | 1.41 br s | 60.0 d | 1.67 s |
| 6 | 79.9 d | 5.25 s | 68.0 d | 3.33 s |
| 7 | 90.9 s | - | 70.4 d | 5.44 d (2.8) |
| 8 | 82.9 s | - | 52.5 s | - |
| 9 | 39.8 d | 3.40 m | 47.1 d | 2.43 dd (6.6, 1.5) |
| 10 | 47.9 d | 2.20 m | 50.9 s | - |
| 11 | 49.8 s | - | 75.4 d | 5.28 d (6.4) |
| 12 | 28.7 t | 2.13 m (β) | 40.1 d | 2.28 br s |
| 1.88 dd (α) (10, 5.2) | - | |||
| 13 | 38.7 d | 2.40 m | 28.0 t | 1.43 m |
| 2.34 m (hidden) | ||||
| 14 | 83.4 d | 3.73 t (3.2) | 39.2 d | 2.34 t (9.6) |
| 15 | 33.4 t | 1.85 m (α) | 66.0 d | 4.06 s |
| 2.71 m (β) | - | |||
| 16 | 81.3 d | 3.28 t (5.2) | 150.8 s | - |
| 17 | 63.5 d | 4.16 br s | 112.2 t | 5.04 d (0.9) |
| 18 | 22.1 q | 1.17 s | 28.9 q | 0.97 s |
| 19 | 169.9 d | 7.46 br s | 62.7 t | 2.49 s |
| 20 | - | - | 74.1 d | 2.75 s |
| 1-OCH3 | 55.4 q | 3.22 s | - | - |
| 14-OCH3 | 57.7 q | 3.45 s | - | - |
| 16-OCH3 | 56.4 q | 3.34 s | - | - |
| -OCH2O- | 93.9 t | 4.93 s | - | - |
| 4.97 s | - | |||
| OAc | 169.5 s | - | 170.3 s | - |
| 21.5 q | 2.06 s | 21.4 q | 2.06 s | |
| ArCO | 167.1 s | - | ||
| 1’ | 129.7 s | - | ||
| 2’, 6’ | 130.1 d | 8.14 d (6.8) | ||
| 3’ ,5’ | 128.4 d | 7.45 m | ||
| 4’ | 133.4 d | 7.58 m | ||
Figure 2.
key 1H-1H COSY ( ) and HMBC (
) and HMBC ( ) correlations of 3 and 4.
) correlations of 3 and 4.
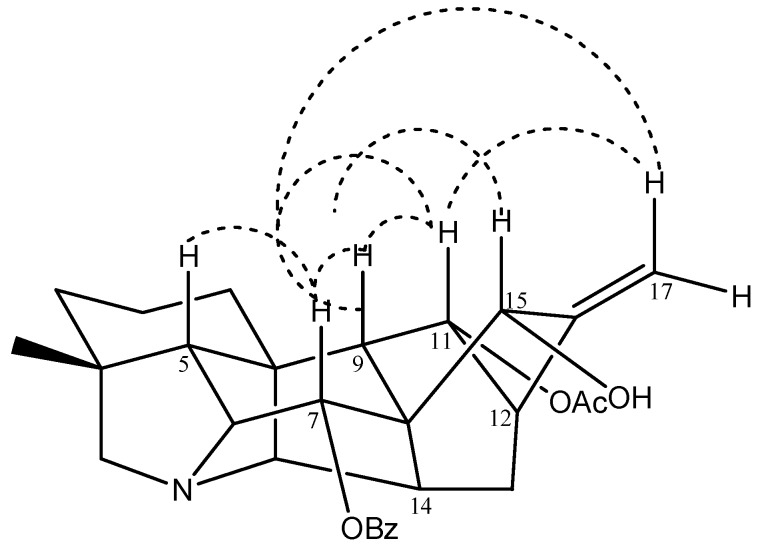
Tiantaishandine (4) mp 248-249 °C, was obtained as an amorphous powder. The HR-ESIMS at m/z 476.2439 corresponded to the pseudo-molecular ion [M+H]+ (C29H34NO5 calc. 476.2431). In the NMR spectrum, some characteristic signals of a C20-diterpene alkaloid having a hetisine-type skeleton [C-4 (37.5 s), C-8 (52.5 s), C-10 (50.9, s), C-16 (150.8 s)] [5], together with some important proton signals [H3-18 (δH 0.97, s), H2-17 (δH 5.04, d, J = 0.9 Hz), H-6 (δH 3.33, 1H, s), H-20 (δH 2.75, 1H, s), and H2-19 (δH 2.49, s)], were observed. In addition to these data, two downfield singlets at δC 170.3 s, 167.1 s, and the IR absorptions at 1736 and 1714 cm-1, showed the presence of one acetyl and one benzoyl group in this compound. The one-proton signal (δH 5.28, d, J = 6.4 Hz) could be attributed to H-11, based on the HMBC corrections of H-11 (δH 5.28) and C-9 (δC 47.1), C-10 (δC 50.9), C-12 (δC 40.1), as well as 1H-doublet at δH 5.44, assigned to H-7 due to the correlations of H-7 (δH 5.44) and C-8 (δC 52.5), C-14 (δC 39.2), C-15 (δC 66.0). The acetyl and benzyl groups could be located at C-11 and C-7, respectively, based on the correlations between H-11 (δH 5.28)/11-OCOCH3 (δC 170.3) and H-7 (δH 5.44) /7-PhCO (δC 167.1). The 13C-NMR spectrum of compound 4 displayed three oxygenated carbon signals (δC 66.0 d, 70.4 d, 75.4 d), suggesting the presence of additional hydroxyl group, which could be placed at C-15 based on the HMBC spectrum (Figure 2). The configuration of this hydroxyl group was determined as 15α-OH, based on the NOESY relationships of H-15 (δH 4.06) with H-17 (δH 5.04), H-9 (δH 2.43) and H-12 (δH 2.28) (Figure 3). Meanwhile the 11-OAc and 7-OBz were also assigned α-configurations due to the NOESY experiments (Figure 3). Consequently, the structure of tiantaishandine was determined as 4.
Figure 3.
Key NOESY correlations of 4.
Experimental
General
Melting points were measured on a Thermal Values analytical microscope and were uncorrected. Optical rotations were recorded on a Perkin-Elmer 341 polarimeter. IR spectra were recorded on a Nicolet FI-IR 200SXY spectrophotomer. 1H- and 13C-NMR spectra were measured in CDCl3 with TMS as the internal standard on a Varian Unity INOVA 400/54 NMR or Bruker AV-600 spectrometers. HR-ESI-MS were measured by a VG AutoSpec 3000 instrument. Silica gel GF254 and H (Qingdao Sea Chemical Factory, China) were used for TLC and column chromatography, respectively. Spots on TLC were detected with modified Dragendorff ’s reagent.
Plant Material
Delphinium tiantaishanense W. J. Zhang et G. H. Chen was collected in the Tiantaishan mountains of Sichuan Province, P.R. China. The plant was identified taxonomically by Wen-Jing Zhang (Disease Prevention and Control Center of Pengzhou City). A voucher speciman (No. 20050818) was deposited in the West China College of Pharmacy, Sichuan University.
Extraction and Isolation
Air-dried powered roots (4.4 kg) of D. tiantaishan were percolated with 0.1M HCl (50 L). The filtrate was then made alkaline to pH>9 with 28% aqueous NH4OH (1.5 L) and extracted five times with ethyl acetate (each 20 L), and the extracts evaporated to give the total crude alkaloids (58 g, yield 1.31%). The crude alkaloids (58 g) were chromatographed over a silica gel (500 g) column eluting with a 7:1→1:1 petroleum ether-acetone gradient to give fractions A (7.5 g), B (9.6 g), C (18.7 g), and D (20.4 g). Fraction A (7.5 g) was rechromatographed on a silica gel column eluting with petroleum ether-acetone (8:1) to afford 2 (120 mg, yield 0.003%). Fraction B (9.6 g) was rechromatographed on a silica gel column eluting with petroleum ether-acetone (5:1) gave 4 (90 mg, yield 0.002%). Fraction C (18.7 g) was rechromatographed on a silica gel column eluting with petroleum-acetone (3:1) to afford 1 (100 mg, yield 0.002%) and 3 (40 mg, yield 0.001%), respectively.
Tiantaishansine (1). White amorphous powder, mp 94-96 °C; 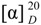 +35.4° (c=0.84, CHCl3); IR (KBr) cm-1: 3425, 2943, 2879, 1707, 1638, 1460; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3): see Table 1; HR-ESI-MS: m/z [M+H]+ 424.2351, calcd for C22H34NO7, 424.2330.
+35.4° (c=0.84, CHCl3); IR (KBr) cm-1: 3425, 2943, 2879, 1707, 1638, 1460; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3): see Table 1; HR-ESI-MS: m/z [M+H]+ 424.2351, calcd for C22H34NO7, 424.2330.
Tiantaishannine (2). White amorphous powder, mp 206-208 °C; 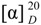 -23.9° (c=0.73, CHCl3); IR (KBr) cm-1: 3396, 2932, 1738, 1457, 1367, 1084; 1H-NMR (400 MHz, CDCl3) and 13C- NMR (100 MHz, CDCl3): see Table 1; HR-ESI-MS: m/z 478.2801 [M+H]+, calcd for C26H40NO7, 478.2799.
-23.9° (c=0.73, CHCl3); IR (KBr) cm-1: 3396, 2932, 1738, 1457, 1367, 1084; 1H-NMR (400 MHz, CDCl3) and 13C- NMR (100 MHz, CDCl3): see Table 1; HR-ESI-MS: m/z 478.2801 [M+H]+, calcd for C26H40NO7, 478.2799.
Tiantaishanmine (3). White amorphous powder, mp 251-253 °C; 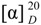 +24.2° (c=0.26, CHCl3); IR (KBr) cm-1: 3396, 2926, 1741, 1637, 1461, 1365; 1H-NMR (600 MHz, CDCl3) and 13C-NMR (150 MHz, CDCl3): see Table 2; HR-ESI-MS: m/z 462.2473 [M+H]+, calcd for C25H36NO7, 462.2486.
+24.2° (c=0.26, CHCl3); IR (KBr) cm-1: 3396, 2926, 1741, 1637, 1461, 1365; 1H-NMR (600 MHz, CDCl3) and 13C-NMR (150 MHz, CDCl3): see Table 2; HR-ESI-MS: m/z 462.2473 [M+H]+, calcd for C25H36NO7, 462.2486.
Tiantaishandine (4).White amorphous powder, mp 248-249 °C; 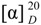 +35.6° (c=0.85, CHCl3); IR (KBr) cm-1: 3446, 2943, 1736, 1714, 1243, 1108; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3): see Table 2; HR-ESI-MS: m/z 476.2439 [M+H]+, calcd for C29H34NO5, 476.2431.
+35.6° (c=0.85, CHCl3); IR (KBr) cm-1: 3446, 2943, 1736, 1714, 1243, 1108; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3): see Table 2; HR-ESI-MS: m/z 476.2439 [M+H]+, calcd for C29H34NO5, 476.2431.
Acknowledgments
This work was supported by the National Science Foundation of China (No. 30472075)
Footnotes
Sample Availability: Available from the authors.
References
- 1.Zhang W. J., Chen G. H. A new species of Delphinium L. from Sichuan, China. West China J. Pharm Sci. 2006;21:560–561. [Google Scholar]
- 2.Pelletier S. W., Mody N. V., Joshi B. S., Schramn L. C. In: Alkaloids: Chemical and Biological Perspectives. Pelletier S. W., editor. Vol. 2. John Wiley. & Sons Press; New York: 1984. pp. 206–210. [Google Scholar]
- 3.Pelletier S. W., Joshi B. S. In: Alkaloids: Chemical and Biological Perspectives. Pelletier S. W., editor. Vol. 7. John Wiley & Sons Press; New York: 1991. p. 297. [Google Scholar]
- 4.Boido V., Edwards O. E., Handa K. L., Kolt R. J., Purushothaman K. K. Alkaloids of Acontitum columbianum Nutt. Can. J. Chem. 1984;62:778–784. [Google Scholar]
- 5.Wang F. P., Liang X. T. In: Alkaloids: Chemistry and Biology. Cordell G. A., editor. Vol. 59. Elsevier Press; New York: 2002. pp. 1–280. [DOI] [PubMed] [Google Scholar]