ABSTRACT
Forisomes in legumes are responsible for fast sieve-element occlusion in response to injury to the vascular system. This prevents uncontrolled leakage of phloem sap and protects against invasion of pathogens. Here we compared forisomes of four different legumes (Pisum sativum, Vicia faba, Trifolium pratense and Medicago sativa) by their location (basal, central, apical) in the sieve element and reactivity to a distant heat stimulus. In each species, the majority of forisomes was located basally. Yet, we found differences in intracellular location: forisomes are distributed more evenly in the sieve elements of Pisum. After burning, basally located forisomes of the four species reacted with dispersion, followed by a spontaneous recondensation with similar reaction times. The results suggest universal forisome behaviour in fabacean species.
KEYWORDS: Calcium, forisome, Medicago sativa, Pisum sativum, sieve elements, sieve-element occlusion, Trifolium pratense, Vicia faba
Introduction
Sieve elements of legumes contain forisomes, highly ordered protein bodies up to 100 micron in length.1 These forisomes respond to local injury by sudden dispersion that occludes the sieve plates.
Forisome mediated sieve-plate occlusion in response to local injury2 is commonly accompanied by callose mediated sieve-pore constriction.3–6 Both mechanisms are ascribed to the effect of Ca2+ ions set free from damaged Ca2+ storage compartments.7,8 Serious damage of sieve elements induces a reversible forisome dispersion in distant intact sieve elements.9 Remote dispersion is caused by the propagation of electrical potential waves, departing from the site of injury,9 that are associated with Ca2+ influx via Ca2+ permeable channels residing in sieve-element plasma membrane.10 Remote forisome dispersion is followed by a re-condensation as result of Ca2+ removal by putative Ca2+ pumps.9–11
In accordance with the location of Ca2+ permeable channels, forisome reactivity (dispersion and re-condensation) to distant damaging stimuli is correlated with their location inside sieve elements.12 The majority of Vicia faba forisomes (82%) is located in the proximity of the basal (downstream) sieve plates.12,13 A small proportion of the forisomes (7.5%) is located near the apical (upstream) sieve plates, while the remaining part (central forisomes, 10.5%) does not co-localize with the sieve plates.12,13 Basal forisomes reacted more frequently to damage caused by distant burning than central or upstream forisomes.12 Dispersion (approx. 22 sec) and re-condensation (approx. 240 sec) time lapses after distant burning were similar for the three forisome locations inside sieve elements.
Whether this uneven distribution of forisomes inside the sieve elements and their reactivity is a universal phenomenon among legumes was not investigated so far. Therefore, forisome locations were documented in four legume species (M. sativa, P. sativum, T. pratense, V. faba).
Furthermore, the behavior of the forisomes was monitored by continuous optical surveillance so that the dispersion and re-condensation reactivity times could be calculated. The forisome features turned out to be roughly similar in all four species under investigation.
Results
In line with previous work12 we examined four different legumes for forisome location inside the sieve elements (basal, central, apical) and the reactivity of basal forisomes to a burning stimulus. In all tested species we found significant differences in the forisome distribution (V. faba – χ2 = 91.975, df = 2, p < 0.001; P. sativum – χ2 = 66.296, df = 2, p < 0.001; M. sativa – χ2 = 68.977, df = 2, p < 0.001; T. pratense – χ2 = 92.435, df = 2, p < 0.001). V. faba was investigated as a methodological control and the forisome distribution was similar to the one published before12 (see Figure 1 a). The main portion (83.7%) of forisomes was located at the basal side of the sieve element, whereas significantly less forisomes were located centrally (11.25%) or at the apical side (5%). A similar pattern was observed for M. sativa (see Figure 1 b). T. pratense (Figure 1 c) showed a different distribution, albeit not statistically significant, from that in V. faba and M. sativa in that more forisomes were located apically (13%) than in the centre (6.5%). Forisomes in sieve elements of P. sativum were distributed more evenly than in V. faba and M. sativa (Figure 1 d). About half (49.5%) can be found at the basal side and a third (32.1 %) in a central location. In all species, however, the majority of forisomes resided in the vicinity of the basal sieve plates (Figure 1).
Figure 1.
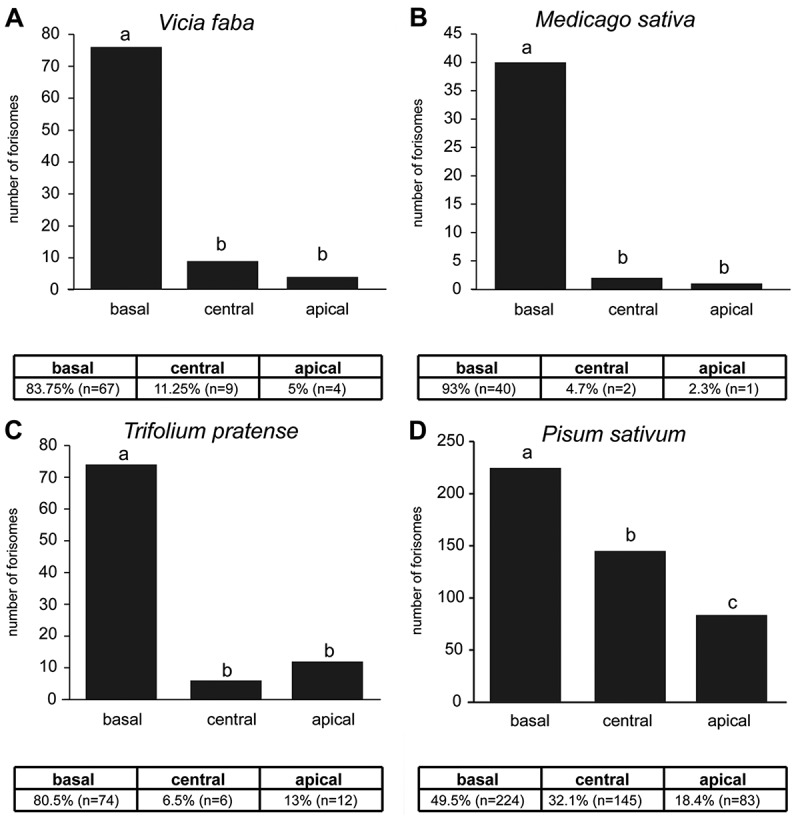
Absolute numbers and percentage of forisome distribution at different locations (basal, central, apical) in sieve elements of A Vicia faba, B Medicago sativa, C Trifolium pratense and D Pisum sativum. Different letters indicate significant differences of the number of forisomes in this location. The forisome location was analysed with a Chi-square test, followed by multiple comparisons, while the familywise error rate was controlled by the Bonferroni correction.
The optical “behavior” of forisomes in response to burning was identical for all species. A few seconds after burning, distant forisomes appeared to vanish abruptly. After some minutes, however, they suddenly showed up again as reconstituted bodies (Figure 2). At re-appearance, the position of the forisome was frequently slightly changed, which was adjusted later so that the forisome finally returned to the original position.
Figure 2.

Dispersion and recondensation of forisomes in response to a remote heat stimulus. A Vicia faba, B Medicago sativa, C Trifolium pratense and D Pisum sativum. The forisomes are marked by asterisks; SE – sieve element; SP – sieve plate.
To reduce the location-dependent variability we only examined the reactivity of basal forisomes (e.g. Figure 2) at a distance of 0.5 to 1.5 cm from the burning side (Figure 3). The percentage of dispersing forisomes did not significantly differ between the respective plant species (χ2 = 3.552, df = 3, p = 0.314, Figure 3a), albeit only 59% reacted in M. sativa as compared to 91% in V. faba. The times from heat stimulus till dispersion and re-condensation were similar between plant species (dispersion: F(3,31) = 1.044, p = 0.387; re-condensation: F(3,29) = 1.636, p = 0.203). Furthermore, the plant species did not influence the duration the forisomes stayed in their dispersed form (F(3,29) = 1.472, p = 0.243).
Figure 3.

Reactivity (dispersion and re-condensation) in response to a remote heat stimulus of basally located forisomes in intact Vicia faba, Medicago sativa, Trifolium pratense and Pisum sativum. A Percent of dispersed forisomes, B duration from burning until dispersion, C duration from dispersion until re-condensation and D duration from burning until re-condensation.
Discussion
As forisomes are unique to legumes, we investigated three additional legume species to broaden the knowledge of these phloem protein bodies. In connection with previous results,12 this short communication allows three principal conclusions as for the universality of forisome behavior:
1. The spatial distribution of forisomes was nearly identical in M. sativa, T. pratense, and V. faba: the majority (around 85%) of the forisomes was located in the proximity of the downstream sieve plates. The forisome distribution in P. sativum was distinctly different with only 50% of the forisomes located near the downstream sieve plates (Figure 1).
2. The forisomes in the species investigated showed the same optical shift to invisibility in the dispersed state (Figure 2).
3. The dispersion reaction times (the time lapse between burning and dispersion) were largely similar in the four species, while the re-condensation reaction times were tended longer in T. pratense (Figure 3).
During this study, preliminary observations asked for further investigations.
The forisome length appeared to be different between the species. Are the forisome dimensions commensurate with interspecific differences in sieve-element diameter?
Are forisome dimensions adapted to the potentially increasing sieve-element diameters towards the petiole?
Does the dispersion reactivity correlate with the distance to the leaf-tip burning site?
Do forisomes disperse abruptly throughout the entire protein body or does the structure gradually collapse?
Does the presence of upstream located forisomes indicate that they are anchored somehow to membrane structures?
What are the implications of the deviating forisome distribution in P. sativum for their behavior?
These and other issues will be tackled in future work.
Material and methods
Plant Material
Vicia faba cv. ‘The Sutton’, Pisum sativum cv. ‘Baccara’, Triofolium pratense cv. ‘Dajana’ and Medicago sativa cv. ‘Giulia’ plants were cultivated in 10 cm diameter plastic pots with a standardized soil mixture (7:20 mixture of Klasmann Tonsubstrat and Klasmann Kultursubstrat TS1, Klasmann-Deilmann GmbH, Geeste, Germany) in a climate chamber (York Refrigeration York, USA) under standard conditions (20 to 22°C, 60 to 70% relative humidity, and a 16/8-hour light/dark period with an irradiance level of 100 to 150 µmol m−2 sec−1 at the plant apex). Test plants were selected in the vegetative phase just before flowering.
Preparation of intact plants and microscopy
For observation of living sieve tubes, cortical cell layers from the lower side of the main vein of a mature (source) leaf were removed down to the phloem, while the leaf was still attached to an intact plant. A fresh razor blade was used to remove the top cell layers by manual slicing.2 The leaf was mounted on a microscope slide with double-sided adhesive tape and the free-lying phloem tissue covered with a bathing medium containing 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 50 mM mannitol and 2.5 mM MES/NaOH buffer, pH 5.7. The viability of the phloem tissue was checked using a microscope (AXIO Imager.M2, Zeiss, Jena, Germany) with a water immersion objective. After exposition of the free-lying phloem to the bathing medium for one hour, forisome reaction was triggered by a heat shock (careful burning of a leaf tip with a match) at a distance of approximately 0.5 to 1.5 cm from the observation window. The events in response to burning were recorded with a color camera (AXIOCAM 503 color Zeiss, Jena, Germany). Digital images were processed with the ZEN software (Zeiss, Jena, Germany) and edited with Adobe® PhotoShop to optimize brightness, contrast and coloring.
Statistics
The forisome location was analysed with a Chi-square test, followed by multiple comparisons, while the familywise error rate was controlled by the Bonferroni correction.
Whether the percentage of forisomes that dispersed after the heat stimulus, differed between different legume species was tested using the test for equality of proportions.
To test whether the time from application of the heat stimulus until forisome dispersion and until recondensation, and the time forisomes stayed in the dispersed form were dependent on the plant species one-way anovas were conducted. In cases of non-homogeneous variances (time from heat stimulus until recondensation, time forisomes stayed in dispersed form), square-root transformations were applied.
All data were analysed using R 3.4.4.14
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft (Grant No. FU 969/2-1 to ACUF) and partly financed by the Max Planck Society.
References
- 1.Peters WS, Haffer D, Hanakam CB, van Bel AJE, Knoblauch M.. Legume phylogeny and the evolution of a unique contractile apparatus that regulates phloem transport. Am J Bot. 2010;97:797–808. doi: 10.3732/ajb.0900328. [DOI] [PubMed] [Google Scholar]
- 2.Knoblauch M, van Bel AJE. Sieve tubes in action. Plant Cell. 1998;10:35–50. [Google Scholar]
- 3.Kauss H. Callose biosynthesis as a calcium-regulated process and possible relations to the induction of other metabolic changes. J Cell Sci. 1985;2:89–103. doi: 10.1242/jcs.1985. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima J, Laosinchai W, Cui X, Brown RM Jr. New insight into the mechanism of cellulose and callose biosynthesis: proteases may regulate callose biosynthesis upon wounding. Cellulose. 2003;10:369–389. doi: 10.1023/A:1027336605479. [DOI] [Google Scholar]
- 5.Delmer DP. Cellulose biosynthesis. Annu Rev Plant Physiol. 1987;38:259–290. doi: 10.1146/annurev.pp.38.060187.001355. [DOI] [Google Scholar]
- 6.Kudlicka K, Brown RM. Cellulose and callose biosynthesis in higher plants. Plant Physiol. 1997;115:643–656. doi: 10.1104/pp.115.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thonat C, Boyer N, Penel C, Courduroux JC, Gaspar T. Cytological indication of the involvement of calcium and calcium-related proteins in the early responses of Bryonia dioica to mechanical stimulus. Protoplasma. 1993;176:133–137. doi: 10.1007/BF01378949. [DOI] [PubMed] [Google Scholar]
- 8.Knoblauch M, Noll GA, Müller T, Prüfer D, Schneider-Hüther I, Scharner D, van Bel AJE, Peters WS. ATP-independent contractile proteins from plants. Nat Mat. 2003;2:600–603. doi: 10.1038/nmat960. [DOI] [PubMed] [Google Scholar]
- 9.Furch ACU, Hafke JB, Schulz A, van Bel AJE. Ca2+-mediated remote control of reversible sieve tube occlusion in Vicia faba. J Exp Bot. 2007;58:2827–2838. doi: 10.1093/jxb/erm143. [DOI] [PubMed] [Google Scholar]
- 10.Furch ACU, van Bel AJE, Fricker MD, Felle HH, Fuchs M, Hafke JB. Sieve element Ca2+ channels as relay stations between remote stimulus and sieve tube occlusion. Plant Cell. 2009;21:2118–2132. doi: 10.1105/tpc.108.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Bel AJE, Furch ACU, Will T, Buxa SV, Musetti R, Hafke JB. Spread the news: systemic dissemination and local impact of Ca2+ signals along the phloem pathway. J Exp Bot. 2014;65:1761–1787. [DOI] [PubMed] [Google Scholar]
- 12.Furch ACU, Buxa SV, van Bel AJE. Similar intracellular location and stimulus reactivity, but differential mobility of tailless (Vicia faba) and tailed forisomes (Phaseolus vulgaris) in intact sieve tubes. PLoS ONE. 2015; doi: 10.1371/journal.pone.0143920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters WS, van Bel AJE, Knoblauch M. The geometry of the forisome-sieve element-sieve plate complex in the phloem of Vicia faba L. leaflets. J Exp Bot. 2006;57:3091–3098. doi: 10.1093/jxb/erl072. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. https://www.R-project.org/ [Google Scholar]


