Abstract
The objectives of this study were to use soybean cake as the raw material for the production of isoflavone powder and compare the effects of different carriers as well as drying methods on the powder quality. Results showed that with spray drying, a level of 40 % maltodextrin as carrier produced the highest yield (mass) of isoflavone powder, followed by 10 % gelatin and 1 % sodium alginate. However, a reversed trend was observed for the isoflavone content. With 1 % sodium alginate, freeze drying generated the greatest yield of isoflavone powder, followed by vacuum drying and spray drying. The isoflavone content also exhibited the same tendency. With poly-γ-glutamic acid (γ-PGA) as carrier, all six levels studied (0.57, 0.28, 0.14, 0.028, 0.014 and 0.003 %) were capable of forming powder containing high amounts of total isoflavone, which was comparable to that using 1% sodium alginate by freeze drying. Both high- and low-molecular-weight γ-PGA showed similar effects in terms of powder yield and isoflavone content.
Keywords: Isoflavone powder, soybean cake, γ-PGA, sodium alginate, maltodextrin
Introduction
Isoflavones, a major class of flavonoids in soybeans, have received great attention in recent years because of their possible roles in the prevention of chronic diseases such as osteoporosis and hypercholesterolemia, as well as alleviation of postmenopausal syndromes [1,2,3]. Twelve isoflavones have been found in soybeans and are divided into four groups, i.e., malonylglucoside, glucoside, acetylglucoside and aglycone, in which the glucoside forms are dominant [4,5]. Soybean cake (defatted soybean meal), a by-product obtained during processing of soybean oil, was found to contain high amounts of isoflavone [6,7]. Thus, it would be a great advantage to the health food industry if the isoflavone in soybean cake could be extracted and processed into powder.
Powder products are often processed by vacuum, freeze or spray drying, but the powder yield and quality can vary depending upon the drying methods and conditions used [8]. For instance, freeze drying is generally considered the most appropriate method to maintain high powder quality, but the capital cost is high. Conversely, spray drying is suitable for commercial powder production, however, this method requires control of several variables like both inlet and outlet temperatures, and the powder quality may be affected during high-temperature drying [9].
Different carriers like gelatin, gum arabic, maltodextrin, cellulose and glycerol monostearate have been used commercially to obtain nonsticky and free flowing powders [10,11], but these carriers suffer a major drawback, namely, a large quantity has to be used for powder formation, which may increase production cost substantially.
Poly-γ-glutamic acid (γ-PGA), a nontoxic and biodegradable polymer consisting of many glutamic acid units linked by amide bonding between α-amino and γ-carboxylic acid groups, is produced from Bacillus sp. [12,13,14]. Both high-molecular-weight γ-PGA (H-γ-PGA) and low-molecular-weight γ-PGA (L-γ-PGA) ranging from 10,000 to 2 million Daltons have been commercialized and they are produced in different ionic forms, i.e., Ca, Na and H-forms. The applications of γ-PGA in removing heavy metals, toxic compounds and artificial dyes have been well documented [12,13,14,15], but the feasibility of using a low level of γ-PGA as carrier for powder formation remains unknown and requires further investigation. The objectives of this study were to isolate isoflavones from soybean cake and determine the effects of various drying methods and carriers, especially γ-PGA, on the yield of isoflavone powder product. The isoflavone content changes as affected by drying conditions were also investigated.
Results and Discussion
Isoflavone content in soybean cake
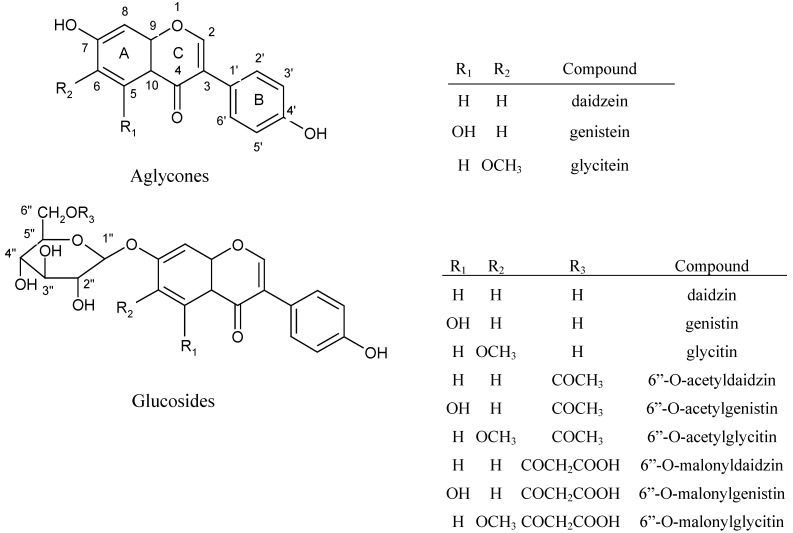
In a previous study we demonstrated that all 12 isoflavones in soybean cake plus the internal standard formononetin could be separated by HPLC within 15 min using a Vydac 201TP54 C18 column [7]. This method is much faster than those reported in the literature [6,16,17]. Figure 1 shows the chemical structures of the 12 isoflavones present in soybean cake. High r2 values ranging from 0.9894~1 were observed for all the calibration curves of the 12 isoflavone standards. The linear regression equations of the 12 isoflavones were: y = 0.2049x–0.0016 (malonyldaidzin), y = 0.1686x+0.0218 (malonylglycitin), y = 0.4492x+0.0081 (malonylgenistin), y = 0.6777x+0.0018 (daidzin), y = 0.2729x–0.0164 (glycitin), y = 0.5138x+0.007 (genistin), y = 0.7899x+0.0048 (acetyldaidzin), y = 0.3716x+0.0087 (acetylglycitin), y = 0.9027x+0.022 (acetylgenistin), y = 0.8639x+0.0313 (daidzein), y = 0.4298x–0.0008 (glycitein) and y = 1.3841x+0.0679 (genistein), respectively. Malonylglucoside was the compound present in the largest amount in soybean cake (2,411 μg/g), followed by glucoside (2,184 μg/g), acetylglucoside (256 μg/g) and aglycone (159 μg/g) for a combined total of 5,010 μg/g [7]. These levels were much higher than those reported by Wang et al. [18] or Coward et al. [19], who found malonylglucoside, glucoside, acetylglucoside and aglycone levels to be 1,013 and 1,340 μg/g, 475 and 249 μg/g, 0 and 207 μg/g, and 24 and 11 μg/g, respectively. These differences may be accounted for by variations in cultivar, growth environment, harvest time and storage conditions [6,20].
Figure 1.
Chemical structures of 12 isoflavones in soybean cake.
Processing of isoflavone powder by spray drying
With gelatin as carrier, it was shown a 5 % level would make spray drying difficult because of the resulting low solids contents. By raising the gelatin level to 15 or 20 %, the isoflavone powder could be processed by spray drying, but the isoflavone content was then too low. Thus, the optimum level of gelatin was chosen to be 10 %. With the inlet temperature set lower than 170 °C, the isoflavone homogenate stuck to the inner tube in the spray dryer and complete drying could only be attained when the inlet temperature reached 180 °C or above with the outlet temperature maintained at 120°. For feed rate, a high value (>10 %) resulted in incomplete drying, while a low one (<10 %) required a longer drying time. Therefore, a value of 10 % was selected as the most suitable feed rate. By choosing the most favorable conditions shown above, that is, gelatin level 10 %, inlet temperature 180 °C, outlet temperature 120 °C and feed rate 10 %, a total amount of 3.6 g powder (6.5% H2O) containing 10,009 μg/g isoflavone was produced after drying.
With maltodextrin as carrier, a 10 % solution was prepared by dissolving 10 g of maltodextrin in 90 g of isoflavone extract and homogenizing for 10 min before spray drying, but the spray-dried powder was found to have a low solids content. By comparing maltodextrin concentrations of 20, 30 and 40 %, a level of 40 % was shown to be the most appropriate. Four feed rates of 10, 15, 20 and 25 % were also compared and the optimum level was found to be 20 %. By controlling the inlet temperature at 180 °C and outlet temperature at 120 °C, a total amount of 7.9 g powder (6.1% H2O) containing 1,887 μg/g isoflavone was obtained after spray drying.
Because of its poor solubility in isoflavone extract, sodium alginate was first dissolved in deionized water and then mixed with isoflavone extract. A concentration of 4 % sodium alginate in water was found to be adequate to maintain a medium viscosity. A final concentration of 1 % sodium alginate in isoflavone extract was prepared by mixing 25 g of aqueous sodium alginate solution and 75 g of isoflavone extract. After homogenization for 10 min, the mixture was subjected to spray drying. By comparing four inlet temperatures (110, 130, 150 and 180 °C), it was shown that higher temperatures (150 and 180 °C) may cause degradation of the isoflavone powder particles, whereas a low temperature (110 °C) could result in inadequate drying. Therefore, the most favorable inlet and outlet temperatures were 130 and 100 °C, respectively. As for feed rate, a 7 % level was optimum as a higher feed rate (≥10 %) led to incomplete drying. A total amount of 1.9 g powder (6.9 % H2O) containing 23,847 μg/g isoflavone was thus generated after spray drying (Table 1).
Table 1.
Effect of drying methods on isoflavone contents (μg/g) A in a powder with 1 % sodium alginate as carrier B.
| Isoflavone | Spray drying | Vacuum drying | Freeze drying |
|---|---|---|---|
| Mdin | 4,619±383c | 6,745±135b | 10,246±333a |
| Mglin | 3,321±331c | 3,788±261b | 4,625±137a |
| Mgin | 4,501±142b | 6,739±22a | 6,640±242a |
| Sub total | 12,441±572c | 17,272±657b | 21,510±437a |
| Din | 1,896±24b | 2,689±82a | 2,736±75a |
| Glin | 1,844±41b | 2,657±213a | 2,765±65a |
| Gin | 5,606±61b | 10,075±113a | 9,964±183a |
| Sub total | 9,346±44b | 15,421±408a | 15,464±322a |
| Adin | 285±4b | 635±38a | 638±42a |
| Aglin | 232±10c | 429±4b | 502±21a |
| Agin | 702±30b | 1,192±74a | 1,161±46a |
| Sub total | 1,219±44b | 2,255±208a | 2,300±67a |
| Dein | 428±44c | 1,138±20b | 1,255±10a |
| Glein | 182±10c | 946±18a | 606±47b |
| Gein | 230±59b | 1,408±33a | 1,288±128a |
| Sub total | 840±5c | 3,492±71a | 3,148±92b |
| Total | 23,847±577c | 38,240±1,061b | 42,423±734a |
A Average of duplicate analyses±standard deviation. B Symbols bearing different letters (a-c) in the same row are significantly different (P<0.05). Mdin: malonyldaidzin; Mglin: malonylglycitin; Mgin: malonylgenistin; Din: daidzin; Glin: glycitin; Gin: genistin; Adin: acetyldaidzin; Aglin: acetylglycitin; Agin: acetylgenistin; Dein: daidzein; Glein: glycitein; Gein: genistein.
Evidently, the yield of isoflavone powder can be affected by both the type of carrier and spray-drying conditions. Fernandez-Perez et al. [21] studied the effects of inlet temperature (100-200 °C) and feed rate (2.5-10 mL/min) on the quality of spray-dried instant soup powder and found that the powder yield increased as the inlet temperature increased (<160 °C). However, a high inlet temperature (200 °C) would decrease the yield of powder, probably due to powder particle degradation. In addition, these authors pointed out that the faster the feed rate and the lower the outlet temperature, the lower the yield of powder. Goula and Adamopoulos [22] also reported that the lower the inlet temperature and the slower the hot air rate, the higher density of the powder was. Likewise, the higher the inlet temperature and the slower the hot air rate, the lower the moisture content of the powder was. Some other authors have suggested that a high feed rate may increase the volume of liquid droplets to thus produce a low yield of powder [23]. Krishnan et al. [24] further stated that carriers such as gelatin and sodium alginate possessed higher viscosity than maltodextrin, which was less efficient in terms of active material or core material encapsulation. Theoretically, the higher the solid content, the larger the yield of powder [21]. But, a high solids content would result in a low amount of active material being encapsulated into the powder. For example, in our study the highest yield of powder (7.9 g) was produced with 40 % maltodextrin as carrier, followed by 10 % gelatin (3.6 g) and 1 % sodium alginate (1.9 g). In contrast, the isoflavone content in the powder followed an increasing trend, with values of 1,887, 10,009 and 23,847 μg/g, respectively.
Production of isoflavone powder by vacuum drying
The results shown above clearly indicate that with 1 % sodium alginate as carrier, a powder product containing high level of isoflavone could be obtained by spray drying. Thus, we further investigated the possibility of processing isoflavone extract into powder with sodium alginate as carrier by vacuum drying. Of the five proportions studied, a powder product of 5.3 and 4.9 g was obtained for the isoflavone solutions containing 1 and 0.4 % sodium alginate, respectively, while the other three levels (0.2, 0.1 and 0.05 %) failed to form powder, probably because of low solids content. In contrast, powder products of 5.9, 5.6, 5.5 and 5.4 g resulted from the isoflavone solutions containing 1, 0.4, 0.2 and 0.1 % H-γ-PGA, respectively, whereas the level of 0.05 % H-γ-PGA failed to produce powder. The isoflavone content in the powder increased with the decreasing concentration of both carriers (Table 2). A concentration of 0.1% H-γ-PGA in the isoflavone extract produced the highest yield (51,564 μg/g) of total isoflavone, followed by the other three concentrations of 0.2, 0.4 and 1 %, which gave 49,900, 46,689 and 38,165 μg/g, respectively. Similarly, a level of 0.4 % sodium alginate in the isoflavone extract generated a greater yield (49,041 μg/g) of total isoflavone than that of 1% (38,661 μg/g). All four groups of isoflavones, namely, malonylglucosides, glucosides, acetylglucosides and aglycones showed the same trend.
Table 2.
Isoflavone contents (μg/g) A in the vacuum-dried powder with sodium alginate and H-γ-PGA (Na) B as carrier C.
| Isoflavones | Carriers | |||||
|---|---|---|---|---|---|---|
| Sodium alginate | H-γ-PGA (Na)B | |||||
| 1 % | 0.4 % | 1 % | 0.4 % | 0.2 % | 0.1 % | |
| Mdin | 8,345±57c | 9,857±91b | 8,170±264c | 9,986±270b | 10,855±255a | 11,315±197a |
| Mglin | 2,853±40e | 3,696±166c | 2,865±23e | 3,137±2d | 4,357±196b | 4,793±32a |
| Mgin | 5,017±148c | 6,410±101a | 4,912±3c | 5,791±117b | 6,507±88a | 6,385±112a |
| Sub total | 16,214±246e | 19,963±358c | 15,947±286e | 18,914±389d | 21,719±29b | 22,492±341a |
| Din | 3,407±12c | 4,480±113a | 3,327±12c | 4,290±24b | 4,345±30ab | 4,338±3ab |
| Glin | 4,217±21d | 5,401±119ab | 3,983±45d | 4,830±96c | 5,277±121b | 5,657±170a |
| Gin | 9,315±42c | 12,356±416a | 9,417±79c | 12,147±165ab | 11,743±126b | 12,087±15ab |
| Sub total | 16,939±51c | 22,238±410a | 16,728±135c | 21,266±237b | 21,365±35b | 22,083±153a |
| Adin | 509±4b | 665±26a | 501±8b | 652±13a | 651±11a | 679±2a |
| Aglin | 482±5d | 787±9a | 466±10d | 567±17c | 734±49ab | 689±17b |
| Agin | 898±99b | 1,098±46a | 873±20b | 1,123±23a | 1,101±29a | 1,124±22a |
| Sub total | 1,889±98c | 2,550±64a | 1,840±19c | 2,342±7b | 2,486±67a | 2,493±37a |
| Dein | 546±5c | 729±13ab | 537±6c | 765±6a | 741±33ab | 714±27b |
| Glein | 365±6c | 580±43a | 357±3c | 461±0b | 445±51b | 479±19b |
| Gein | 2,708±64d | 2,983±82c | 2,757±6d | 2,941±26c | 3,144±31b | 3,304±3a |
| Sub total | 3,618±53d | 4,291±52c | 3,651±3d | 4,167±32c | 4,330±115b | 4,497±22a |
| Total | 38,661±448d | 49,041±168b | 38,165±172d | 46,689±652c | 49,900±117b | 51,564±478a |
A Average of duplicate analyses±standard deviation. B γ-PGA (Na) with high molecular weight. C Symbols bearing different letters (a-e) in the same row are significantly different (P<0.05). Mdin: malonyldaidzin; Mglin: malonylglycitin; Mgin: malonylgenistin; Din: daidzin; Glin: glycitin; Gin: genistin; Adin: acetyldaidzin; Aglin: acetylglycitin; Agin: acetylgenistin; Dein: daidzein; Glein: glycitein; Gein: genistein.
The above results indicated the most appropriate concentrations for sodium alginate and H-γ-PGA in isoflavone extract were 0.4 and 0.1 %, respectively, with the proportion of carrier to isoflavone extract being 1:4 and 1:19 (w/w). Therefore, it is necessary to investigate the possibility of producing powder product by lowering the levels of both carriers. Four levels of 0.4, 0.2, 0.04 and 0.02 % of sodium alginate, and 0.1, 0.05, 0.01 and 0.005 % of both H-γ-PGA and L-γ-PGA, were each prepared in isoflavone extract as described above.
A powder product of 4.9, 4.7 and 4.6 g was formed for alginate levels of 0.4, 0.2 and 0.04 %, respectively. For both H-γ-PGA and L-γ-PGA levels at 0.1, 0.05 and 0.01 %, a powder product of 5.4, 5.2 and 5.2 g was generated for the former and 5.6, 5.3 and 5.3 g for the latter. Levels of 0.02 % sodium alginate and 0.005 % of H-γ-PGA or L-γ-PGA both failed to form powder, which may be due to the presence of a low solids content. The highest amount of malonylglucoside was found for 0.01 % H-γ-PGA and L-γ-PGA, which equaled 27,052 and 26,868 μg/g, respectively, followed by 0.05 % H-γ-PGA and L-γ-PGA (25,498 and 25,059 μg/g), and 0.04 and 0.2 % sodium alginate (24,764 and 21,978 μg/g). The same trend also occurred for both acetylglucoside and aglycone. However, only minor differences were shown for glucoside (Table 3). For total isoflavone, it also followed a similar trend as the four groups of isoflavones.
Table 3.
Isoflavone contents (μg/g) A in the vacuum-dried powder with H-γ-PGA (Na) B and L-γ-PGA (Na) C or sodium alginate as carrier D.
| Isoflavones | Carriers | |||||
|---|---|---|---|---|---|---|
| Sodium alginate | H-γ-PGA (Na)B | L-γ-PGA (Na)C | ||||
| 0.20% | 0.04% | 0.05% | 0.01% | 0.05% | 0.01% | |
| Mdin | 10,915±47e | 12,459±110c | 12,934±247b | 13,613±187a | 12,971±283b | 13,693±74a |
| Mglin | 5,287±39a | 5,377±187a | 5,389±110a | 5,558±26a | 5,323±64a | 5,444±135a |
| Mgin | 5,776±407d | 6,928±21b | 7,176±171b | 7,882±77a | 6,765±187bc | 7,731±248a |
| Sub total | 21,978±415c | 24,764±318b | 25,498±529b | 27,052±290a | 25,059±534b | 26,868±309a |
| Din | 3,992±235b | 4,365±115a | 4,471±128a | 4,390±175a | 4,419±24a | 4,448±144a |
| Glin | 5,073±294b | 5,099±366b | 5,104±64b | 5,182±80ab | 5,073±19a | 5,148±33a |
| Gin | 1,2051±58b | 12,731±352a | 12,798±217a | 12,772±93a | 12,513±186a | 12,669±214a |
| Sub total | 21,117±587b | 22,195±101a | 18,777±326a | 22,344±348a | 22,005±182ab | 22,266±324a |
| Adin | 849±44b | 951±24a | 759±28c | 887±29ab | 778±28c | 868±2b |
| Aglin | 680±28c | 717±20bc | 667±8c | 856±64a | 657±14d | 880±31a |
| Agin | 1,121±22b | 1,211±71ab | 954±32c | 1,313±61a | 968±15d | 1,360±6a |
| Sub total | 2,650±6b | 2,879±114a | 2,380±53c | 3,056±154a | 2,403±57d | 3,109±27a |
| Dein | 637±51c | 688±6bc | 636±20c | 806±27a | 652±16bc | 816±40a |
| Glein | 558±1b | 595±27ab | 632±15ab | 664±47a | 623±15a | 614±51a |
| Gein | 3,287±35b | 3,397±59ab | 3,402±156ab | 3,518±95a | 3,355±9ab | 3,458±56a |
| Sub total | 4,482±86bc | 4,679±91b | 4,670±151b | 4,989±169a | 4,630±8b | 4,888±67a |
| Total | 50,227±92d | 54,517±625b | 54,922±150b | 57,441±962a | 54,098±765b | 57,130±673a |
A Average of duplicate analyses±standard deviation. B γ-PGA (Na) with high molecular weight. C γ-PGA (Na) with low molecular weight. D Symbols bearing different letters (a-f) in the same row are significantly different (P<0.05). Mdin: malonyldaidzin; Mglin: malonylglycitin; Mgin: malonylgenistin; Din: daidzin; Glin: glycitin; Gin: genistin; Adin: acetyldaidzin; Aglin: acetylglycitin; Agin: acetylgenistin; Dein: daidzein; Glein: glycitein; Gein: genistein.
Processing of isoflavone powder by freeze drying
Following the same approach, a mixture (100 g) containing sodium alginate and isoflavone extract was freeze dried at –40° under a 60 millitorr vacuum and with a drying time of 24 h. A powder product of 6.6 g (9.1 % H2O) was obtained which contained the highest level of malonylglucoside, glucoside, acetylglucoside and aglycone (Table 1). Of the various dried products, freeze-dried powder contained the highest content of total isoflavone, followed by vacuum-dried and spray-dried powder (Table 1). However, for aglycone, vacuum drying was shown to produce a higher level than freeze drying, probably caused by conversion from glucose-containing isoflavone during extensive vacuum drying at 60 °C. In a study dealing with the conversion and degradation of isoflavone during heating, Chien et al. [5] demonstrated that aglycone can be formed from malonylglucoside, glucoside or acetylglucoside depending on heating temperature and time. Compared to vacuum drying, freeze drying showed a higher level of malonylglucoside, but no significant difference was found in the contents of acetylglucoside (Table 1). Obviously, the highest yield of total isoflavone was accomplished by freeze drying, since both conversion and degradation of isoflavones were minimized. Nevertheless, vacuum drying generated a higher yield of powder (7.2 g) than either freeze drying (6.6 g) or spray drying (1.9 g).
A similar outcome was reported by Monsoor [25], who evaluated the effects of several drying methods on the functional properties of soy hull pectin, the lowest yield of powder being observed for spray drying, whereas there is no significant difference between freeze drying and vacuum drying. In our study, freeze-dried powder contained a higher amount of moisture (9.1 %) than vacuum dried (7.2 %) and spray dried (6.9 %), which may be explained by formation of porous structures in the powder during sublimation, which should be more prone to moisture absorption after drying [26]. The same phenomenon was reported by Tsami et al. [27], who found that freeze-dried powder could absorb moisture more readily than vacuum-dried powder, which can be attributed to the former being capable of forming a large number of pores with much smaller diameter.
By comparing the results shown above, freeze drying was proven to be the best method for production of powder with high-levels of isoflavone. Thus, we further investigated the effects of H-γ-PGA and L-γ-PGA on the production of isoflavone powder by freeze drying. Because of the high moisture absorption capacity and poor solubility in isoflavone extract, both H-γ-PGA and L-γ-PGA were dissolved separately in water to obtain a medium viscosity and a concentration of 2 %. In the beginning a mixture containing 25 g of H-γ-PGA or L-γ-PGA solution and 75 g of isoflavone extract was placed in a freezer (–30°) for 24 h. However, this mixture failed to freeze, mainly due to the presence of ethanol in the isoflavone extract. After various studies, a mixture containing 40 g of H-γ-PGA or L-γ-PGA solution and 100 g of isoflavone extract (0.28 % H-γ-PGA or L-γ-PGA in isoflavone extract) was frozen after 24 h at –30 °C. Six concentrations of 0.57, 0.28, 0.14, 0.028, 0.014 and 0.003 % H-γ-PGA or L-γ-PGA in isoflavone extract were prepared separately and placed in a freezer until frozen, after which each mixture was freeze dried at –40 °C under 60 millitorr for 24 h, and powder products of 8.4, 7.8, 7.9, 7.9, 7.6 and 7.5 g were produced, respectively, for H-γ-PGA, whereas for L-γ-PGA, a powder product of 8.4, 8.1, 7.7, 7.1, 7.5 and 7.7 g was formed. Of the four groups of isoflavones, both malonylglucosides and glucosides dominated for both powders containing H-γ-PGA and L-γ-PGA at all six levels (Table 4 and Table 5). Only slight differences between acetylglucoside and aglycone were noted, probably because they are present in much smaller amounts in isoflavone extract. With 0.003 % H-γ-PGA or L-γ-PGA, the contents of total isoflavone in the freeze dried powder were 48,144 and 48,155 μg/g, respectively (Table 4 and Table 5). Only minor differences were observed for 0.014 % H-γ-PGA and L-γ-PGA, which gave 48,204 and 47,245 μg/g, respectively. The same phenomenon also applied to H-γ-PGA and L-γ-PGA with levels at 0.28 and 0.57 %. No significant difference was found between 1 % sodium alginate and 0.57% H-γ-PGA or L-γ-PGA (Table 4 and Table 5) for total isoflavone in the freeze dried powder. This result further demonstrated that a high yield of powder containing a large amount of isoflavone could be attained by using an extremely low level of 0.003 % H-γ-PGA or L-γ-PGA, and was comparable to that using 1 % sodium alginate as carrier. As mentioned before, with γ-PGA as carrier the powder production cost could be reduced greatly.
Table 4.
Isoflavone contents (μg/g) A in the freeze-dried powder with H-γ-PGA (Na) B or sodium alginate as carrier C.
| Isoflavones | Carriers | ||||||
|---|---|---|---|---|---|---|---|
| 1 % sodium alginate | H-γ-PGA (Na)B | ||||||
| 0.57 % | 0.28 % | 0.14 % | 0.028 % | 0.014 % | 0.003 % | ||
| Mdin | 9,160±92d | 9,562±258d | 10,527±166c | 10,768±13c | 12,476±273ab | 12,802±454a | 12,055±195b |
| Mglin | 3,118±141ef | 3,620±442bcd | 3,930±10b | 3,848±1bc | 3,436±365cde | 3,868±43bc | 3,982±39b |
| Mgin | 7,176±25d | 7,404±162d | 7,732±311c | 7,791±45bc | 7,958±75abc | 7,867±122bc | 8,056±29ab |
| Sub total | 19,455±258e | 20,586±538d | 22,189±466c | 2,2407±30c | 23,870±563b | 24,537±534ab | 24,093±205ab |
| Din | 3,579±18a | 3,707±27d | 3,902±64bc | 3,950±3abc | 4,030±85a | 3,896±41bc | 3,984±16ab |
| Glin | 4,071±21a | 3,917±131a | 4,106±135a | 4,241±52a | 4,253±67a | 4,159±184a | 4,323±10a |
| Gin | 10,437±77e | 11,094±157def | 11,770±332a | 11,648±336ab | 11,441±3abcde | 11,174±102cdef | 11,315±37bcde |
| Sub total | 18,088±116cd | 18,719±1cde | 19,779±531a | 19,838±385a | 19,724±156a | 19,230±327abc | 19,622±43ab |
| Adin | 651±8ab | 696±22abcd | 716±46ab | 731±20a | 699±24abcd | 679±14abcd | 726±6a |
| Aglin | 641±5d | 672±68ab | 643±5ab | 667±23ab | 719±119ab | 795±130a | 735±50ab |
| Agin | 1,078±2b | 1,148±38bcd | 1,183±41abc | 1,192±5abc | 1,218±2ab | 1,184±67abc | 1,193±1abc |
| Sub total | 2,370±11a | 2,516±127ab | 2,543±93ab | 2,590±3ab | 2,637±74a | 2,658±210a | 2,654±55a |
| Dein | 715±8b | 740±46a | 768±61a | 784±18a | 727±24a | 718±43a | 764±5a |
| Glein | 441±9abc | 462±40b | 474±46b | 523±51ab | 486±45b | 470±42b | 591±12a |
| Gein | 539±29abcde | 542±28abc | 573±14a | 554±19ab | 429±12d | 430±5d | 419±2d |
| Sub total | 1,694±27e | 1,744±58abcde | 1,814±120abc | 1,860±52a | 1,642±81cde | 1,618±79de | 1,774±19abcd |
| Total | 41,607±126e | 43,565±352d | 46,325±1210bc | 46,695±306abc | 47,873±400ab | 48,204±923a | 48,144±198a |
A Average of duplicate analyses±standard deviation. B γ-PGA (Na) with high molecular weight. C Symbols bearing different letters (a-f) in the same row are significantly different (P<0.05). Mdin: malonyldaidzin; Mglin: malonylglycitin; Mgin: malonylgenistin; Din: daidzin; Glin: glycitin; Gin: genistin; Adin: acetyldaidzin; Aglin: acetylglycitin; Agin: acetylgenistin; Dein: daidzein; Glein: glycitein; Gein: genistein.
Table 5.
Isoflavone contents (μg/g)A in the freeze-dried powder with L-γ-PGA (Na)B or sodium alginate as carrier C.
| Isoflavones | Carriers | ||||||
|---|---|---|---|---|---|---|---|
| 1 % sodium alginate | L-γ-PGA (Na)B | ||||||
| 0.57 % | 0.28 % | 0.14 % | 0.028 % | 0.014 % | 0.003 % | ||
| Mdin | 9,160±92d | 9242±125d | 10256±320c | 10665±66c | 12230±75b | 11949±637b | 12386±570ab |
| Mglin | 3,118±141ef | 2940±111f | 3402±289de | 3936±93b | 3955±99b | 4760±83a | 4718±38a |
| Mgin | 7,176±25d | 7186±58d | 8200±127a | 7891±92bc | 7945±57abc | 7455±160d | 7946±23abc |
| Sub total | 19,455±258e | 19368±179e | 21857±736c | 22491±251c | 24130±231ab | 24164±880ab | 25050±632a |
| Din | 3,579±18a | 3606±42e | 3864±29c | 3958±5abc | 4048±50a | 3857±88c | 3864±40c |
| Glin | 4,071±21a | 4080±441a | 4075±53a | 4114±70a | 4125±207a | 4052±108a | 4132±144a |
| Gin | 10,437±77e | 10796±30fg | 11508±199bcd | 11590±19abc | 11550±109abc | 11039±285ef | 11123±63def |
| Sub total | 18,088±116cd | 18481±453de | 19447±222ab | 19661±94ab | 19723±148a | 18948±480cde | 19119±247abcd |
| Adin | 651±8ab | 699±46abcd | 711±10abc | 689±27abcd | 696±35abcd | 642±12d | 659±23bcd |
| Aglin | 641±5d | 648±15ab | 682±98ab | 652±43ab | 597±7b | 767±25a | 587±37b |
| Agin | 1,078±2b | 1123±47cd | 1188±1abc | 1242±14a | 1175±24abc | 1124±24cd | 1154±28bc |
| Sub total | 2,370±11a | 2470±79ab | 2581±109ab | 2582±2ab | 2468±65ab | 2533±60ab | 2399±89b |
| Dein | 715±8b | 746±52a | 783±38a | 768±6a | 712±23a | 703±37a | 711±29a |
| Glein | 441±9abc | 495±41ab | 501±9ab | 506±70ab | 451±38b | 472±44b | 448±54b |
| Gein | 539±29abcde | 523±18bc | 546±11abc | 554±15ab | 509±12c | 424±19d | 427±2d |
| Sub total | 1,694±27e | 1,764±111abcde | 1830±58ab | 1829±91ab | 1672±28bcde | 1599±100de | 1586±85e |
| Total | 41,607±126e | 42083±465de | 45715±347c | 46564±435abc | 47993±46ab | 47245±1521abc | 48155±1052a |
A Average of duplicate analyses±standard deviation. B γ-PGA (Na) with low molecular weight.C Symbols bearing different letters (a-g) in the same row are significantly different (P<0.05). Mdin: malonyldaidzin; Mglin: malonylglycitin; Mgin: malonylgenistin; Din: daidzin; Glin: glycitin; Gin: genistin; Adin: acetyldaidzin; Aglin: acetylglycitin; Agin: acetylgenistin; Dein: daidzein; Glein: glycitein; Gein: genistein.
Table 6 shows the characteristics of various isoflavone powders made by different drying methods. Both spray-dried and freeze-dried powder possessed a slightly yellow appearance, while vacuum-dried powder was deep yellow, which may be due to long-time drying at 60 °C.
Table 6.
Characteristics of the various isoflavone powder made by different drying methods.
| Drying method | Carrier | Carrier content (%) | Color | Powder weight (g) |
Water content (%) | Isoflavone content (μg/g) |
|---|---|---|---|---|---|---|
| Spray-drying | Gelatin | 10 | Light yellow | 3.6 | 6.5 | 10,009 |
| Maltodextrin | 40 | Light yellow | 7.9 | 6.1 | 1,887 | |
| Sodium Alginate | 1 | Light yellow | 1.9 | 6.9 | 23,847 | |
| Freeze-drying | Sodium Alginate | 1 | Yellow to white | 6.6 | 9.1 | 43,423 |
| H-γ-PGA (Na) A | 0.570 | Light yellow | 8.4 | 14.0 | 43,565 | |
| H-γ-PGA (Na) | 0.280 | Light yellow | 7.8 | 14.8 | 46,325 | |
| H-γ-PGA (Na) | 0.140 | Light yellow | 7.9 | 16.1 | 46,695 | |
| H-γ-PGA (Na) | 0.028 | Light yellow | 7.9 | 17.5 | 47,873 | |
| H-γ-PGA (Na) | 0.014 | Light yellow | 7.6 | 16.7 | 48,204 | |
| H-γ-PGA (Na) | 0.003 | Light yellow | 7.5 | 17.9 | 48,144 | |
| L-γ-PGA (Na)B | 0.570 | Light yellow | 8.4 | 13.2 | 42,083 | |
| L-γ-PGA (Na) | 0.280 | Light yellow | 8.1 | 14.7 | 45,715 | |
| L-γ-PGA (Na) | 0.140 | Light yellow | 7.7 | 15.9 | 46,564 | |
| L-γ-PGA (Na) | 0.028 | Light yellow | 7.1 | 17.1 | 47,993 | |
| L-γ-PGA (Na) | 0.014 | Light yellow | 7.5 | 17.4 | 47,245 | |
| L-γ-PGA (Na) | 0.003 | Light yellow | 7.7 | 17.9 | 48,155 | |
| Vacuum-drying | Sodium Alginate | 1 | Yellow | 5.3 | 4.4 | 38,601 |
| Sodium Alginate | 0.40 | Yellow | 4.9 | 4.5 | 49,041 | |
| Sodium Alginate | 0.20 | Yellow | 4.7 | 4.6 | 50,227 | |
| Sodium Alginate | 0.04 | Yellow | 4.6 | 4.9 | 54,517 | |
| H-γ-PGA (Na) | 0.10 | Yellow | 5.4 | 5.7 | 51,564 | |
| H-γ-PGA (Na) | 0.05 | Yellow | 5.2 | 5.8 | 54,922 | |
| H-γ-PGA (Na) | 0.01 | Yellow | 5.2 | 5.8 | 57,441 | |
| L-γ-PGA (Na) | 0.10 | Yellow | 5.6 | 5.4 | 52,542 | |
| L-γ-PGA (Na) | 0.05 | Yellow | 5.3 | 5.6 | 54,098 | |
| L-γ-PGA (Na) | 0.01 | Yellow | 5.3 | 5.5 | 57,130 |
A γ-PGA (Na) with high molecular weight. Bγ-PGA (Na) with low molecular weight.
Tsami et al. [27] also reported that pectin-sugar powder made by vacuum drying exhibited deeper color than with freeze drying. With spray drying, the maltodextrin carrier produced the highest yield of powder, followed by gelatin and sodium alginate. With sodium alginate as carrier, freeze drying generated the greatest yield of powder, followed by vacuum drying and spray drying. It was also observed that the powder weight increased following a rise in carrier content, which resulted in a decrease of isoflavone level because of the dilution effect.
Conclusions
With spray drying, use of a level of 40 % maltodextrin as carrier results in a higher yield of isoflavone powder than with 10 % gelatin or 1 % sodium alginate as carriers. Freeze drying can generate a larger amount of isoflavone powder than vacuum drying and spray drying with 1 % sodium alginate as carrier. Compared to sodium alginate, an extremely low level of γ-PGA (0.003 %) as carrier can be used to produce powder containing high amount of total isoflavone. The results of this study may provide a basis for possible commercial production of isoflavone powder in the food industry. Further research is necessary to study the stability of isoflavone powder during storage.
Experimental Section
Materials
Soybean cake (50 kg) was purchased from Chung-Lian Oil Co. (Taichung, Taiwan), ground into powder and stored at -20° for use. Twelve isoflavone standards were obtained from LC Laboratories (Woburn, MA, USA), Sigma (St. Louis, MO, USA) and Nacalai (Kyoto, Japan). Formononetin (internal standard) was from Sigma. HPLC-grade acetonitrile was from Merck (Darmstadt, Germany). Analytical-grade ethanol (95%) was from Taiwan Tobacco and Wine Co. (Tainan, Taiwan). Deionized water was made using a Milli-Q water purification system (Millipore Co., Bedford, MA, USA). Gelatin, maltodextrin and sodium alginate were from Cheng-Fang Co. (Taipei, Taiwan). Both high-molecular-weight-γ-PGA (H-γ-PGA, MW 800 kDa) and low-molecular-weight-γ-PGA (L-γ-PGA, MW 200-400 kDa) in Na form were provided by Vedan Enterprise Corp. (Taichung, Taiwan).
Instrumentation
The HPLC instrument was composed of a Rheodyne 7161 injector (CA, USA), two Jasco pumps (PU 980 and PU 1980) and a Jasco MD-915 photodiode-array detector (Tokyo, Japan). Borwin computer software was used to process data. A Vydac 201TP54 C18 column (250 x 4.6 mm I.D., 5 μm) was used to separate the 12 isoflavones in soybean cake and powder. The Büchi Mini B290 spray dryer was from Büchi Co. (Flawil, Switzerland). The FD24 freeze dryer was from Ching-Ming Co. (Taipei, Taiwan). The KVED-1 vacuum dryer was from Tong-Yuan Co. (Taipei, Taiwan). The Sorvall RC5C high-speed centrifuge was from Du Pont Co. (Wilmington, Delaware, USA). The PT-MR 3000 homogenizer was from Kinematica Co. (Switzerland).
Extraction of isoflavone from soybean cake
Soybean cake (3 kg) was mixed with deionized water-ethanol (1:1, v/v, 9-L) in a flask, and the mixture was shaken for 2 h. Then the crude extract was divided into 10 portions and each subjected to centrifugation at 6,000 rpm for 20 min (25 °C), after which all the supernatants were collected and filtered through a glass filter paper. All the filtrates were combined to give approximately 4500-mL of filtrate that was used as raw material for processing the powder.
Processing of isoflavone powder by spray drying
Because of differences in solubility of the carriers in isoflavone extract, various proportions of gelatin, maltodextrin or sodium alginate to isoflavone extract have to be tried to find the optimum concentration. Four concentrations of 5, 10, 15 and 20 % gelatin in isoflavone extract were prepared by mixing 5, 10, 15 and 20 g of gelatin with 95, 90, 85 and 80 g of isoflavone extract, respectively. Similarly, four concentrations of 10, 20, 30 and 40 % maltodextrin in isoflavone extract were prepared by mixing 10, 20, 30 and 40 g of maltodextrin with 90, 80, 70 and 60 g of isoflavone extract, respectively. For sodium alginate, it was first dissolved in deionized water for a concentration of 4 % because of its poor solubility in isoflavone extract. Then a sodium alginate solution (25-g) was mixed with isoflavone extract (75-g) to obtain a final concentration of 1 %. Each carrier-containing isoflavone solution was subjected to homogenization for 10 min and then spray dried under the following conditions: feed rate 5, 10, 15 and 20 % and inlet temperatures 150, 160, 170 and 180 °C for gelatin solution; feed rates 10, 15, 20 and 25 % and inlet temperatures 150, 160, 170 and 180 °C for maltodextrin solution; feed rates 5, 7 and 10 %, and inlet temperatures 110, 130, 150 and 180 °C for sodium alginate solution. These conditions were chosen based on the different characteristics of carrier solutions like viscosity and solid content. After processing into powder, a sample (0.05 g) of either gelatin- and sodium alginate-containing powder or a sample (0.25 g) of maltodextrin-containing powder was separately dissolved in deionized water (5-mL). Then each solution (500 μL) was mixed with formononetin internal standard (200 μg/mL, 100 μL), followed by addition of deionized water (400-μL) and filtration through a 0.2-μm membrane filter. A 20-μL sample was injected for HPLC analysis.
Processing of isoflavone powder by vacuum drying
As the powder formation during vacuum drying can be affected by solids content, it is necessary to find out the optimum proportion of carrier to isoflavone extract. A concentration of 2 % sodium alginate and H-γ-PGA in deionized water was prepared separately, following by collecting 50, 20, 10, 5 and 2.5 g and mixing with 50, 80, 90, 95 and 97.5 g of isoflavone extracts, respectively. Proportions of carrier (sodium alginate or H-γ-PGA) to isoflavone extract at 1:1, 1:4, 1:9, 1:19 and 1:39 (w/w) were thus obtained with final concentrations of carrier of 1.0, 0.4, 0.2, 0.1 and 0.05%, respectively. After homogenization for 10 min, each solution was vacuum dried at 60 °C under 4 cm Hg for 48 h and then ground into powder. A powder sample (0.05 g) was dissolved in deionized water (5-mL), of which an aliquot (500-μL) was collected and mixed with formononetin internal standard (200 μg/mL, 100 μL) and diluted with deionized water (400 μL). The solution was filtered through a 0.2-μm membrane filter and 20 μL was injected for HPLC analysis.
To investigate the possibility of using a low level of carrier for production of isoflavone powder, four concentrations of 2, 1, 0.2 and 0.1 % of aqueous sodium alginate solution were prepared, and 20-g of each was collected and mixed with 80 g of isoflavone extract to obtain final concentrations of 0.4, 0.2, 0.04 and 0.02 %, respectively. Each solution was homogenized for 10 min and then vacuum dried at 60 °C under 4 cm Hg for 36 h. Likewise, four concentrations of 2, 1, 0.2 and 0.1 % aqueous solutions of both H-γ-PGA and L-γ-PGA were prepared, in which 5-g of each was collected and mixed with 95-g of isoflavone extract for the final concentrations at 0.1, 0.05, 0.01 and 0.005 %, respectively, and each solution was subjected to homogenization and vacuum drying. A 0.05-g of sample powder was collected for HPLC analysis following the same procedure described above.
Processing of isoflavone powder by freeze drying
For freeze drying, both sodium alginate and γ-PGA were not directly dissolved in isoflavone extract containing 50 % ethanol because of poor solubility. Instead, they were dissolved in deionized water to make freezing possible. Six concentrations of 4, 2, 0.4, 0.2, 0.04 and 0.02 % of sodium alginate in water were prepared, and 25-g of each was mixed with 75-g of isoflavone extract for the final concentrations of 1, 0.5, 0.1, 0.05, 0.01 and 0.005 %, respectively. After homogenization for 10 min, each solution was placed in a freezer (–30 °C) for 24 h for freezing. Similarly, six concentrations of 2, 1, 0.5, 0.1, 0.05 and 0.01 % of γ-PGA dissolved in water were prepared, and 40-g of each was mixed with 100-g of isoflavone extract giving final concentrations of 0.57, 0.28, 0.14, 0.028, 0.014 and 0.003 %, respectively. Each solution was homogenized for 10 min and then placed in a freezer (–30 °C) for 24 h. Next, all the sodium alginate and γ-PGA solutions were freeze dried at –40° under 60 millitorr for 36 h and subjected to grinding for powder production. A powder sample (0.05-g) was dissolved in deionized water (5-mL), of which 500 μL was collected and mixed with 100-μL internal standard formononetin (200 μg/mL) and 400-μL deionized water. The solution was filtered through a 0.2-μm membrane filter and a 20-μL sample was injected for HPLC analysis.
Identification and quantitation of isoflavone
The various isoflavones in soybean cake and powder were identified by comparing retention times and absorption spectra of unknown peaks with reference standards and co-chromatography with added standards. For quantitation, each isoflavone standard was dissolved in methanol for a concentration of 200 or 2000 μg/mL, while the internal standard (formononetin) was dissolved in methanol for a concentration of 200 μg/mL. Three standard concentrations of 1, 5 and 10 μg/mL of the 12 isoflavones were each prepared by mixing 25, 125 and 250 μL (from 200 μg/mL), respectively, with 500 μL internal standard (from 200 μg/mL) and diluting to 5 mL with methanol. Likewise, the other three standard concentrations of 20, 50 and 100 μg/mL for the 12 isoflavones were each prepared by mixing 50, 125 and 250 μL (from 2000 μg/mL), respectively, with 500 μL internal standard (from 200 μg/mL) and diluting to 5 mL with methanol. The final concentration of internal standard in each standard solution was 20 μg/mL. A 20-μL isoflavone standard solution of each was injected and 12 standard curves were prepared by plotting concentration ratio against area ratio, and the linear regression equation and correlation coefficient (r2) of each standard curve was calculated. A gradient mobile phase developed by Hsieh et al. [28] was used to separate the 12 isoflavones: a mixture of acetonitrile (A) and deionized water (B) (8:92, v/v) was used initially, increased to 10% A in 2 min, 12% A in 3 min, 22% A in 10 min, 23% A in 11 min, 35% A in 12 min, 50% A in 13 min, maintained for 3 min and returned to 8% A in 20 min. All 12 isoflavones were adequately resolved within 15 min with a flow rate of 2.0 mL/min and a column temperature of 35 °C and detection wavelength at 262 nm. Each isoflavone was quantified using a formula as described by Kao and Chen [6]. Duplicate analyses were performed, and the data were processed using SAS [29] and subjected to analysis of variance and Duncan’s multiple range test for comparison (α=0.05).
Footnotes
Sample Availability: Available from the authors.
References
- 1.Setchell K.D.R., Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J. Nutr. 1999;129:758s–767s. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 2.Messina M., Gugger E.T., Alekel D.L. Handbook of Nutraceuticals and Functional Foods. CRC Press LLC; Boca Raton, FL, USA: 2001. Soy protein, soybean isoflavones and bone health: a review of the animal and human data; pp. 77–98. [Google Scholar]
- 3.Demonty I., Lamarche B., Jones P.J.H. Role of isoflavones in the hypocholesterolemic effect of soy. Nutr. Rev. 2003;61:189–203. doi: 10.1301/nr.2003.jun.189-203. [DOI] [PubMed] [Google Scholar]
- 4.Kao T.H., Lu. Y.F., Hsieh H.C., Chen B.H. Stability of isoflavone glucosides during processing of soymilk and tofu. Food Res. Intl. 2004;37:891–900. [Google Scholar]
- 5.Chien J.T., Hsieh H.C., Kao T.H., Chen B.H. Kinetic model for studying the conversion and degradation of isoflavones during heating. Food Chem. 2005;91:425–434. doi: 10.1016/j.foodchem.2004.06.023. [DOI] [Google Scholar]
- 6.Kao T.H., Chen B.H. An improved method for determination of isoflavones in soybean powder by liquid chromatography. Chromatographia. 2002;56:423–430. doi: 10.1007/BF02492004. [DOI] [Google Scholar]
- 7.Kao T.H., Lu Y.F., Chen B.H. Preparative chromatography of four groups of isoflavones from soybean cake. Euro. Food Res. Technol. 2005;221:459–465. doi: 10.1007/s00217-005-1206-4. [DOI] [Google Scholar]
- 8.Wang C.Y., Chen B.H. Tomato pulp as source for the production of lycopene powder containing high proportion of cis isomers. Euro. Food Res. Technol. 2006;222:347–353. doi: 10.1007/s00217-005-0058-2. [DOI] [Google Scholar]
- 9.Bhandari B.R., Dumoulin E.D., Richard H.M.J., Noleau I., Lebert A.M. Flavor encapsulation by spray drying: application to citral and linalyl acetate. J. Food Sci. 1992;57:217–220. doi: 10.1111/j.1365-2621.1992.tb05459.x. [DOI] [Google Scholar]
- 10.Jaya S., Das H. Effect of maltodextrin, glycerol monostearate and tricalcium phosphate on vacuum dried mango powder properties. J. Food Eng. 2004;63:125–134. doi: 10.1016/S0260-8774(03)00135-3. [DOI] [Google Scholar]
- 11.Cano-Chauca M.P., Stringheta P.C., Ramos A.M., Cal-Vidal J. Effect of carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. Technol. 2005;6:420–428. doi: 10.1016/j.ifset.2005.05.003. [DOI] [Google Scholar]
- 12.Inbaraj B.S., Chiu C.P., Ho G.H., Yang J., Chen B.H. Removal of cationic dyes from aqueous solution using an anionic poly-γ-glutamic acid-based adsorbent. J. Hazard. Mater. 2006;137:226–234. doi: 10.1016/j.jhazmat.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 13.Inbaraj B.S., Chiu C.P., Chiu Y.T., Ho G.H., Yang J., Chen B.H. Effect of pH on binding of mutagenic heterocyclic amines by the natural biopolymer poly(γ-glutamic acid) J. Agric. Food Chem. 2006;54:6452–6459. doi: 10.1021/jf061300o. [DOI] [PubMed] [Google Scholar]
- 14.Inbaraj B.S., Chien J.T., Ho G.H., Yang J., Chen B.H. Equilibrium and kinetic studies on sorption of basic dyes by a natural biopolymer poly(γ-glutamic acid) Biochem. Eng. J. 2006;31:204–215. doi: 10.1016/j.bej.2006.08.001. [DOI] [Google Scholar]
- 15.Gutnick D.L., Bach H. Engineering bacterial biopolymer for the biosorption of heavy metals: new products and novel formulations. Appl. Microbiol. Biotechnol. 2000;54:451–460. doi: 10.1007/s002530000438. [DOI] [PubMed] [Google Scholar]
- 16.Murphy P.A., Song T., Buseman G., Barua K. Isoflavones in soy-based infant formulas. J. Agric. Food Chem. 1997;45:4635–4638. [Google Scholar]
- 17.Franke A.A., Hankin J.H., Yu M.C., Maskarinec G., Low S.H., Cluster L.J. Isoflavone levels in soy foods consumed by multiethnic populations in Singapore and Hawaii. J. Agric. Food Chem. 1999;47:977–986. doi: 10.1021/jf9808832. [DOI] [PubMed] [Google Scholar]
- 18.Wang C., Ma Q., Pagadala S., Sherrard M.S., Krishnan P.G. Changes of isoflavones during processing of soy protein isolates. JAOCS. 1998;75:337–341. [Google Scholar]
- 19.Coward L., Smith M., Kirk M., Barnes S. Chemical modification of isoflavones in soyfoods during cooking and processing. Am. J. Clin. Nutr. 1998;68:1468s–1491s. doi: 10.1093/ajcn/68.6.1486S. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.J., Ahn J.K., Kim S.H., Kim J.T., Han S.J., Jung M.Y., Chung I.M. Variation in isoflavone of soybean cultivars with location and storage duration. J. Agric. Food Chem. 2003;51:3382–3389. doi: 10.1021/jf0261405. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Perez V., Tapaidor J., Martin A., Luque de Castro M.D. Optimization of the drying step for preparing a new commercial powdered soup. Innov. Food Sci. Emerg. Technol. 2004;5:361–368. doi: 10.1016/j.ifset.2004.05.001. [DOI] [Google Scholar]
- 22.Goula A.M., Adamopoulos K.G. Spray drying of tomato pulp in dehumidified air: II. The effect on powder properties. J. Food Eng. 2005;66:35–42. doi: 10.1016/j.jfoodeng.2004.02.031. [DOI] [Google Scholar]
- 23.Maury M., Murphy K., Kumar S., Shi L., Lee G. Effects of process variables on the powder yield of spray-dried trehalose on a laboratory spray-dryer. Euro. J. Pharm. Biopharm. 2005;59:565–573. doi: 10.1016/j.ejpb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan S., Bhosale R., Singhal R.S. Microencapsulation of cardamon oleoresin: evaluation of blends of gum Arabic, maltodextrin and modified startch as wall materials. Carbohydr. Polym. 2005;61:95–102. doi: 10.1016/j.carbpol.2005.02.020. [DOI] [Google Scholar]
- 25.Monsoor M.A. Effect of drying methods on the functional properties of soy hull pectin. Carbohydr. Polym. 2005;61:362–367. doi: 10.1016/j.carbpol.2005.06.009. [DOI] [Google Scholar]
- 26.Cohen J.S., Yang T.C.S. Progress in food dehydration. Trends Food Sci. Technol. 1995;6:20–25. doi: 10.1016/S0924-2244(00)88913-X. [DOI] [Google Scholar]
- 27.Tsami E., Krokida M.K., Drouzas A.E. Effect of drying method on the sorption characteristics of model fruit powder. J. Food Eng. 1999;38:381–392. [Google Scholar]
- 28.Hsieh H.C., Kao T.H., Chen B.H. A fast HPLC method for analysis of isoflavones in soybean. J. Liquid Chrom. Related. Technol. 2004;27:315–324. doi: 10.1081/JLC-120027102. [DOI] [Google Scholar]
- 29.SAS Instruments; Cary, NC, USA: 2004. SAS/STAT Guide for Personal Computers. version 6. [Google Scholar]