Abstract
Nine acetamidochalcones were synthesized and evaluated as antinociceptive agents using the mice writhing test. Given intraperitoneally all the compounds were more effective than the two reference analgesic drugs (acetylsalicylic acid and acetaminophen) used for comparison. N-{4-[(2E)-3-(4-nitrophenyl)prop-2-enoyl]phenyl}acetamide (6) was the most effective compound and was therefore selected for more detailed studies. It caused dose-related inhibition in the writhing test, being about 32 to 34-fold more potent than the standard drugs. It was also effective in the second phase of the formalin test and the capsaicin test. These acetamidochalcones, especially compound 6, might be further used as models to obtain new and more potent analgesic drugs.
Keywords: Acetamidochalcones, antinociception, mice
Introduction
Naturally-occurring and synthetic chalcone compounds have shown promising biological activity and safety profiles and have shown potential for use as lead compounds for the discovery of antioxidant, anti-inflammatory, anticancer or anti-infective agents [1]. A number of chalcones and their derivatives have also been found to inhibit the synthesis of nitric oxide (NO) and prostaglandins (PG), which are products of the nitric oxide synthase (NOS) and cyclooxygenase (COX) pathways, respectively [2,3]. Recently, Nowakovska published a review of the anti-infective and anti-inflammatory activity of chalcones, which constitute a unique template that is associated with diverse biological activities [4].
Previous studies carried out by our research group have revealed that some simple synthetic chalcones, or those derived from the abundant natural product 2-hydroxy-4,6-dimethoxyacetophenone, exhibit pronounced antinociceptive effects in the mice writhing test [5,6]. The present study reports on the antinociceptive effects of some synthetic acetamidochalcones in this test. The most active compound was evaluated in more detail in other pain models. The results of two well-known analgesic agents, acetylsalicylic acid and acetaminophen, were included as positive controls for comparison purposes.
Results and Discussion
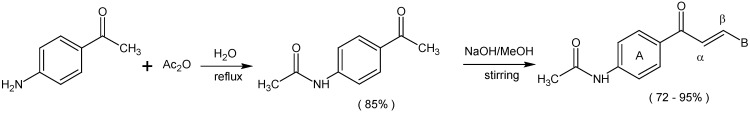
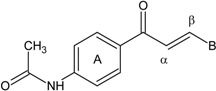
A series of chalcones which possess an acetamido substituent at the 4’-position of ring A and different substituent groups in the 3 or 4-positions of ring B (compounds 1-7) or different aromatic heterocycles in the ring B position (compounds 8 and 9) were synthesized (Scheme 1). All the compounds were obtained in good yields (72-95 %), and were characterized by conventional spectral data. Inspection of the 1H-NMR spectra suggested that the chalcones were geometrically pure and presented trans configurations (J = 15-16 Hz) [7].
Scheme 1.
Synthesis of 4’-acetamidochalcones.
B = -C6H5 (1); 4-OCH3C6H4 (2); 4-CH3C6H4 (3); 4-ClC6H4 (4); 3,4-Cl2C6H3 (5); 4-NO2C6H4 (6); -N(CH3)2C6H4 (7); -C5H3S (8); -C5H3O (9).
In this study, the antinociceptive activity for all the compounds indicated in Table 1 was initially evaluated using the acetic acid-induced writhing test in mice after intraperitoneal administration of a 10 mg/kg dose. Among the synthesized compounds, the most effective were chalcones 2, 3, 6, 7 and 9, which caused inhibitions of 91.6, 85.4, 94.1, 84.1 and 90.9 %, respectively, although all the chalcones significantly inhibited the induced abdominal constrictions and were more effective than the two analgesic drugs, (acetylsalicylic acid and acetaminophen), which caused inhibitions of 35% and 38%, respectively, in the same model and at the same dose. Analyzing the more outstanding results, especially compounds 2, 6 and 9, it can be observed that the electronic parameters (by induction, or resonance, or both) in the ring B do not appear to influence the analgesic activity.
Table 1.
Antinociceptive activity of 4’-acetamidochalcones, acetylsalicylic acid (ASA) and acetaminophen (ACE) against acetic acid-induced abdominal constrictions in mice.
 | ||
|---|---|---|
| Structure (B) | Chalcones | % Inhibition |
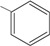 |
1 | 65.6 ± 4.9 ** |
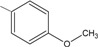 |
2 | 91.6 ± 2.9 ** |
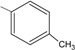 |
3 | 85.4 ± 2.2 ** |
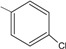 |
4 | 70.3 ± 3.8 ** |
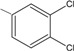 |
5 | 71,1 ± 4,0 ** |
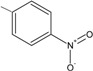 |
6 | 94.1 ± 2.5 ** |
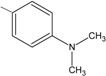 |
7 | 84.1 ± 0.6 ** |
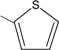 |
8 | 64.8 ± 2.8 ** |
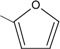 |
9 | 90.9 ± 2.2 ** |
| ASA | - | 35.0 ± 2.0 * |
| ACE | - | 38.0 ± 1.0 ** |
Each group represents the mean ± s.e.m. of 6-8 experiments. Compounds, acetylsalicylic acid and acetaminophen were administered intraperitoneally at 10 mg/kg; **p < 0.01 and * p < 0.05 compared with the corresponding control value.
A preliminary attempt to correlate the activity with some structural parameters, such as the hydrophobic, steric or electronic effects of the substituent groups in ring B using the HyperChem software program proved unsuccessful. Nevertheless, further studies are in progress to determine the possible involvement of other structural parameters (molecular, topological, geometrical, etc.), and these results will be published elsewhere in due course.
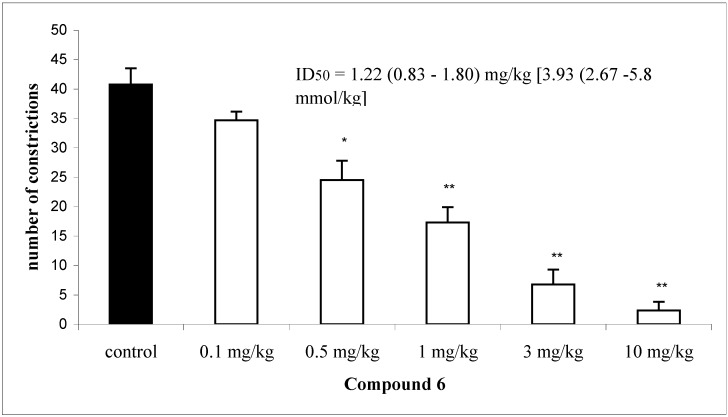
Compound 6, which possesses a nitro group in the 4-position of ring B, showed the most significant inhibitory activity, causing a 94.1% inhibition in the writhing test. This compound was therefore selected for more detailed studies in this and other tests. The ID50 of this chalcone was determined and the result is shown in Figure 1. It was observed that this compound, administered intraperitoneally, caused a potent and dose-dependent antinociceptive effect, with a ID50 value of 1.22 mg/kg (3.93 µmol/kg, i.p.), being about 32 to 34-fold more potent than the standard drugs (acetaminophen and acetylsalicylic acid) used as references, which presented ID50 values of 125 and 133 μmol/kg, i.p., respectively, in the same experimental model.
Figure 1.
Effect of compound 6 (0.1, 0.5, 1, 3 and 10 mg/kg), given intraperitoneally, against acetic acid 0.6 % induced abdominal constrictions in mice. Each column represents the mean s.e.m. of six to eight experimental values. * p < 0.05; **p < 0.01.
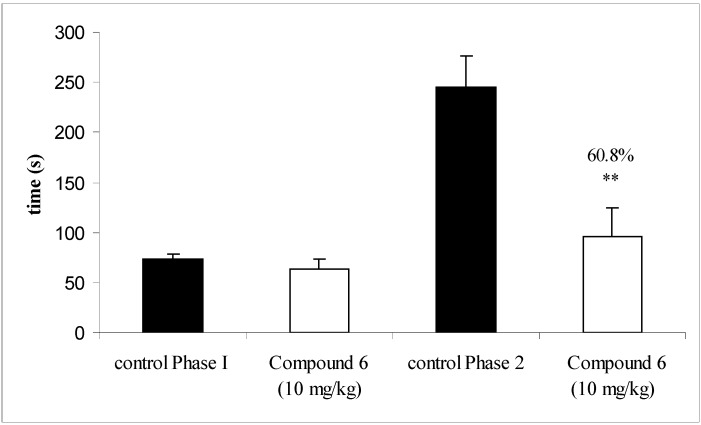
Compound 6 was also examined in the formalin-induced pain test, a reported behavior model characterized by neurogenic (first) and inflammatory (second) phases [8,9]. The results (Figure 2) revealed that both the chalcone and acetylsalicylic acid (control drug), were inactive in preventing the first phase of formalin-induced (neurogenic) pain at 10 mg/kg, i.p. However, compound 6 significantly inhibited the second phase (inflammatory) pain, with a 60.8 % inhibition, whereas acetylsalicylic acid only displayed a 39.0 % inhibition.
Figure 2.
Effect of compound 6 (10 mg/kg), given intraperitoneally, against the first (0 to 5 min) and second (15 to 30 min) phases in a formalin test in mice. Each column represents the mean s.e.m. of six to eight experimental values. * p < 0.05; **p < 0.01.
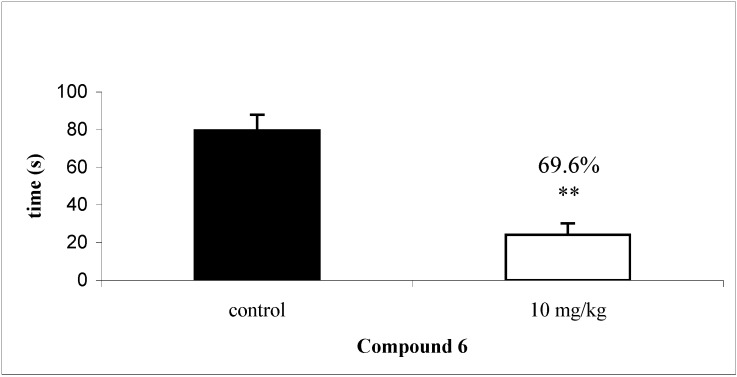
On the other hand, chalcone 6 (10 mg/kg, i.p.) exhibited considerable antinociceptive activity in the capsaicin test, causing a 70.0 % inhibition of capsaicin induced licking and thus providing more direct evidence of the effects of this compound on neurogenic pain. This suggests its involvement with the antagonism of the vanilloid receptor (Figure 3).
Figure 3.
Effect of compound 6 (10 mg/kg), on licking/biting response induced by intraplantary injection of capsaicin in mice. Each group represents the mean of six to eight experiments. ** p < 0.01, compared with the corresponding control value.
While compound 6, like acetylsalicylic acid and acetaminophen (and several non-steroidal anti-inflammatory and analgesic drugs), was effective in suppressing acetic acid-induced pain in a non-opioid pathway, a lack of antinociceptive effects (results not shown) was observed in the hot-plate test, a technique that is selective for opioid-derived analgesics [10]. Although the presence of the nitro group in ring B (compound 6) may suggest a possible toxic action, in accordance with the literature [11,12], our experiments did not indicate any signs of toxicity in the animals. Preliminary tests using Artemia saline (Brine Shrimp) larvae demonstrated low toxicity for these molecules. In this context, some clinically used drugs possess nitro group(s) in their structure, such as the well-documented nimesulide, a non-steroidal anti-inflammatory drug used to treat inflammation and pain [13], which encourages us to continue these investigations.
Conclusions
In summary, the acetamidochalcones evaluated in this work demonstrated significant antinociceptive activity against acetic acid-induced abdominal constrictions. When given intraperitoneally, all the chalcones synthesized were more active than the reference drugs, acetylsalicylic acid and acetaminophen, in particular compound 6, which indicated an interesting antinociceptive profile. Pharmacological studies are in progress to confirm the antinociceptive potential of the most active compounds in other models and routes of administration and to characterize the precise mechanism(s) of action of these acetamidochalcones. Finally, the findings of this study suggest that acetamidochalcones may represent an important class of novel and potent analgesic agents. The precise mechanism of action remains to be determined, but it does not involve the opioid system, and probably involves interaction with the vanilloid system and/or the mediators of the inflammatory process induced by formalin.
Experimental
General
The melting points were determined using a Microquimica WG APF-301 apparatus, and are uncorrected. The infrared (IR) spectra were recorded in KBr disks on a Perkin–Elmer 16 PC spectrometer. The 1H- and 13C-NMR spectra were recorded in DMSO-d6 using a Bruker WM 300 MHz spectrometer. Results are expressed in ppm downfield from the signal of TMS used as internal standard. Elemental analysis C and H percentages of were in agreement with the product formulae (within ± 0.4 % of theoretical values). The purity of the synthesized substances was monitored by thin-layer chromatography (TLC) using silica gel 60 F254 pre-coated aluminum sheets (Merck®) with several solvent systems of different polarities. The compounds were visualized with ultraviolet light (254 nm) and purified by recrystallization from ethyl alcohol and water or by column chromatography on silica gel (Merck, 60–120 mesh) eluting with hexane/ethyl acetate solvent systems of different polarities.
Chemistry: Synthesis of N-(p-acetylphenyl)acetamide
A solution of p-aminoacetophenone (2.2 g, 16.27 mmol) in water was stirred at room temperature, followed by the addition of acetic anhydride (4.1 mL, 43.43 mmol). The reaction mixture was then heated at reflux for 2 h. The solution was cooled in an ice bath and the resulting crystals were washed with cool water and filtered under vacuum, giving an 85.0 % yield of the title compound.
Synthesis of acetamidochalcones 1-9
A 50% w/v aq. NaOH solution (1.5 mL) was added to a well stirred solution of N-(p-acetyl-phenyl)acetamide (1.7 mmol) and the appropriate substituted benzaldehyde (1.7 mmol) in methanol (50 mL). The reaction mixture was stirred overnight at room temperature, according to the previously described methodology [14]. It was then neutralized with 1N HCl and the product filtered and extracted with chloroform. The combined organic layers were dried (Na2SO4), filtered and evaporated. The products were purified by column chromatography or recrystallization from ethyl alcohol or ethyl alcohol and water.
N-{4-[(2E)-3-phenylprop-2-enoyl]phenyl}acetamide (1): Yield: 80 %; m.p.: 161.7–162.2 oC; FT-IR (ν, cm-1): 1676 (-NH-C=O), 1648 (-C=O), 1598 (-C=C); 1H-NMR: 7.69 (d, Hα, J =15.6), 7.98 (d, Hβ, J = 15.6), 7.81–7.39 (m, Ar); 2.20 (s, OCCH3); 13C-NMR: 189.3 (C=O), 121.7 (Cα), 144.7 (Cβ), 142.5 (C-N), 169.1 (N-C=O), 24.6 (-CH3); Anal. Calc. for C17H15NO2 (MW = 265.3) C: 76.96, H: 5.70, N: 5.28, found C: 76.91, H: 5.64, N: 5.20.
N-{4-[(2E)-3-(4-metoxyphenyl)prop-2-enoyl]phenyl}acetamide (2): Yield: 84 %; m.p.: 206.5–207.0 oC; FT-IR (ν, cm-1): 1668 (-NH-C=O), 1597 (-C=O), 1532 (-C=C); 1H-NMR: 7.41 (d, Hα, J=15.6), 7.73 (d, Hβ, J=15.6), 6.92–8.02 (m, Ar), 3.85 (s, -OCH3), 2.23 (s, OCCH3), 1.6 (NH); 13C-NMR: 189.3 (C=O), 119.1 (Cα), 144.7 (Cβ), 142.1 (C-N), 168.7 (N-C=O), 55.7 (OCH3), 25.1 (-CH3); Anal. Calc. for C18H17NO3 (MW = 295.3) C: 73.20, H: 5.80, N; 4.74, found C: 73.11, H: 5.76, N: 4.69.
N-{4-[(2E)-3-(4-methylphenyl)prop-2-enoyl]phenyl}acetamide (3): Yield = 79 %; m.p.: 197.5-199.0 °C; FT-IR (ν, cm-1): 1676 (-NH-C=O), 1645 (-C=O), 1590 (-C=C); 1H-NMR: 7.45 (d, Hα, J=15.6), 7.79 (d, Hβ, J=15.6), 7.21–8.03 (m, Ar), 2.39 (s, CH3), 2.23 (s, OCCH3); 13C-NMR: 189.3 (C=O), 120.9 (Cα), 144.9 (Cβ), 141.3 (C-N), 25.1(OCCH3), 21.8 (-CH3); Anal. Calc. for C18H17NO2 (MW = 279.33) C: 77.40, H: 6.13, N: 5.01, found C: 77. 33, H: 6.09, N: 4.92.
N-{4-[(2E)-3-(4-chlorophenyl)prop-2-enoyl]phenyl}acetamide (4): Yield = 83 %; m.p.: 215.0-215.7 °C; FT-IR (ν, cm-1): 1662 (-NH-C=O), 1607 (-C=O), 1534 (-C=C); 1H-NMR: 7.69 (d, Hα, J=15.6), 7.94 (d, Hβ, J=15.6), 7.50 – 8.15 (m, Ar), 2.1 (s, OCCH3); 13C-NMR: 188.1 (C=O), 123.4 (Cα), 142.5 (Cβ), 144.5 (C-N), 169.7 (N-C=O), 135.6 (C-Cl), 24.9 (-CH3). Anal. Calc. for C17H14ClNO2 (MW = 299.75) C: 68.12, H: 4.71, N: 4.67, found C: 68.08, H: 4.69, N: 4.61.
N-{4-[(2E)-3-(3,4-dichlorophenyl)prop-2-enoyl]phenyl}acetamide (5): Yield = 95 %; m.p.: 210.4-211.0 °C, FT-IR (ν, cm-1): 1663 (-NH-C=O), 1599 (-C=O), 1536 (-C=C); 1H-NMR: 7.64 (d, Hα, J=15.6), 8.0 (d, Hβ, J=15.6), 7.65 – 8.23 (m, Ar), 2.07 (s, OCCH3); 13C-NMR: 187.9 (C=O), 169.7 (N-C=O), 124.7 (Cα), 141.1 (Cβ), 144.6 (C-N), 131.6–130.7 (C-Cl), 24.9 (-CH3); Anal. Calc. for C17H13Cl2NO2 (MW = 334.20) C: 61.10, H: 3.92, N: 4.19, found C: 61.0, H: 3.87, N: 4.14.
N-{4-[(2E)-3-(4-nitrophenyl)prop-2-enoyl]phenyl}acetamide (6): Yield = 89 %; m.p.: 239.7-241.0 °C, FT-IR (ν, cm-1): 1678 (-NH-C=O), 1596 (-C=O), 1517 (-C=C); 1H-NMR: 7.64 (d, CH=CH, J=15.6), 7.81 (d, CH=CH, J=15.6), 7.49 – 8.29 (m, Ar), 2.23 (s, OCCH3); 13C-NMR: 189.0 (C=O), 119.2 (Cα), 142.7 (Cβ), 141.5 (C-N), 25.1 (OCCH3); Anal. Calc. for C17H14N2O4 (MW = 310.30) C: 65.80, H: 4.55, N: 9.03, found C: 65.76, H: 4.51, N: 8.98.
N-(4-{(2E)-3-[4-(dimethylamino)phenyl]prop-2-enoyl}phenyl)acetamide (7): Yield = 72 %; m.p.: 150.9 -154.7 °C, FT-IR (ν, cm-1): 1684 (-NH-C=O), 1646 (-C=O), 1597 (-C=C); 1H-NMR: 7.59 (d, Hα, J=15.6), 7.62 (d, Hβ, J=15.6), 6.69–8.08. (m, Ar), 2.96 (s, 2CH3), 2.07 (s, OCCH3); 13C-NMR: 189.0(C=O), 120.4 (Cα), 145.64 (Cβ), 144.34 (C-N), 168.9 (N-C=O), 39.5 (N-CH3), 24.5 (-CH3); Anal. Calc. for C18H17NO2 (MW = 279.33) C: 77.40, H: 6.13, N: 5.01, found C: 77.31, H: 6.10, N: 5.03.
N-{4-[(2E)-3-(2-thienyl)prop-2-enoyl]phenyl}acetamide (8): Yield = 90 %; m.p.: 147.8-149.3 °C; FT-IR (ν, cm-1): 1677 (-NH-C=O), 1647 (-C=O), 1600 (-C=C); 1H-NMR: 7.57 (d, Hα, J=16), 7.93 (d, Hβ, J=16), 7.82 – 8.10 (d, Ar), 7.66–7.17 (m, thienyl) 3.08 (s, OCCH3); 13C-NMR: 187.8 (C=O), 121.2 (Cα), 136.8 (Cβ), 144.6 (C-N), 169.5 (N-C=O), 141.2, 130.0 (S-C), 23.8 (-CH3); Anal. Calc. for C15H13NO2S (MW = 271.33) C: 66.40, H: 4.83, N:5.16, found C: 66.37, H: 4.79, N: 5.13.
N-{4-[(2E)-3-(2-furyl)prop-2-enoyl]phenyl}acetamide (9): Yield = 95 %; m.p.: 118.1-119.0 °C; FT-IR (ν, cm-1): 1697 (-NH-C=O), 1645 (-C=O), 1597 (-C=C); 1H-NMR: 7.57 (d, Hα, J=15.8), 7.58 (d, Hβ, J=15.8), 7.82 – 8.09 (d, Ar), 7.76 – 6.63 (m, furyl), 3.01 (s, OCCH3); 13C-NMR: 187.2 (C=O), 119.1 (Cα), 129.9 (Cβ), 144.0 (C-N), 168.9 (N-C=O), 145.6, 152.1 (O-C), 23.8 (-CH3); Anal. Calc. for C15H13NO3 (MW = 255.27) C: 70.58, H: 5.13, N: 5.49, found C: 70.55, H: 5.08, N: 5.37.
Pharmacology: Animals
Male Swiss mice (25–35 g) were used, housed at 22 ± 2 oC under a 12 h light/12 h dark cycle, with access to food and water ad libitum. The experiments were performed during the light phase of the cycle. The animals were acclimatized to the laboratory for at least 2 h before testing, and were used once throughout the experiments. All the experiments reported in this study were carried out in accordance with the current guidelines for the care of laboratory animals and the ethical guidelines for investigation of experimental pain in conscious animals [15].
Drugs
The following chemicals were used: acetic acid, formalin, capsaicin (Calbiochem, San Diego, CA, USA), and morphine hydrochloride (Merck, Darmstadt, Germany). All the studied compounds, as well as the reference drugs, were dissolved in Tween® 80 (E. Merck) plus 0.9% of NaCl solution, with the exception of the capsaicin, which was dissolved in ethanol. The final concentrations of Tween® 80 and ethanol did not exceed 5% and did not cause any effects per se.
General pharmacological assay procedures
Acetic acid-induced writhing
Abdominal constriction was induced in mice by intraperitoneal injection of acetic acid (0.6 %), as described by Collier et al. with minor modifications [16,17]. The animals were pre-treated intraperitoneally with the studied compounds (3, 6 and 10 mg/kg, 30 min before), except in the case of compound 6 (0.1, 0.5, 1, 3, 10 mg/kg). The control animals received a similar volume of saline solution (10 mL/kg). The number of abdominal constrictions (full extension of both hind paws) was cumulatively counted over a period of 20 min. Antinociceptive activity was expressed as the reduction in the number of abdominal constrictions between the control animals and the mice pre-treated with the compounds.
Formalin test
The observation chamber was a glass cylinder of 20 cm in diameter, equipped with a mirror placed at a 45° angle to allow clear observation of the animals’ paws. The mice were treated with 0.9 % saline solution (i.p.) or compound 6 (10 mg/kg, i.p.) 30 min before formalin injection. Each animal was placed in the chamber for 5 min before treatment, in order to allow acclimatization to the new environment. The formalin test was carried out as described by Hunskaar and Hole, with minor modifications [17,18]. A 2.5 % formalin solution (0.92 % formaldehyde, 20 μL) in 0.9 % saline solution were injected intraplantarly into the right hind paw. The animal was then returned to the chamber and the amount of time spent licking the injected paw was considered as indicative of pain. Two distinct phases of intensive licking activity were identified: an early acute phase and a late or tonic phase (0–5 and 15–30 min after formalin injection, respectively).
Capsaicin-induced nociception
The procedure used was similar to that described previously [19]. After the adaptation period capsaicin (20 μL, 1.6 μg/paw) was injected intraplantarly into the right hindpaw. The animals were observed individually for 5 min following capsaicin injection. The amount of time spent licking the injected paw was timed with a chronometer and was considered as indicative of nociception. The animals were treated with the compound 6 via i.p. (10 mg/kg) 30 min prior to capsaicin injection, respectively. The control animals received a similar volume of saline, intraperitoneally.
Hot-plate test
The hot-plate test was used to measure response latencies, according to the method described by Eddy and Leimback [20]. The mice were treated with saline solution, morphine (10 mg/kg, s.c.) or compound 6 (10 mg/kg, i.p.), and placed individually on a hot plate maintained at 56±1 oC. The time between placing the animal on the hot plate and the occurrence of either the licking of the hind paws, shaking the paw or jumping off the surface was recorded as response latency. Mice with baseline latencies of more than 20 s were eliminated from the study and the cut-off time for the hot-plate latencies was set at 30 s. The animals were treated 30 min before the assay.
Statistical analysis
The results are presented as mean ± S.E.M., except for the ID values (i.e. the dose of compound 6 reducing the nociceptive response by 50 %, relative to the control value), which are reported as geometric means, accompanied by their respective 95 % confidence limits. The ID50 value was determined by linear regression from individual experiments using the GraphPad software (GraphPad software, San Diego, CA). The statistical significance of the differences for the comparison between the groups and the control was detected by ANOVA, followed by Dunnett’s multiple comparison test. P-values of less than 0.05 (P < 0.05) were considered indicative of significance.
Acknowledgments
The authors are grateful to CNPq and ProPPEC/UNIVALI (Brazil) for financial support.
Footnotes
Sample Availability: Contact the authors.
References
- 1.Ni L., Meng Q. M., Siroski J. A. Recent advances in therapeutic chalcones. Expert Opin. Ther. Pat. 2004;14:1669–1691. doi: 10.1517/13543776.14.12.1669. [DOI] [Google Scholar]
- 2.Ahmad S., Israf D. A., Hj. Lajis N., Shaari K., Mohamed H., Wahab A. A., Ariffin K. T., Hoo W. Y., Aziz N. A., Kadir A. A., Sulaiman M. R., Somchit M. N. Cardamonin, inhibits pro-inflammatory mediators in activated RAW 264.7 cells and whole blood. Eur. J. Pharmacol. 2006;538:188–194. doi: 10.1016/j.ejphar.2006.03.070. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K., Seung B. H., Sung L. S., Sanghyun L., Hoon J S, Sil L. Y., Hyun S. K., Ohuchi K. Synthesis and biological evaluation of new biphenyl ether thiazine derivatives. Ensho Saisei. 2005;25:130–136. doi: 10.2492/jsir.25.130. [DOI] [Google Scholar]
- 4.Nowakowska Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007;42:125–137. doi: 10.1016/j.ejmech.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Corrêa R., Pereira M. A. S., Buffon D., Santos L., Cechinel-Filho V., Santos A. R. S., Nunes R. J. Antinociceptive properties of chalcones. Structure-activity relationships. Arch. Pharm. 2001;334:167–172. doi: 10.1002/1521-4184(200110)334:10<332::aid-ardp332>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Campos-Buzzi F., de Campos J. P., Tonini P. P., Corrêa R., Yunes R. A., Boeck P., Cechinel-Filho V. Antinociceptive effects of synthetic chalcones obtained from xanthoxyline. Arch. Pharm. 2006;339:361–365. doi: 10.1002/ardp.200600049. [DOI] [PubMed] [Google Scholar]
- 7.Ram V. J., Saxena A. S., Srivastava S., Chandra S. Bioorg. Oxygenated chalcones and bischalcones as potential antimalarial agents. Med. Chem. Lett. 2000;10:2159–2161. doi: 10.1016/S0960-894X(00)00409-1. [DOI] [PubMed] [Google Scholar]
- 8.Hunskaar S., Fasmer O. B., Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J. Neurosci. Methods. 1985;14:69–76. doi: 10.1016/0165-0270(85)90116-5. [DOI] [PubMed] [Google Scholar]
- 9.Shibata M., Ohkubo T., Takahaschi H., Inoki R. Modified formalin test: characteristic biphasic pain response. Pain. 1989;38:347–352. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- 10.Abbott F.V., Franklin K.B.J. Noncompetitive antagonism of morphine analgesia by diazepam in the formalin test. Pharmacol. Biochem. Behav. 1986;24:319–321. doi: 10.1016/0091-3057(86)90358-8. [DOI] [PubMed] [Google Scholar]
- 11.Rosesnkranz H. S., Mermelstein R. Mutagenicity and genotoxicity of nitroarenes. All nitro-containing chemicals were not created equal. Mutat. Res. 1983;114:217–267. doi: 10.1016/0165-1110(83)90034-9. [DOI] [PubMed] [Google Scholar]
- 12.Chung K. T., Murdock C. A., Zhou Y., Stevens S. E., Jr., Li Y. S., Wei C. I., Fernando S. Y., Chou M. W. Effects of the nitro-group on the mutagenicity and toxicity of some benzamines. Environ. Mol. Mutagen. 1996;27:67–74. doi: 10.1002/(SICI)1098-2280(1996)27:1<67::AID-EM9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 13.Michaux C., Charlier C., Julémont F., de Leval X., Dogné J. M., Pirotte B., Durant F. A new potential cyclooxygenase-2 inhibitor, pyridinic analogue of nimesulide. Eur. J. Med. Chem. 2005;40:1316–1324. doi: 10.1016/j.ejmech.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Satyanarayana M., Tiwari P., Tripathi B. K., Srivastava A. K., Pratap R. Synthesis and antihyperglycemic activity of chalcone based aryloxypropanolamines. Bioorg. Med. Chem. 2004;12:883–889. doi: 10.1016/j.bmc.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 16.Collier H. D. J., Dinnin L. C., Johnson C. A., Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos-Buzzi F., Corrêa R., De Souza M. M., Yunes R. A., Nunes R. J., Cechinel-Filho V. Studies on new cyclic imides obtained from aminophenazone with analgesic properties. Drug Res. 2002;52:455–461. doi: 10.1055/s-0031-1299914. [DOI] [PubMed] [Google Scholar]
- 18.Hunskaar A. T., Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–104. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 19.Sakurada T., Katsumata K., Tan-No K., Sakurada S., Kisara K. The capsaicin test in mice for evaluating tachykinin antagonist in the spinal cord. Neuropharmacology. 1992;31:1279–1285. doi: 10.1016/0028-3908(92)90057-V. [DOI] [PubMed] [Google Scholar]
- 20.Eddy N. B., Leimback D. Synthetic analgesic. II. Dithienylbutenyl and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953;107:385–393. [PubMed] [Google Scholar]