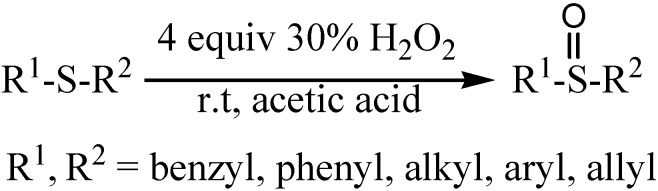Abstract
A “green” highly selective oxidation of organic sulfides to the corresponding sulfoxides was developed using hydrogen peroxide and glacial acetic acid under transition metal-free and mild conditions. The oxidation procedure is very simple and the products are easily isolated in excellent yields (90-99%).
Keywords: Sulfide, glacial acetic acid, hydrogen peroxide, selective oxidation, sulfoxide
Introduction
The growth in the chemistry of organic sulfoxides during last decade was due to their importance as synthetic intermediates for the production of a wide range of chemically and biologically active molecules. They often perform a major function as therapeutic agents such as anti-ulcer (proton pump inhibitor) [1], antibacterial, antifungal, anti-atherosclerotic [2], antihypertensive [3] and cardiotonic agents [4], as well as psychotonics [5] and vasodilators [6].
The oxidation of sulfides to sulfoxides is the most straightforward synthetic route to the latter, and numerous reagents and oxidative procedures are available for this transformation. However, many of them cause overoxidation to the corresponsing sulfones. Therefore, control of the reaction conditions, that is, time, temperature and the relative amount of oxidants, plays an important role in avoiding the formation of oxidation side products, but this is often hard to achieve and therefore there is still considerable interest in the development of selective oxidants for this transformation [7,8,9,10,11,12].
Much of the current work in this area focuses on the use of transition-metal catalyzed processes [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. However, a large number of such oxidation reactions often require the use of toxic metal reagents or catalysts. Consequently, from a Green Chemistry standpoint it is very important to develop a “green” oxidation system for chemical manufacturing. Hydrogen peroxide is considered as an ideal “green” oxidant due to its strength and lack of toxic by-products. Among the research on the oxidation of sulfides to the corresponding sulfoxides under transition metal-free conditions [23,24,25,26,27,28,29,30,31,32,33] some most promising results were reported by Ravikumar et al. [34], in which hydrogen peroxide in solvent of hexafluoro-2-propanol was used for the selective oxidation of sulfides to sulfoxides. Although the yields of these reactions were quite high, hexafluoro-2-propanol is poisonous, expensive and volatile, which severely restrict its practical use in organic synthesis. In continuation of our current studies on the selective oxidation of hydrocarbons [35,36,37,38,39,40] we wish to report herein a very efficient and simple method for the oxidation of sulfides to the corresponding sulfoxides using H2O2 under mild conditions, as shown in Scheme 1.
Scheme 1.
Oxidation of sulfides to the corresponding sulfoxides.
Results and Discussion
Methyl phenyl sulfide was selected as a model substrate for optimization, and the oxidation of this substrate in various solvents was studied. Among the solvents examined, glacial acetic acid was the most effective (Table 1, entries 1-5). The reaction was also carried out at different temperatures under the same conditions. As the results indicate (Table 1, entries 6-9), the oxidation proceeded to completion faster at elevated temperatures. Further, when the oxidation of methyl phenyl sulfide was carried out using different concentrations of hydrogen peroxide, the reaction did not reach completion using less than 8 mmol of H2O2. However, when a large concentration was employed neither the conversion nor selectivity of the reaction was improved (Table 1, entries 10-13). The large excess of hydrogen peroxide required was a result of its decomposition in acetic media. The oxygen released in the decomposition played little role in the oxidation of the sulfides, as proven by the fact that the oxidation occurred in poor yield when carried out in the absence of hydrogen peroxide by bubbling molecular oxygen through the reaction mixture under similar reaction conditions (Table 1, entry 14). In an independent experiment the oxidation of methyl phenyl sulfoxide to the corresponding sulfones was investigated under the same reaction conditions; no oxidation took place during 120 min and the starting material was recovered.
Table 1.
Oxidations of methyl phenyl sulfide a.
| Entry | H2O2
(mmol) |
Solvent (2 mL) |
Time (min) | Temp. (oC) | Conversion (%) b |
Sulfoxide Selectivity (%) b |
|---|---|---|---|---|---|---|
| 1 | 8 | CH3COOH | 80 | 25 | 100 | >99 |
| 2 | 8 | CH2Cl2 | 90 | 25 | 0 | 0 |
| 3 | 8 | CH3CN | 90 | 25 | <5 | >99 |
| 4 | 8 | CH3NO2 | 90 | 25 | <5 | >99 |
| 5 | 8 | CH3OH | 240 | 25 | 20 | >99 |
| 6 | 8 | CH3COOH | 440 | 0 | 93 | >99 |
| 7 | 8 | CH3COOH | 35 | 45 | 100 | >99 |
| 8 | 8 | CH3COOH | 20 | 60 | 100 | >99 |
| 9 | 8 | CH3COOH | 8 | 80 | 100 | >99 |
| 10 | 1 | CH3COOH | 80 | 25 | 8 | >99 |
| 11 | 2 | CH3COOH | 80 | 25 | 13 | >99 |
| 12 | 5 | CH3COOH | 80 | 25 | 23 | >99 |
| 13 | 8 | CH3COOH | 80 | 25 | 100 | >99 |
| 13 | 10 | CH3COOH | 75 | 25 | 100 | >99 |
| 14c | - | CH3COOH | 600 | 25 | 7 | >99 |
a In all experiments 2 mmol of substrate were used.
b Isolated yield on the basis of the weight of the pure product obtained.
c The reaction was carried out under an atmosphere of O2 instead of in the presence of 30% H2O2.
The oxidations of other sulfides such as diaryl, dibenzyl, dialkyl, diallyl, arylbenzyl, arylalkyl, benzyl -alkyl, allylphenyl and cyclic sulfides were then examined using the optimized reaction conditions (Table 2). In all cases, the reactions resulted in 100% conversion of the sulfides. In the case of benzyl sulfides (Table 2, entries 2 and 6), no oxidation was observed at the benzylic C-H bond. Similarly, the carbon-carbon double bonds in allyl sulfides remained intact during the oxidation (Table 2, entries 5 and 8). This procedure can also be applied to the oxidation of cyclic sulfides (Table 2, entry 10).
Table 2.
Oxidation of sulfides to sulfoxides with hydrogen peroxide a.
| Entry | Substrate | Time (min) | Conversion (%) | Sulfoxide b | Yield (%)c |
|---|---|---|---|---|---|
| 1 | 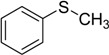 |
80 | 100 | 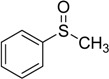 |
99 |
| 2 | 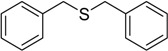 |
120 | 100 | 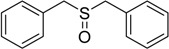 |
93 |
| 3 | 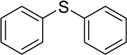 |
75 | 100 | 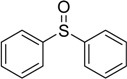 |
94 |
| 4 | 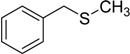 |
50 | 100 | 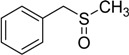 |
92 |
| 5 | 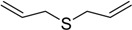 |
35 | 100 | 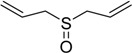 |
90 |
| 6 | 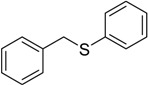 |
70 | 100 | 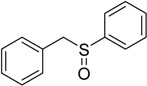 |
93 |
| 7 | 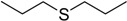 |
50 | 100 | 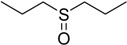 |
91 |
| 8 | 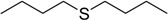 |
60 | 100 | 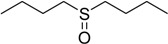 |
92 |
| 9 | 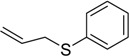 |
48 | 100 | 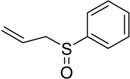 |
90 |
| 10 | 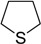 |
30 | 100 | 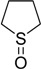 |
96 |
| 11 | 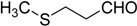 |
26 | 100 | 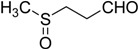 |
93 |
| 12 | 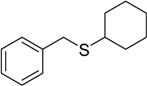 |
45 | 100 | 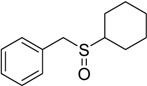 |
91 |
a 2 mmol of substrate at room temperature and 8 mmol of 30% aqueous H2O2, in glacial acetic acid (2 mL) at room temperature.
b The products were identified by comparison of physical and spectroscopic properties with authentic compounds.
c Isolated yields on the basis of the weight of the pure product obtained.
Large scale oxidation of methyl phenyl sulfoxide (2 mol) was also investigated and the results demonstrated that oxidation took place in good yield (typically less than 2% yield was lost). The kinetic studies show that the oxidation of sulfide is a second order reaction and is not acid catalyzed, so the reaction times remained constant as a result of increasing the acidities of the medium by the addition of trichloroacetic acid at fixed content of acetic acid. However, experiments demonstrate that dry hydrogen peroxide (percarbonate) in glacial acetic acid does not generate peracetic acid on standing. Further, kinetic study with peracetic acid under identical reaction conditions reveal that the oxidation of sulfides is very fast; too fast to follow by titrimetry. Although the exact mechanism of this transformation is still unclear, the oxidation probably involves the electophilic attack of the peroxide oxygen on sulfur. Despite the fact that, protonated hydrogen peroxide is a powerful oxidizing agent the oxidation of sulfides is rule out by this oxidizing mediator as the reaction is not acid catalyzed; acetic acid and trichloroacetic acid fail to protonate hydrogen peroxide.
Conclusions
As shown, the proposed system was found to be a selective method for the hydrogen peroxide oxidation at room temperature of a variety of sulfides to the corresponding sulfoxides. Although the precise mechanism of this transformation is still uncertain, the oxidation probably involves the electophilic attack of the peroxide oxygen on the sulfide sulfur atom. Moreover, this oxidation system is clean, safe and operationally simple and the yields of the products are high, so the oxidation method meets the needs of contemporary “green chemistry” and is suitable for practical synthesis.
Experimental
General
All chemicals were used without further purification as received from different commercial sources (Merck, Aldrich, Fluka). All the isolated sulfoxide compounds were identified through comparison of their GLC (retention time) IR, NMR and melting points with literature data.
General method for the oxidation of sulfides to sulfoxides
Hydrogen peroxide (8 mmol, 30%) was slowly added to the sulfide (2 mmol) in glacial acetic acid (2 mL). The reaction mixture was then stirred at room temperature until thin layer chromatography indicated the reaction was complete. The resulting solution was neutralized with aqueous NaOH (4 M) and the product was extracted with CH2Cl2. The organic layer was dried over anhydrous Na2SO4 and then concentrated under reduced pressure to yield analytically pure product. Rate measurements were made in glacial acetic acid under second order conditions at constant temperature. For each kinetic run a fresh solution of the oxidant in glacial acetic acid was prepared and standardized iodometrically. The progress of the oxidation, with the sulfides present in excess over the oxidant, was followed by iodometric estimation of the unconsumed oxidizing agent.
Acknowledgments
We are grateful for the financial support of Mazandaran University of the Islamic Republic of Iran.
Footnotes
Sample Availability: Available from authors.
References
- 1.Lai S. K. C., Lam K., Chu K. M., Wong B. C., Hui W. M., Hu W. H., Lau G. K., Wong W. M., Yuen M. F., Chan A. O., Lai C. L., Wong J. N. Lansoprazole for the Prevention of Recurrences of Ulcer Complications from Long-Term Low-Dose Aspirin Use. New Engl. J. Med. 2002;346:2033–2038. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- 2.Sovova M., Sova P. Pharmaceutical significance of Allium sativum L. Antifungal effects. Ceska Slov. Farm. 2003;52:82–87. [PubMed] [Google Scholar]
- 3.Kotelanski B., Grozmann R. J., Cohn J. N. C. Positive inotropic effect of oral esproquin in normal subjects. Pharmacol. Ther. 1973;14:427–433. doi: 10.1002/cpt1973143427. [DOI] [PubMed] [Google Scholar]
- 4.Schmied R., Wang G. X., Korth M. Intracellular Na+ activity and positive inotropic effect of sulmazole in guinea pig ventricular myocardium. Comparison with a cardioactive steroid. Circ. Res. 1991;68:597–604. doi: 10.1161/01.RES.68.2.597. [DOI] [PubMed] [Google Scholar]
- 5.Nieves A. V., Lang A. E. Treatment of excessive daytime sleepiness in patient with Parkinson’s disease with modafinil. Clin. Neuropharmacol. 2002;25:111–114. doi: 10.1097/00002826-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Padmanabhan S., Lavin R. C., Durant G. J. Asymmetric synthesis of a neuroprotective and orally active N-methyl-D-aspartate receptor ion-channel blocker, CNS 5788. Tetrahedron: Asymmetr. 2000;11:3455–3645. doi: 10.1016/S0957-4166(00)00328-1. [DOI] [Google Scholar]
- 7.Kaczorowska K., Kolarska Z., Mitka K., Kowalski P. Oxidation of sulfides to sulfoxides. Part 2: Oxidation by hydrogen peroxide. Tetrahedron. 2005;61:8315–8327. [Google Scholar]
- 8.Wang S. H., Mandimutsira B. S., Todd R., Ramdhanie B., Fox J. P., Goldberg D. P. Catalytic sulfoxidation and epoxidation with a Mn(III) triazacorrole: Evidence for a "third oxidant" in high-valent porphyrinoid oxidations. J. Am. Chem. Soc. 2004;126:18–19. doi: 10.1021/ja038951a. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hashimi M., Roy G., Sullivan A. C., Wilson J. R. H. Selective oxidations of sulfides to sulfoxides using immobilised cerium alkyl phosphonate. Tetrahedron Lett. 2005;46:4365–4398. doi: 10.1016/j.tetlet.2005.04.079. [DOI] [Google Scholar]
- 10.Venkataramanan N. S., Kuppuraj G., Rajagopal S. Metal-salen complexes as efficient catalysts for the oxygenation of heteroatom containing organic compounds - synthetic and mechanistic aspects. Coord. Chem. Rev. 2005;249:1249–1268. doi: 10.1016/j.ccr.2005.01.023. [DOI] [Google Scholar]
- 11.Du G. D., Espenson J. H. Oxidation of triarylphosphines and aryl methyl Sulfides with hydrogen peroxide catalyzed by dioxovanadium(V) ion. Inorg. Chem. 2005;44:2465–2471. doi: 10.1021/ic048290y. [DOI] [PubMed] [Google Scholar]
- 12.Velusamy S., Kumar A. V., Saini R., Punniyamurthy T. Copper catalyzed oxidation of sulfides to sulfoxides with aqueous hydrogen peroxide. Tetrahedron Lett. 2005;46:3819–3822. doi: 10.1016/j.tetlet.2005.03.194. [DOI] [Google Scholar]
- 13.Shul’pin G. B., Suss-Fink G., Shul’pina L. S. Oxidations by the system "hydrogen peroxide-manganese(IV) complex-carboxylic acid" Part 3. Oxygenation of ethane, higher alkanes, alcohols, olefins and sulfides. J. Mol. Catal. A: Chem. 2001;170:17–34. doi: 10.1016/S1381-1169(01)00052-8. [DOI] [Google Scholar]
- 14.Shabani A., Lee D. G. Solvent free permanganate oxidations. Tetrahedron Lett. 2001;42:5833–5838. doi: 10.1016/S0040-4039(01)01129-7. [DOI] [Google Scholar]
- 15.Barker J. E., Ren T. Facile oxygenation of organic sulfides with H2O2 catalyzed by Mn-Me(3)TACN compounds. Tetrahedron Lett. 2004;45:4681–4685. doi: 10.1016/j.tetlet.2004.04.125. [DOI] [Google Scholar]
- 16.Mirkhani V., Tangestaninejad S., Moghadam M., Mohammadpoor-Baltork I., Kargar H. Efficient oxidation of sulfides with sodium periodate catalyzed by manganese(III) Schiff base complexes. J. Mol. Catal. A: Chem. 2005;242:251–255. doi: 10.1016/j.molcata.2005.07.045. [DOI] [Google Scholar]
- 17.Okun N. M., Tarr J. C., Hilleshiem D. A., Zhang L., Hardcastle K. I., Hill C. L. Highly reactive catalysts for aerobic thioether oxidation - The Fe-substituted polyoxometalate/hydrogen dinitrate system. J. Mol. Catal. A: Chem. 2006;246:11–17. doi: 10.1016/j.molcata.2005.10.006. [DOI] [Google Scholar]
- 18.Barnes I., Hjorth J., Mihalopoulos N. Dimethyl sulfide and dimethyl sulfoxide and their oxidation in the atmosphere. Chem. Rev. 2006;106:940–975. doi: 10.1021/cr020529+. [DOI] [PubMed] [Google Scholar]
- 19.Memarian H. R., Mohammadpoor-Baltork I., Bahrami K. Photoinduced electron transfer reactions of aryl benzyl sulfides promoted by 2,4,6-triphenylpyrilium tetrafluoroborate (TP+BF4-) Bull. Kor. Chem. Soc. 2006;27:106–110. doi: 10.5012/bkcs.2006.27.1.106. [DOI] [Google Scholar]
- 20.Carson E. C., Lippard S. J. Dioxygen-Initiated oxidation of heteroatomic substrates incorporated into ancillary pyridine ligands of carboxylate-rich diiron(II) complexes. Inorg. Chem. 2006;45:837–848. doi: 10.1021/ic051476s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith J. R. L., Murray J., Walton P. H., Lowdon T. R. Organosulfur oxidation by hydrogen peroxide using a dinuclear Mn-1,4,7-trimethyl-1,4,7-triazacyclononane complex. Tetrahedron Lett. 2006;47:2005–2008. doi: 10.1016/j.tetlet.2006.01.054. [DOI] [Google Scholar]
- 22.Park E. S., Lee S. H., Lee J. H., Rhee H. J., Yoon C. M. Deoxygenation of sulfoxide and aza-aromatic N-oxide using a protocol of indium and acyl chloride. Synthesis-Stuttgart. 2005;20:3499–3501. [Google Scholar]
- 23.Nehlsen J., Benziger J., Kevrekidis I. Oxidation of aliphatic and aromatic sulfides using sulfuric acid. Ind. Eng. Chem. Res. 2006;45:518–524. doi: 10.1021/ie050901m. [DOI] [Google Scholar]
- 24.Reddy K. R., Rajasekhar C. V., Ravindra A. L-proline-H2O2: A new chemoselective approach for oxidation of sulfides to sulfoxides. Synth. Commun. 2006;36:3761–3766. doi: 10.1080/00397910600946363. [DOI] [Google Scholar]
- 25.Coulomb J., Certal V., Fensterbank L., Lacote E., Malacria M. Formation of cyclic sulfinates and sulfinamides through homolytic substitution at the sulfur atom. Angew. Chem. Int. Edit. Engl. 2006;45:633–637. doi: 10.1002/anie.200503369. [DOI] [PubMed] [Google Scholar]
- 26.Tang R.Y., Zhong P., Lin Q. L. A convenient conversion of pyrazolyl disulfide to sulfides by sodium dithionite and synthesis of sulfoxides. J. Fluorine Chem. 2006;127:948–953. doi: 10.1016/j.jfluchem.2006.04.002. [DOI] [Google Scholar]
- 27.He X., Chan T. H. New non-volatile and odorless organosulfur compounds anchored on ionic liquids. Recyclable reagents for Swern oxidation. Tetrahedron. 2006;62:3389–3394. doi: 10.1016/j.tet.2006.01.045. [DOI] [Google Scholar]
- 28.Hajipour A. R., Guo L. W., Ruolho A. E. Nitric acid in the presence of supported P2O5 on silica gel affords an efficient and mild system for oxidation of organic compounds under solvent-free conditions. Mol. Cryst. Liq. Cryst. 2006;456:85–93. doi: 10.1080/15421400600786363. [DOI] [Google Scholar]
- 29.Choghamarania A. G. Iodic acid (HIO3) Synlett. 2006:2347–2348. doi: 10.1055/s-2006-949618. [DOI] [Google Scholar]
- 30.Joseph J. K., Jain S. L., Sain B. N-methylpyrolidin-2-one hydrotribromide (MPHT) as a new and efficient reagent for the oxidation of sulfides to sulfoxides and sulfones. Synth. Commun. 2006;36:2743–2747. doi: 10.1080/00397910600764790. [DOI] [Google Scholar]
- 31.Ji H. B., Hu X. F., Shi D. P., Li Z. Controllable oxidation of sulfides to sulfoxides and sulfones with aqueous hydrogen peroxide in the presence of beta-cyclodextrin. Russ. J. Org. Chem. 2006;42:959–961. doi: 10.1134/S1070428006070049. [DOI] [Google Scholar]
- 32.Qian W. X., Pei L. Efficient and highly selective oxidation of sulfides to sulfoxides in the presence of an ionic liquid containing hypervalent iodine. Synlett. 2006:709–712. doi: 10.1055/s-2006-933109. [DOI] [Google Scholar]
- 33.Firouzabadi H., Iranpoor N., Jafari A. A., Riazymontazer E. Metal-free chemoselective oxidation of sulfides to sulfoxides by hydrogen peroxide catalyzed by in situ generated dodecyl hydrogen sulfate in the absence of organic co-solvents. Adv. Synth. Catal. 2006;348:434–438. doi: 10.1002/adsc.200505312. [DOI] [Google Scholar]
- 34.Ravikumar K. S., Begue J.-P., Bonnet-Delpon D. A selective conversion of sulfide to sulfoxide in hexafluoro-2-propanol. Tetrahedron Lett. 1998;39:3141–3144. doi: 10.1016/S0040-4039(98)00498-5. [DOI] [Google Scholar]
- 35.Golchoubian H., Nemati Kharat A. Hydrogen peroxide oxidation of mono- and disubstituted alkylarenes catalyzed by dinuclear Co-III-Cu-II macrocyclic complex. Pol. J. Chem. 2005;79:825–830. [Google Scholar]
- 36.Mardani H. R., Golchoubian H. Effective oxidation of benzylic and aliphatic alcohols with hydrogen peroxide catalyzed by a manganese(III) Schiff-base complex under solvent-free conditions. Tetrahedron Lett. 2006;47:2349–2352. doi: 10.1016/j.tetlet.2006.02.007. [DOI] [Google Scholar]
- 37.Mardani H. R., Golchoubian H. Selective and efficient C-H oxidation of alkanes with hydrogen peroxide catalyzed by a manganese(III) Schiff base complex. J. Mol. Catal. A: Chem. 2006;259:197–200. doi: 10.1016/j.molcata.2006.06.029. [DOI] [Google Scholar]
- 38.Hosseinpoor F., Golchoubian H. Mild and highly efficient transformation of thiols to symmetrical disulfides using urea-hydrogen peroxide catalyzed by a Mn(III)-salen complex. Catal. Lett. 2006;111:165–168. doi: 10.1007/s10562-006-0141-8. [DOI] [Google Scholar]
- 39.Hosseinpoor F., Golchoubian H. Mn(III)-catalyzed oxidation of sulfides to sulfoxides with hydrogen peroxide. Tetrahedron Lett. 2006;47:5195–5197. doi: 10.1016/j.tetlet.2006.05.012. [DOI] [Google Scholar]
- 40.Hosseinpoor F., Golchoubian H. Aerobic oxidation of thiols to disulfides catalyzed by a manganese(III) Schiff-base complex. Catal. Commun. 2007;8:697–700. doi: 10.1016/j.catcom.2006.08.036. [DOI] [Google Scholar]