Table 2.
Oxidation of sulfides to sulfoxides with hydrogen peroxide a.
| Entry | Substrate | Time (min) | Conversion (%) | Sulfoxide b | Yield (%)c |
|---|---|---|---|---|---|
| 1 | 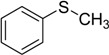 |
80 | 100 | 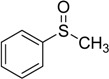 |
99 |
| 2 | 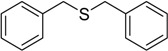 |
120 | 100 | 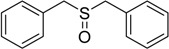 |
93 |
| 3 | 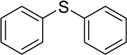 |
75 | 100 | 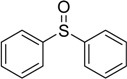 |
94 |
| 4 | 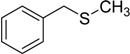 |
50 | 100 | 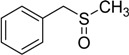 |
92 |
| 5 | 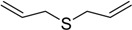 |
35 | 100 | 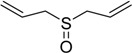 |
90 |
| 6 | 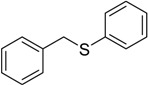 |
70 | 100 | 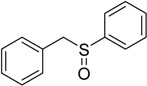 |
93 |
| 7 | 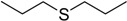 |
50 | 100 | 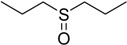 |
91 |
| 8 | 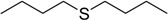 |
60 | 100 | 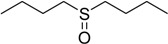 |
92 |
| 9 | 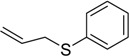 |
48 | 100 | 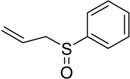 |
90 |
| 10 | 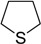 |
30 | 100 | 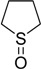 |
96 |
| 11 | 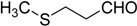 |
26 | 100 | 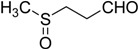 |
93 |
| 12 | 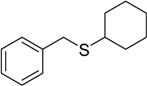 |
45 | 100 | 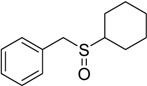 |
91 |
a 2 mmol of substrate at room temperature and 8 mmol of 30% aqueous H2O2, in glacial acetic acid (2 mL) at room temperature.
b The products were identified by comparison of physical and spectroscopic properties with authentic compounds.
c Isolated yields on the basis of the weight of the pure product obtained.
