Abstract
Cocoa contains high levels of different flavonoids. In the present study, the enantioseparation of catechin and epicatechin in cocoa and cocoa products by chiral capillary electrophoresis (CCE) was performed. A baseline separation of the catechin and epicatechin enantiomers was achieved by using 0.1 mol·L−1 borate buffer (pH 8.5) with 12 mmol·L-1 (2-hydroxypropyl)-γ-cyclodextrin as chiral selector, a fused-silica capillary with 50 cm effective length (75 μm I.D.), +18 kV applied voltage, a temperature of 20 °C and direct UV detection at 280 nm. To avoid comigration or coelution of other similar substances, the flavan-3-ols were isolated and purified using polyamide-solid-phase-extraction and LC-MS analysis. As expected, we found (-)-epicatechin and (+)-catechin in unfermented, dried, unroasted cocoa beans. In contrast, roasted cocoa beans and cocoa products additionally contained the atypical flavan-3-ol (-)-catechin. This is generally formed during the manufacturing process by an epimerization which converts (-)-epicatechin to its epimer (-)-catechin. High temperatures during the cocoa bean roasting process and particularly the alkalization of the cocoa powder are the main factors inducing the epimerization reaction. In addition to the analysis of cocoa and cocoa products, peak ratios were calculated for a better differentiation of the cocoa products.
Keywords: Flavonoids, Cocoa, Chocolate, Catechin, Epicatechin, Epimerization, Enantioseparation, Capillary Electrophoresis, LC-MS, Isolation, Purification
Introduction
In recent years the analysis of polyphenolic compounds in raw and processed food became more important with regard to their numerous physiological properties [1,2,3]. Due to their various effects, the flavan-3-ols group is of major interest. In plants they can act as chemical signals to attract or deter insects and provide a defense against pathogens and environmental stress [4]. Regarding their health benefits in humans, they show positive cardiovascular, anticancer or antiviral effects [5,6]. However, some studies have dealt with a negative physiological potential [7,8]. Even though catechin and epicatechin have been analyzed by different analytical methods and tested in model systems, some aspects have not been investigated yet [9,10]. Regarding their structure, catechin and epicatechin posess two chiral centers. Therefore, it is important to remember that the properties of chiral substances like (+)-catechin and (-)-epicatechin and their enantiomers depend on the conformation of the molecules (Figure 1).
Figure 1.
Structures of the catechin /epicatechin enantiomers and procyanidin B2.
Consequently, some studies have shown totally different physiological effects of each enantiomer [10,11,12,13]. During biosynthesis catechin and epicatechin are predominantly synthesized as (+)-catechin and (-)-epicatechin [14]. The atypical flavan-3-ol enantiomers (-)-catechin and (+)-epicatechin, often referred to as ent-catechin and ent-epicatechin, rarely occur in nature and their presence seems to be induced by enzymatic action [15]. Vivanco and co-workers found racemic catechin in the roots of spotted knapweed (Centaurea maculosa, Lam.) and showing a high phytotoxic potential induced by (-)-catechin [16,17]. Lately, the natural occurrence of all flavan-3-ol enantiomers, both (+/-)-catechin and (+/-)-epicatechin was described in guaraná seeds (Paullinia cupana var. sorbilis) [18]. Additionally, atypical flavan-3-ol enantiomers can also be formed by non-enzymatic action. High temperature and/or an increasing pH value are able to induce an epimerization reaction. The epimers of catechin and epicatechin participate in equilibrium reactions [19,20]. Regarding processed food, monomeric flavan-3-ols could also be converted to their epimers. They were found in tea beverages and recently in cocoa and cocoa products [21,22]. Donovan et al. separated both (+)- and (-)-catechin and (+)- and (-)-epicatechin by chiral HPLC [10]. In a previous attempt, we also tried to separate the flavan-3-ol enatiomers by chiral HPLC, applying a similar separation system, but the separation only succeeded for (+)- and (-)-catechin and failed for (+)- and (-)-epicatechin [23]. Furthermore, technological methods to control the epimerization and the loss of the beneficial flavan-3-ols in cocoa products and other food by controlling the temperature and pH values during food processing have been developed [24,25,26].
Based on our earlier studies, we were interested in analyzing the atypical flavan-3-ol enantiomers focusing on their alteration in cocoa and cocoa products. We used chiral capillary electrophoresis (CCE) to separate the flavan-3-ols with cyclodextrins as chiral selectors [18]. Additionally we confirmed our results by isolating and purifying the flavan-3-ol monomers and performing LC-MS experiments.
Results and Discussion
Analysis of cocoa and cocoa products by CCE
Based on preliminary studies, we were able to separate both catechin and epicatechin into their two corresponding enantiomers using only one chiral selector added to the background electrolyte [18]. As shown in the electropherograms (see Figure 2, Figure 3, Figure 4 and Figure 5), we were able to separate the flavan-3-ols in nearly 8 minutes. All samples were prepared under gentle and mild conditions, avoiding undesirable reactions of the analytes (see Experimental section). We applied the method to different cocoa and cocoa products. We thus confirmed that unfermented, dried, unroasted cocoa beans predominantely contain (-)- epicatechin and small amounts of (+)-catechin (see Figure 2). The analysis of different samples of roasted cocoa beans and commercial cocoa products like chocolate provides evidence for the occurrence of (-)-catechin besides (-)-epicatechin and (+)-catechin (see Figure 3 and Figure 4). In cocoa powder and products containing cocoa powder, we even found increased levels of (-)-catechin (see Figure 5).
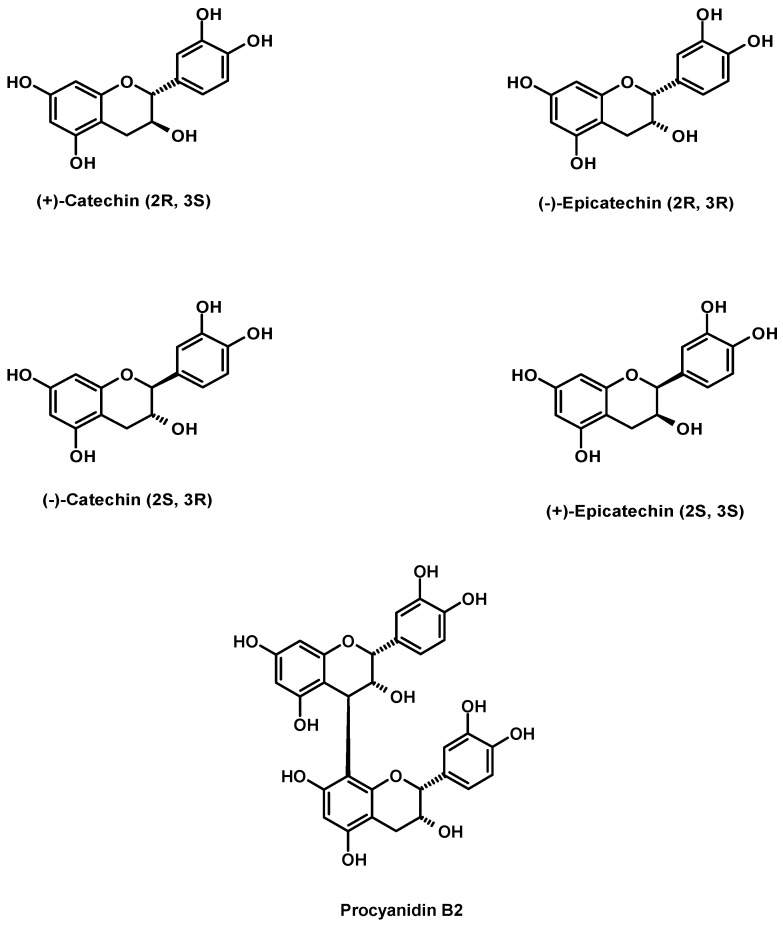
Figure 2.
Electropherogram showing the enantioselective separation of catechin and epicatechin in a) an extract of unfermented, dried, unroasted cocoa beans and b) isolated from this extract. 1: (-)-Epicatechin, 2: (+)-Catechin, P: Procyanidin B2. Experimental conditions: uncoated fused-silica capillary [60 cm (effective length 50 cm) × 75 µm I.D.]; electrolyte: 0.1 mol·L-1 borate buffer, pH 8.5; chiral selector: 12.0 mmol·L-1 HP-γ-CD; voltage: 18 kV; UV-Detection: 280 nm; temperature: 20 °C; injection: pressure, 3 s, 0.3 p.s.i..
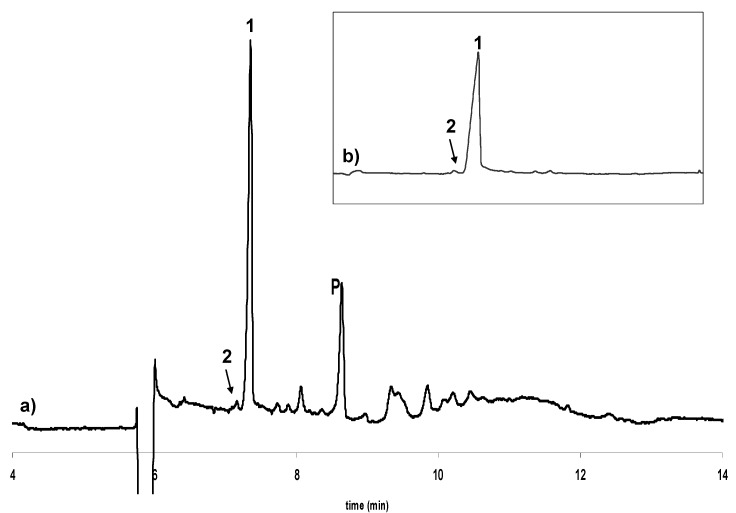
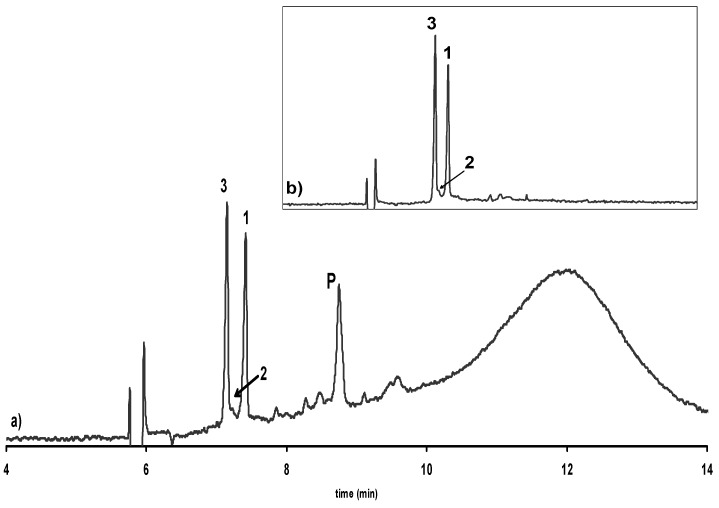
Figure 3.
Electropherogram showing the enantioselective separation of catechin and epicatechin in a) an extract of roasted cocoa beans and b) isolated from this extract. 1: (-)-Epicatechin, 2: (+)-Catechin, 3: (-)-Catechin, P: Procyanidin B2. Experimental conditions as in Figure 2.
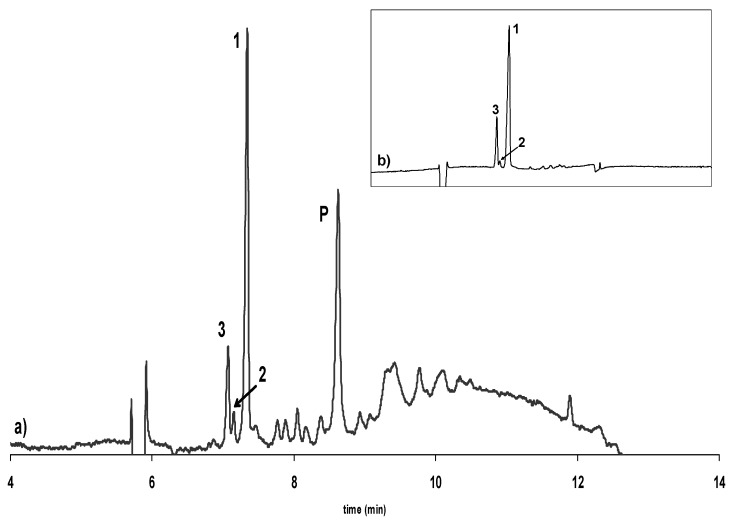
Figure 4.
Electropherogram showing the enantioselective separation of catechin and epicatechin in a) an extract of dark chocolate and b) isolated from this extract. 1: (-)-Epicatechin, 2: (+)-Catechin, 3: (-)-Catechin, P: Procyanidin B2. Experimental conditions as in Figure 2.
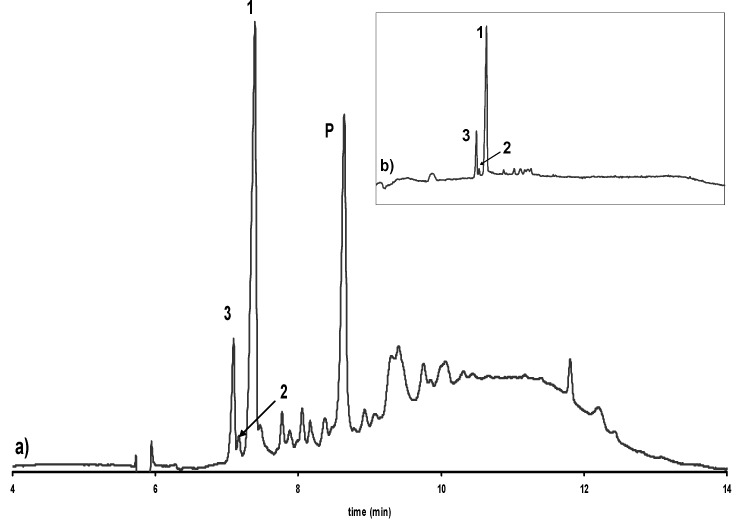
Figure 5.
Electropherogram showing the enantioselective separation of catechin and epicatechin in a) an extract of cocoa powder and b) isolated from this extract. 1: (-)-Epicatechin, 2: (+)-Catechin, 3: (-)-Catechin, P: Procyanidin B2. Experimental conditions as in Figure 2.
Influence of temperature
Our enantioselective analysis of cocoa and cocoa products showed that the occurrence of (-)-catechin is influenced by the cocoa processing conditions used. Our analysis showed that the first initial processing step for the formation of (-)-catechin is the roasting procedure (see Figure 3). Under the influence of high temperature (-)-epicatechin was converted into (-)-catechin, indicating an epimerization reaction. To confirm the influence of heat treatment during the roasting process we roasted unfermented cocoa beans which only contain (-)-epicatechin and (+)-catechin and undectectable levels of (-)-catechin (roasting conditions mentioned below). Analyzing the cocoa beans after the roasting procedure, we found (-)-catechin (results not shown). Therefore the occurrence of (-)-catechin in roasted cocoa beans is clearly induced by the roasting conditions. However, we could not exclude the possibility that in cocoa (-)-catechin additionally derives from the degradation of the procyanidins present.
Analyzing cocoa products, like cocoa liquor or chocolate bars containing different levels of cocoa solids we also detected (-)-catechin (see Figure 3). After the roasting procedure the cocoa beans are ground to cocoa liquor and used as an ingredient for chocolate [27].
Another reason for the occurrence of (-)-catechin could be the epimerization reaction of the flavan-3-ols during sample preparation, which could be induced by high temperature and/or alkaline pH value. We could exclude these possibilities, because the whole sample preparation was accomplished at room temperature. The pH value of the methanolic/aqueous solution at the beginning of the cocoa extraction was 5.3 – 7.1. Additionally we couldn’t detect any influence of oxygen or UV-light during the sample preparation. To confirm this, we conducted experiments under “extreme” conditions. A standard solution of (-)-epicatechin was aerated for 24 hours. After that time we analyzed the sample and could not find any epimerization products. To test the influence of daylight and UV-light, we prepared a (-)-epicatechin standard solution using the standard conditions. Another solution was irradiated by UV-light for 24 hours (experimental conditions mentioned below). Analyzing these samples by CCE, we could not find any epimerization products in the solution subjected by daylight conditions. On the other hand, in the UV irradiated solution we could find (-)-catechin (results not shown). Therefore we can confidently exclude any influence of heat, light and oxygen to the epimerization reaction during sample preparation.
Influence of alkalization
As shown in Figure 5, we detected an increased level of (-)-catechin in cocoa powder and products containing cocoa powder, in contrast to chocolate samples. This fact is caused by the processing of cocoa powder. Cocoa liquor is alkalized to improve its technological and sensory qualities. Thus, it is typically treated for a certain period under basic pH and heat conditions and then neutralized to the desired pH value. The cocoa powder then can be used, for example, in instant chocolate drinks, commercial desserts or chocolate coatings. Under the alkaline conditions the epimerization reaction continues. After the alkalization of the cocoa liquor, it is subjected to pressure to separate the cocoa butter from the cocoa solids [27]. Perhaps the pressure applied (40-50 kPa) could also induce the epimerization reaction of the flavan-3-ols. We confirmed our results by alkalization experiments. Thus, we alkalized dried, unfermented, unroasted cocoa beans, which only contain (-)-epicatechin and (+)-catechin and no detectable (-)-catechin (experimental conditions mentioned below). The prepared extracts were analyzed by CCE. After this treatment we also found (-)-catechin in our samples. From this we infer that the occurrence of (-)-catechin in cocoa powder is first influenced by the roasting conditions and second by the alkalization process.
We could exclude any epimerization during the sample preparation and the CCE analysis. The enantioselective separation was performed with a borate buffer at pH 8.5. We separated the flavan-3-ol enantiomers during nearly 8 minutes and at a temperature of 20 °C. As shown in our previous studies, the analysis time used would be too short for any epimerization reactions to take place under the chosen conditions [18,28]. All results were confirmed by spiking experiments and comparison of the migration times to those obtained using standard solutions.
Isolation, purification and LC-MS analysis
The extracts from the different cocoa products show significant levels of procyanidins that are likely to comigrate with the monomeric flavanols in CCE separation. The monomeric flavanols were therefore isolated from the extracts and the identity and purity were confirmed by non-chiral HPLC–UV-iontrap MS analysis. After isolation and purification, no compounds are present anymore that would likely compromise CCE (see Figure 6).
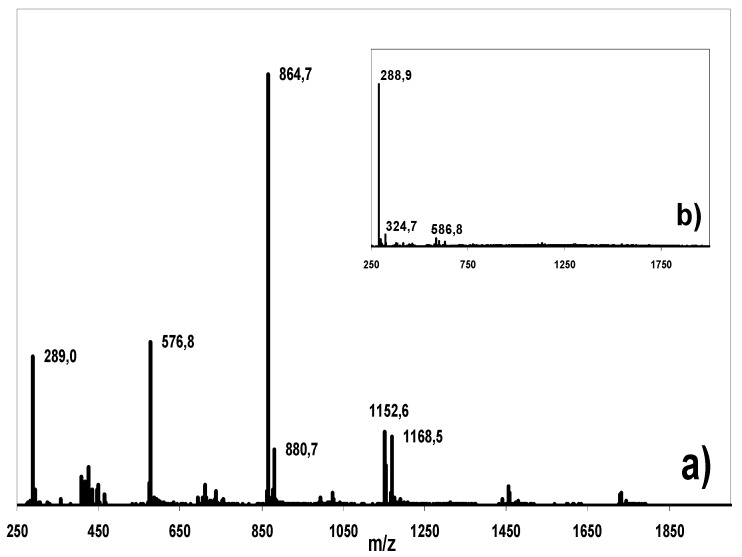
Figure 6.
MS spectra (averaged over the retention time frame of the flavan-3-ols, 20-35 min) of a) the raw cocoa bean extract and b) the isolated and purified flavan-3-ols from this extract. The signals in the extract spectrum correspond to procyanidins. The small signals in the isolated flavanols correspond to ionization-induced adducts and fragments and background noise.
Comparison of the flavan-3-ol peak ratios
Referring to former studies, the peak ratios of polyphenols can be much more interesting than the absolute amounts of flavan-3-ol enantiomers, because it is constant in a cultivar. The content of a particular substance may differ from year to year, but the peak ratios remain nearly constant. Therefore, during the last few years the use of peak ratios of polyphenolic substances has become more popular [29].
For a better comparison of our CCE results we calculated the peak ratio of the flavan-3-ol enantiomers of various cocoa beans, chocolate and cocoa powder samples. Table 1 shows the peak ratios of (-)-catechin and (-)-epicatechin calculated for different cocoa samples after enantioselective separation by CCE. During the processing of cocoa, degradation of catechin and epicatechin is enormous compared to their levels in unfermented cocoa beans, which is caused by the fermentation and the roasting procedure [30]. Additionally, the major flavan-3-ol enantiomer, (-)-epicatechin is epimerized to (-)-catechin. In unfermented, dried, unroasted cocoa beans we were unable to identify (-)-catechin by CCE. However, we found (-)-catechin in roasted cocoa beans and their subsequent products, like cocoa liquor and chocolate. In these products the peak ratios of (-)-catechin and (-)-epicatechin are in a similar range. It can therefore be concluded that one of the main reasons for the epimerization of (-)-epicatechin to (-)-catechin is the roasting procedure. The ratios of the non alkalized cocoa powders are different. By processing non-alkalized cocoa powder the cocoa liquor will be subjected to heat and pressure to extract the cocoa butter from the cocoa solids [27]. We can not exclude the possibility that the epimerization reaction continues after this. Comparing the (-)-catechin and (-)-epicatechin peak ratios of alkalized cocoa powder we observed enormous differences (see Table 1). As mentioned above during the alkalization process the epimerization reaction continues.
Table 1.
Peak ratios of (-)-catechin and (-)-epicatechin for the enantioselective separation of catechin and epicatechin in different cocoa and cocoa product extracts.
| Raw, unfermented cocoa beans | - * |
| Roasted cocoa beans | 18 : 82 |
| Roasted cocoa beans (nibs) | 19 : 81 |
| Cocoa liquor (non-alkalized) | 18 : 82 |
| Chocolate (85% cocoa solids) | 18 : 82 |
| Cocoa Powder (non-alkalized) | 24 : 76 |
| Cocoa Powder (non- alkalized) | 35 : 65 |
| Cocoa Powder (alkalized)** | 42 : 58 |
| Cocoa Powder (alkalized) ** | 65 : 35 |
* undectectable level of (-)-catechin
** grade of alkalization not known
In contrast to (-)-catechin, which displayed increasing levels during cocoa processing, (+)-epicatechin was not detected by CCE in the analyzed samples, which would be expected to occur as a product of the epimerization of (+)-catechin. This finding may be due to several reasons: (1) (+)-catechin is only a minor genuine component of the natural flavanols in cocoa in contrast to the dominating (-)-epicatechin. Consequently, in comparison to the (-)-catechin levels, only small amounts of (+)-epicatechin would likely be formed during the epimerization reaction; (2) generally, the sensitivity of the detection used in CCE is limited and moreover the small amounts of (+)-epicatechin are not sufficiently separated from the close eluting predominant (-)-epicatechin peak; (3) the transoid structure of the substituents at C-2 and C-3 in catechin is thermodynamically favoured over the cisoid structure in epicatechin (see Figure 1). The formation of (-)-catechin from (-)-epicatechin is therefore likely favoured in comparison to the formation of (+)-epicatechin from (+)-catechin [31]. These factors might all together account for this finding.
Conclusions
Cocoa and chocolate samples were analyzed by CCE using the enantioseparation of catechin and epicatechin. (-)-Catechin was found among (-)-epicatechin and (+)-catechin as a result of an epimerization reaction. (+)-Epicatechin, however, could not be determined. It was found that (-)-catechin is formed during the roasting process and especially during the alkalization of the cocoa powder. The results were confirmed by isolating and analyzing the monomeric flavan-3-ols by solid-phase-extraction and LC-MS, respectively. We observed a degradation of (-)-epicatechin during the manufacturing process. Additionally (-)-epicatechin levels decreased caused by its epimerization to (-)-catechin. Comparing the differences of peak ratios between (-)-catechin and (-)-epicatechin, we could characterize the influence of roasting and particularly alkalization.
Outlook
The epimerization of flavan-3-ol enatiomers should be intensively studied with regard to the technological parameters during the roasting and alkalization process. Furthermore, an appropriate chiral HPLC method should be applied, verifying the occurrence of (+)-epicatechin, which is likely formed from (+)-catechin.
Experimental
General
Standards of (+)-catechin, (-)-catechin, (+)-epicatechin, and (-)-epicatechin were obtained from Sigma (St. Louis, MO, USA). Anhydrous sodium tetraborate, boric acid, sodium hydroxide, (2-hydroxypropyl)-γ-cyclodextrin, and sodium dihydrophosphate were purchased from Fluka Chemie (Buchs, Switzerland), and dimethylsulfoxide from Merck (Darmstadt, Germany). Methanol and acetonitrile were purchased from Fisher Scientific (Loughborough, UK). For unfermented, dried, unroasted beans, cocoa fruits were purchased from a Brasilian supermarket (Campinas/Sao Paolo, Brazil) and the raw beans removed manually and sundried. Roasted cocoa beans, chocolate and cocoa products were bought from a local supermarket. Catechin standards were diluted in dimethylsulfoxide (DMSO) to a concentration of 0.5 mg·mL-1 and were stored at 8 °C. The background electrolyte (BGE) was prepared by dissolving appropriate amounts of boric acid and anhydrous sodium tetraborate adjusted to desired pH values by using NaOH [32]. The purified water we used in this study was produced by an Elgastat UHQ II (Elga Ltd., Buds, UK). The chiral selectors were dissolved to different concentrations in the running buffer. Before use, the buffer solutions were filtered through a 0.45 µm regenerated cellulose syringe filter (IVA, Meerbusch, Germany). The cocoa beans, chocolate samples, and cocoa product samples were ground in a pebble mill (MM Retsch, Haan, Germany). An amount (500 mg) was mixed with MeOH+purified water (30 mL, 80:20, v/v) and stirred for 2 hours. Then methanol was evaporated at room temperature at ambient pressure in a fume hood. The residue was diluted in purified water (30 mL), centrifuged at 10000 rpm for 15 min in a Biofuge Stratos (Heraeus Holding GmbH, Hanau, Germany) and filtered through a folded filter paper (Schleicher and Schuell Microscience GmbH, Dassel, Germany). The filtrate was purified and concentrated by solid phase extraction using a previously published method with a 6 mL size Chromabond PA cartridge (Macherey-Nagel, Düren, Germany) packed with 1000 mg of polyamide. The cartridge was conditioned with DMSO+formic acid (2 mL, 99:1, v/v) for 10 min and washed with purified water (10 mL). An aliquot of the above mentioned filtrate (30 mL) was loaded onto the SPE cartridge quantitatively and the cartridge was washed with purified water (20 mL) and DMSO+formic acid (1.5 mL, 99:1, v/v). Finally, the adsorbed analytes were eluted with the latter solvent mixture (2.0 mL) and the eluate was used for the CCE [33].
Roasting, alkalization, temperature and UV experiments
Roasting and alkalization experiments were performed using unfermented, dried, unroasted cocoa beans. The beans were roasted in a Memmert UL 50 drying oven (Memmert, Schwalbach, Germany) at 120 °C for 2 hours or at 160 °C for 2.5 hours. After that time, the preparation of the beans followed the sample preparation mentioned above. For the alkalization experiments, the ground beans were mixed with MeOH+purified water (30 mL, 80:20, v/v) for 2 hours. The methanol was evaporated at room temperature. The residue was diluted with 10 mol·L-1 Na2CO3 solution (30 mL, pH 9.6), centrifuged at 10000 rpm for 15 min in the Biofuge Stratos and filtered through a folded filter paper. Then the solution was heated in a water bath for 1 hour at 75 °C. After that, the solution was cooled down to room temperature, neutralized to pH 6.1 with 0.1 mol·L-1 citric acid and purified and concentrated by the solid phase extraction procedure described above. For the temperature experiments, a standard solution of (-)-epicatechin (0.5 mg·mL-1) was stored in a beaker at room temperature for 24 hours. After that time, the solution was analyzed by CCE. For the daylight and UV-experiments a standard solution of (-)-epicatechin (0.5 mg·mL-1) was exposed to daylight for 24 hours and another was irradiated with UV-light (254, 280 nm) using a “Fluotest Universal” (Original Hanau Quarzlampen GmbH, Hanau, Germany) for 24 hours. After that time, the solution was analyzed by CCE.
Isolation and purification of monomeric flavan-3-ols from extracts
Isolation and purification of the monomeric flavan-3-ols from cocoa and chocolate extracts was performed using 3 mL size ChromaBond solid phase extraction cartridges packed with 500 mg of polyamide (Macherey-Nagel, Düren, Germany), according to a previously described method with slight modifications [18]. The cartridge was conditioned with DMSO+formic acid (2 mL, 99:1, v/v) and purified water (5 mL). The extract (500 µL) was dissolved in purified water (9.5 mL), loaded onto the cartridge and the polyamide was then washed with purified water (10 mL) and MeOH (2 mL). The monomeric flavan-3-ols were recovered from the SPE cartridge by elution with additional MeOH (3 mL). The solvent was evaporated in vacuo and the compounds dissolved in DMSO+formic acid (500 µL, 99:1, v/v) prior to analysis. The extracts as well as the isolated monomeric flavanols were analyzed by HPLC-UV-ion trap MS to verify the identity of the flavan-3-ols and verify the purity. Subsequently, the isolated flavanols were subjected to CCE.
HPLC- MS analysis
Individual polyphenols were identified by multi step mass spectrometric fragmentation after HPLC separation and UV-VIS diode array detection. A Summit HPLC System (Dionex, Idstein, Germany) equipped with an Aqua 3 µm C18, 150 mm x 2 mm I.D. analytical column (Phenomenex, Aschaffenburg, Germany) kept at 35 °C was used. 1% Acetic acid in purified water (mobile phase A) and 1% acetic acid in acetonitrile (mobile phase B) were used at 300 µL·min-1 starting at 0% B with a linear gradient to 30% B after 60 minutes followed by washing (100% B for 10 min) and reequilibration (0% B for 10 min). 5 µL of each sample were injected and the chromatograms monitored at 200–595 nm.
An LCQ Classic ion-trap mass spectrometer (Thermo Fisher Scientific, Dreieich, Germany) with an ESI-interface and a metal needle kit was operated in the negative mode as published earlier [18,33]. The phenolic compounds were detected in their deprotonated form as the quasimolecular ion [M-H]– one mass unit below their molecular mass. Identification of individual compounds was conducted by comparison of their UV-spectra and ion trap fragmentation patterns with a self prepared library from standard substances and known compounds as described in detail previously [33,34,35].
Chiral capillary electrophoretic analysis
All chiral separations were carried out on a Beckman P/ACE MDQ capillary electrophoretic system (Beckman Instruments, Fullerton, CA, USA) equipped with a photo diode array detection system. The scan range was 190 to 350 nm. All electropherograms were recorded at 280 nm. Data was processed on an IBM personal computer with Beckman 32 Karat software version 7.0. The identification of the analytes was achieved comparing the migration times and the UV-spectra of the flavan-3-ols and by spiking with standard solutions of the single enantiomers. Uncoated fused-silica capillaries of 375 µm O.D. × 75 µm I.D. were obtained from Beckman and cut to 60.2 cm (effective length 50 cm). The capillary was first washed by 20 p.s.i. pressure with 0.25 mol·L-1 NaOH for 20 min and finally with purified water for 10 min; between each run with 0.25 mol·L-1 NaOH for 3 min, with purified water for 2 min, and equilibrated with the running buffer for 5 min; for the overnight storage with 0.25 mol L-1 NaOH for 5 min, with purified water for 10 min, and an air stream for 3 minutes to remove the liquid in the capillary. The capillary temperature was set to 20 °C and the applied voltage was 18 kV. Samples were injected hydrodynamically (0.3 p.s.i.) for 3 s. We injected purified water for 2 s (0.2 p.s.i.) after and before injecting the sample into the capillary. In this way the contamination of the sample vial with small amounts of the running buffer was avoided. For every pair of buffer we used a separate vial of purified water. This procedure has no negative effect on the chiral separation system, but solves the problem of sample degradation as studied previously [23].
Analytical characteristics (CCE)
The linearity of the CCE method in the 20-200 µg·mL-1 range was demonstrated by standard curves for (+)-catechin, (-)-catechin and (-)-epicatechin (r2 > 0.999). It has to be mentioned that to achieve a sufficient peak resolution in chiral separations by capillary electrophoresis, the peak height of a chiral substance should be less than 5 mAU [18]. The reproducibility of the migration time was investigated for (+)-catechin (RSD 0.8% intra-day (n=3), RSD 0.97% inter-day (n=9)) and (-)-epicatechin (RSD 0.77% intra-day (n=3), RSD 0.99% inter-day (n=9)). Detection limits: (defined as a signal-to-noise ratio of 3) limit of detection = 5 µg·mL-1 and limit of quantification = 10 µg·mL-1 (defined as a signal-to-noise ratio of 6) for (+)-catechin, (-)-catechin and (-)-epicatechin.
Acknowledgments
The authors acknowledge PD Dr. Friedhelm Marx and Dr. Roberta Rodrigues, University of Bonn, for the kind gift of raw, dried cocoa beans.
Footnotes
Sample Availability: Samples of the compounds are available from the corresponding author.
References
- 1.Arts I.C.W., van de Putte B., Hollman P.C.H. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000;48:1746–1751. doi: 10.1021/jf000025h. [DOI] [PubMed] [Google Scholar]
- 2.Harnley J.M., Doherty R.F., Beecher G.R., Holden J.M., Haytowitz D.B., Bhagwat S., Gebhardt S. Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 2006;54:9966–9977. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- 3.Hooper L., Cassidy A. A review of the health potential of bioactive compounds. J. Sci. Food Agric. 2006;86:1805–1813. doi: 10.1002/jsfa.2599. [DOI] [Google Scholar]
- 4.Treutter D. Significane of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006;4:147–157. doi: 10.1007/s10311-006-0068-8. [DOI] [Google Scholar]
- 5.Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2006;51:116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kermez Z., Chetrit D., Shoseyov O., Regev-Shosani G. Protection of lipids from oxidation by epicatechin, trans-resveratrol, and gallic and caffeic acids in intestinal model systems. J. Agric. Food Chem. 2006;54:10288–10293. doi: 10.1021/jf0621828. [DOI] [PubMed] [Google Scholar]
- 7.Murphy K.J., Chronopoulos A.K., Singh I., Francis M.A., Moriarty H., Pike M.J., Turner A.H., Mann N.J., Sinclair A.J. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cocoa) inhibit platelet function. Am. J. Clin. Nutr. 2003;77:1466–1473. doi: 10.1093/ajcn/77.6.1466. [DOI] [PubMed] [Google Scholar]
- 8.Hurell R.F., Reddy M., Cook J.D. Inhibition of non-haem iron aborption in man by polyphenolic-containing beverages. Brit. J. Nutr. 1999;81:289–295. [PubMed] [Google Scholar]
- 9.Vinson J.A., Proch J., Bose P., Muchler S., Taffera P., Shuta D., Samman N., Agbor G.A. Chocolate is a powerfull ex vivo and in vivo antioxidant, an antiathereosclerotic agent in animal model, and a significant contributor to antioxidants in european and american diets. J. Agric. Food Chem. 2006;54:8071–8076. doi: 10.1021/jf062175j. [DOI] [PubMed] [Google Scholar]
- 10.Donovan J.L., Crespy V., Oliveira M., Cooper K.A., Gibson B.B., Williamson G. (+)-Catechin is more bioavailable than (-)-catechin: Relevance to the bioavailability of catechin from cocoa. Free Radical Res. 2006;40:1029–1034. doi: 10.1080/10715760600868545. [DOI] [PubMed] [Google Scholar]
- 11.Nyfeler F., Moser U.K., Walter P. Stereospecific effects of (+)- and (-)-catechin on glycogen metabolism in isolated rat hepatocytes. Biochim. Biophys. Acta. 1983;763:50–57. doi: 10.1016/0167-4889(83)90024-1. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y., Liang R., Yang B., Wang L., Yang Q., Wang Y. Protection of (-)-catechin gallate and (+)-epicatechin on xanthine-xanthine oxidase system injury in cultered cardiomyocytes. Zhongguo Shiyan Fangjixue Zazhi. 2006;12:36–38. [Google Scholar]
- 13.Cho S.Y., Park P.J., Shin H.J., Kim Y.K., Shin D.W., Shin E.S., Lee H.H., Lee B.G., Baik J.H., Lee T.R. (-)-Catechin suppresses expression of Kruppel-like factor 7 and increases expression and secretion of adiponectin protein in 3T3-L1 cells. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1166–E1172. doi: 10.1152/ajpendo.00436.2006. [DOI] [PubMed] [Google Scholar]
- 14.Pfeiffer J., Kühnel C., Brandt J., Duy D., Punyasiri P.A.N., Forkmann G., Fischer T.C. Biosynthesis of flavan-3-ols by leucoanthocyanidin 4-reductase and anthocyanidin reductase in leaves of grape (Vitis vinifera L.), apple (Malus x domestica Borkh.) and other crops. Plant Physiol. Bioch. 2006;44:323–334. doi: 10.1016/j.plaphy.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Ellis C.J., Yeap Foo L., Porter L.J. Enantiomerism: A characteristic of the proanthocyanidin chemistry of the Monocotyledonae. Phytochem. 1983;22:483–487. doi: 10.1016/0031-9422(83)83030-1. [DOI] [Google Scholar]
- 16.Blair A.C., Nissen S.J., Brunk G.R., Hufbauer R.A. A lack of evidence for an ecological role of the putative allelochemical (+/-)-catechin in spotted knapweed invasion success. J. Chem. Ecol. 2006;32:2327–2331. doi: 10.1007/s10886-006-9168-y. [DOI] [PubMed] [Google Scholar]
- 17.Prithiviraj B., Perry L.G., Badri D.V., Vivanco J. M. Chemical facilitation and induced pathogen resistance mediated by root-secreted phytotoxin. New Phytol. 2007;173:852–860. doi: 10.1111/j.1469-8137.2006.01964.x. [DOI] [PubMed] [Google Scholar]
- 18.Kofink M., Papagiannopoulos M., Galensa R. Enantioseparation of catechin and epicatechin in plant food by chiral capillary electrophoresis. Eur. Food Res. Technol. 2007;225:569–577. doi: 10.1007/s00217-006-0455-1. [DOI] [Google Scholar]
- 19.Haslam E. In: The flavonoids. Harborne J.B., editor. Chapman and Hall; London: 1975. pp. 550–553. Chapter 10. [Google Scholar]
- 20.Kennedy J.A., Munro M.H.G., Powell H. K. J., Porter L.J., Yeap Foo L. The protonation reactions of catechin and epicatechin and related compounds. Aust. J. Chem. 1984;37:885–892. doi: 10.1071/CH9840885. [DOI] [Google Scholar]
- 21.Kodama S., Yamamoto A., Akinobu M., Yanai H. Direct enatioseparation of catechin and epicatechin in tea drinks by 6-O-α-D-glucosyl-β-cyclodextrin-modified micellar electrokinetic chromatography. Electrophoresis. 2004;25:2892–2898. doi: 10.1002/elps.200305902. [DOI] [PubMed] [Google Scholar]
- 22.Gotti R., Furlanetto S., Pinzauti S., Cavrini V. Analysis of catechins in Theobroma cacoa beans by cyclodextrin-modified micellar electrokinetic chromatography. J. Chromatogr. A. 2006;1112:345–352. doi: 10.1016/j.chroma.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 23.Gel-Moreto N. PhD Thesis. University of Bonn; 2003. Enantiomerentrennung von Polyphenolen in Citrus mittels Kapillarelektrophorese (CE) und Hochleistungsflüssigkeitschromatographie (HPLC) [Google Scholar]
- 24.Hammerstone J.F., JR., Acquarone V. Process for controlling the isomerization of (-)-epicatechin and (+)-catechin in edible products. U.S. Pat. Appl. 2007/0003640. 2007.
- 25.Woefel K.A., Robbins R.J. Products containing polyphenols. WO 2007/002883. 2007.
- 26.Hammerstone J.F., JR., Acquarone V. Heat-processed products having altered monomer profiles and processes for controlling the epimerization of (-)-epicatechin and (+)-catechin in the products. WO 2007/002852. 2007.
- 27.Beckett S.T. The science of chocolate. Royal Society of Chemistry; Cambridge: 2000. pp. 31–47. [Google Scholar]
- 28.Komatsu Y., Suematsu S., Hisanobu Y., Saigo H., Matsuda R., Hara K. Effects of pH and temperature on reaction kinetics of catechins in green tea infusion. Biosci. Biotech. Biochem. 1993;57(6):907–910. [Google Scholar]
- 29.Zimmermann B.F., Galensa R. One for all-all for one: Proof of authenticity and tracing of foods with flavonoids. Analysis of proanthocyanidins in barley and malt. Eur. Food Res. Technol. 2006;24:385–393. doi: 10.1007/s00217-006-0333-x. [DOI] [Google Scholar]
- 30.Wollgast J., Anklam E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000;33:423–447. doi: 10.1016/S0963-9969(00)00068-5. [DOI] [Google Scholar]
- 31.Kiatgrajai P., Wellons J.D., Gollob L., White J.D. Kinetics of epimerization of (+)-catechin and its rearrangement to catechinic acid. J. Org. Chem. 1982;47:2910–2912. doi: 10.1021/jo00136a021. [DOI] [Google Scholar]
- 32.Wätzig H., Degenhardt M., Kunkel A. Strategies for capillary electrophoresis: Method development and validation for pharmaceutical and biological applications. Electrophoresis. 1998;19:2695–2752. doi: 10.1002/elps.1150191603. [DOI] [PubMed] [Google Scholar]
- 33.Papagiannopoulos M., Wollseifen H.R., Mellenthin A., Haber B., Galensa R. Identification and quantification of polyphenols in Carob Fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MSn. J. Agric. Food Chem. 2004;52:3784–3791. doi: 10.1021/jf030660y. [DOI] [PubMed] [Google Scholar]
- 34.Friedrich W., Eberhardt A., Galensa R. Investigations of proanthocyanidins by HPLC with electrospray ionization mass spectrometry. Eur. Food Res. Technol. 2000;211:56–64. doi: 10.1007/s002170050589. [DOI] [Google Scholar]
- 35.Papagiannopoulos M., Zimmermann B., Mellenthin A., Krappe M., Maio G., Galensa R. On-line coupling of pressurized liquid extraction, solid-phase-extraction and high-performance liquid chromatography for automated analysis of proanthocyanidins in malt. J. Chromatogr. A. 2002;958:9–16. doi: 10.1016/S0021-9673(02)00364-3. [DOI] [PubMed] [Google Scholar]