Abstract
Eight naturally occurring marine-sponge derived sesquiterpenoid quinones were evaluated as potential inhibitors of pyruvate phosphate dikinase (PPDK), a C4 plant regulatory enzyme. Of these, the hydroxyquinones ilimaquinone, ethylsmenoquinone and smenoquinone inhibited PPDK activity with IC50’s (reported with 95% confidence intervals) of 285.4 (256.4 – 317.7), 316.2 (279.2 – 358.1) and 556.0 (505.9 – 611.0) μM, respectively, as well as being phytotoxic to the C4 plant Digitaria ciliaris. The potential anti-inflammatory activity of these compounds, using bee venom phospholipase A2 (PLA2), was also evaluated. Ethylsmenoquinone, smenospongiarine, smenospongidine and ilimaquinone inhibited PLA2 activity (% inhibition of 73.2 ± 4.8 at 269 µM, 61.5 ± 6.1 at 242 µM, 41.0 ± 0.6 at 224 µM and 36.4 ± 8.2 at 279 µM, respectively). SAR analyses indicate that a hydroxyquinone functionality and a short, hydroxide/alkoxide side-chain at C-20 is preferred for inhibition of PPDK activity, and that a larger amine side-chain at C-20 is tolerated for PLA2 inhibitory activity.
Keywords: Pyruvate phosphate dikinase (PPDK), C4 plant, phospholipase A2 (PLA2), anti-inflammatory activity, sesquiterpene hydroxyquinones/hydroquinones/amino-quinones, ilimaquinone
Introduction
Sesquiterpene quinol/quinone compounds, represented by avarol (1) [1,2] and ilimaquinone (2) [3,4] (Table 1), exhibit a wide range of bioactivity, including mammalian cytotoxicity, antimicrobial, antiviral and anti-inflammatory actions [5,6,7,8,9,10,11,12,13,14,15,16,17,18]. The sesquiterpene quinones share a common drimane rearranged cis- or trans-decalin ring varying at the relative position of the double bond at the C-4 carbon and/or the stereochemical configuration about C-5. The C-9 position is decorated with a variably hydroxylated or heteroatom-substituted benzoquinone side chain. Given the interesting and diverse medicinal activities of this class of compounds, this structural motif has been the subject of several synthetic studies [19].
Table 1.
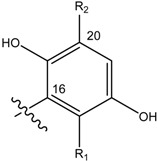
Structures of avarol, 1, the sesquiterpene hydroxyquinones/ amino-quinones/hydroquinones 2 – 9 and bolinaquinone, 10.
| Name | Drimane rearranged decalin ring | X | R1 | R2 |
| (1) [1, 2] Dysidea avara |
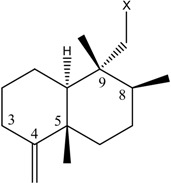 |
 |
H | H |
| (2) [3, 4] Rhopaloeides sp. |
 |
OH | OCH3 | |
| (3) [22] Smenospongia sp. |
OH | OH | ||
| (4) [22, 23] Smenospongia sp. |
OH | OCH2CH3 | ||
| (5) [22] Smenospongia sp. |
OH | NH(CH2)2C6H5 | ||
| (6) [22] Smenospongia sp. |
OH | NH(CH2)2CH(CH3)2 | ||
| (7) [22] Smenospongia sp. |
OH | NHCH2CH(CH3)2 | ||
| (8), [24] Rhopaloeides sp. |
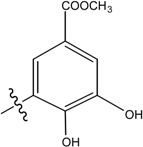 |
- | - | |
| (9) [25] Rhopaloeides sp. |
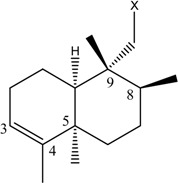 |
 |
- | - |
| (10) [26] Dysidea sp. |
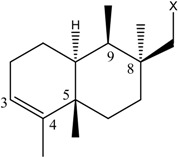 |
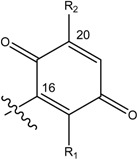 |
OH | OCH3 |
Recently, we reported that ilimaquinone selectively inhibited pyruvate Pi dikinase (PPDK) [20], generally considered the rate limiting enzyme for C4 plant dark reactions [21] and did not adversely affect the other critical enzymes phosphoenolpyruvate carboxylase (PEPC) and malate dehydrogenase (MDH). Furthermore, when ilimaquinone was applied to leaves of a C4 plant, a detrimental effect was observed, demonstrating the translation of the in vitro response to an in vivo response.
One of the major challenges for the agrochemical and pharmaceutical industries alike is to discover molecules, whether novel or known, that are able to bind selectively to an appropriate receptor (e.g. PPDK) within a target species (e.g. C4 plants) and are able to reach that receptor when applied to the whole organism (e.g. C4 plant in the field).
In this study we report on the herbicidal properties of eight sesquiterpene hydroxyquinones, hydroquinones and aminoquinones isolated from marine sponges, in particular their ability to inhibit PPDK and translation of this in vitro activity into a phytotoxic response in whole C4 plants. We also report on the potential anti-inflammatory activity of these compounds against phospholipase A2 (PLA2) isolated from bee venom [18] and propose a putative pharmacophore to explain the activity profiles.
Results and Discussion
Herbicidal activity
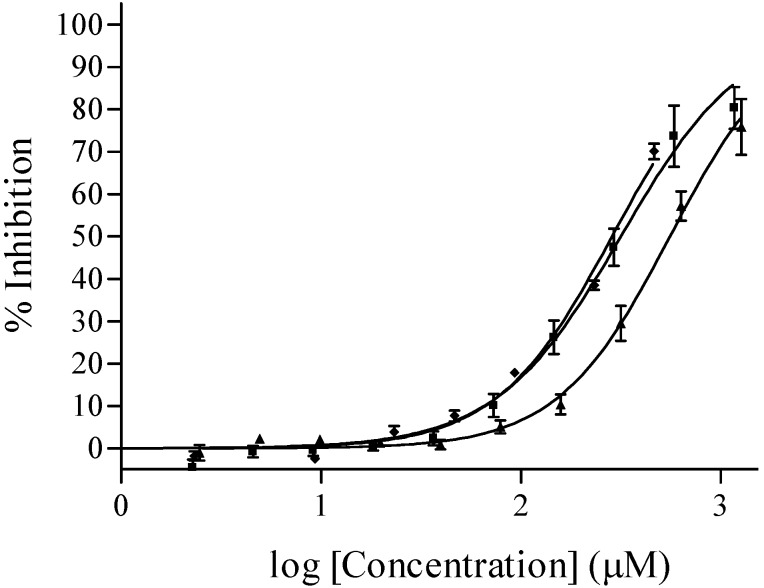
Of the eight marine derived sesquiterpene quinones/hydroquinones tested (Table 1) in the coupled enzyme assay the three sesquiterpene hydroxyquinones – ilimaquinone (2), smenoquinone (3) and ethylsmenoquinone (4) – were identified as inhibiting PPDK activity at 435 μgmL-1. The aminoquinones 5, 6 and 7, and hydroquinones 8 and 9 were inactive at this concentration. Dose response analysis revealed the following ranking, in order of decreasing PPDK inhibitory potency: (2) > (4) > (3) with IC50’s (reported with 95% confidence intervals in parenthesis) of 285.4 (256.4 – 317.7), 316.2 (279.2 – 358.1) and 556.0 (505.9 – 611.0) μM, respectively against PPDK (Figure 1).
Figure 1.
Dose-response titration of ilimaquinone (♦), smenoquinone (▲) and ethylsmenoquinone (■) against PPDK. Data points are the mean and SD of 2 (Ilimaquinone) or 4 (smenoquinone and ethylsmenoquinone) individual experiments. The data was fitted with a sigmoidal (Hill) dose response curve of the form 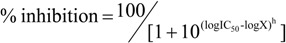 where IC50 is the concentration that causes 50 % inhibition of PPDK, X is the concentration of the compound, h is the Hill slope and curve fitting is constrained between 0 and 100% inhibition.
where IC50 is the concentration that causes 50 % inhibition of PPDK, X is the concentration of the compound, h is the Hill slope and curve fitting is constrained between 0 and 100% inhibition.
The three PPDK inhibitory compounds, 2, 3 and 4, were applied to the leaves of the C4 plant Digitaria ciliaris with detrimental effects noted within the first 24 h. Controls, with formulation only, showed no observable effect. Application of 3 and 4 to the flag leaves resulted in a reduction in the leaf size within 24 h, followed by leaf shrivelling over the area of application after 48 h. Application of 2, however, resulted in leaf death over the area of treatment within the first 24 h. In all three experiments no translocation of activity was observed. It is well documented that post-emergent herbicides must generally be able to penetrate the leaf cuticle to have whole plant activity [27]. The phytotoxic effect of compounds 2 – 4 indicate that they can penetrate the lipophilic leaf cuticle using the formulation 0.5% dimethylsulfoxide (DMSO) and 0.08% Uptake™ in MeOH. Herbicides are generally formulated in mostly aqueous carriers, but compounds 2 – 4, however, have limited solubility in water. This limitation may be overcome by tailoring the carrier with the use of adjuvants such as surfactants, oils, fertilizers and pH modifiers to optimize penetration and ensure the compound is delivered to the target receptor. Further studies are in progress in order to evaluate the herbicidal activity of these compounds.
Theoretical chemical property calculations
All compounds in this study were assessed for their herbicide potential using chemical property calculations according to Tice [28] (Table 2). Modifications to the Lipinski ‘Rule of 5’ [29] have been proposed for post-emergent herbicides, with compounds being more likely to be developed as post-emergent herbicides if the molecular mass is within 150-500; if the theoretical logarithm of the n-octanol/water partition coefficient (alog P), is less than or equal to 5.0; if the molecule’s polar surface area (PSA) is between 50-60 Å2; if the number of hydrogen bond donors is three or less; and if the number of hydrogen bond acceptors is between 2 and 12 inclusive.
Table 2.
Observed biological properties and calculated theoretical properties of the eight sesquiterpene hydroxyquinones/aminoquinones/hydroquinones 2 - 9 as compared to the desired range [28,29,30].
| Molecular mass | clog P | H-bond donors | H-bond acceptors | Rot. Bonds | PSA (Å2) | SMV(Å3) | PPDK IC50 (μM) | C4 phytotoxicity d | % inhibitionof PLA2 | |||
| 24h | 48h | 72h | ||||||||||
| Desired Tice a | 150≤x ≤500 | ≤5.0 | ≤3 | 2≤x≤12 | ≤12 | 50-60 | 6 | 6 | 6 | |||
| Lipinski b | ≤500 | ≤5.0 | ≤5 | ≤10 | ||||||||
| (2) | 358.2 | 6.0 | 1 | 4 | 3 | 63.6 | 351.4 | 285.4 | 4 | 4 | 4 | 36.4 ± 8.2 at 279 µM |
| (3) | 344.2 | 5.9 | 2 | 4 | 2 | 74.6 | 333.8 | 556.0 | 2 | 3 | 3 | - |
| (4) | 372.3 | 6.4 | 1 | 4 | 4 | 63.6 | 368.2 | 316.2 | 2 | 3 | 3 | 73.2 ± 4.8 at 269 µM |
| (5) | 447.3 | 8.9 | 2 | 4 | 6 | 66.4 | 443.2 | Inactive c | - | - | - | 41.0 ± 0.6 at 224 µM |
| (6) | 413.6 | 8.2 | 2 | 4 | 6 | 66.4 | 421.8 | Inactive c | - | - | - | 61.5 ± 6.1 at 242 µM |
| (7) | 399.6 | 7.7 | 2 | 4 | 5 | 63.6 | 405.0 | Inactive c | - | - | - | Inactive at 251 µM |
| (8) | 372.5 | 7.7 | 2 | 4 | 4 | 66.8 | 367.8 | Inactive c | - | - | - | Inactive at 269 µM |
| (9) | 372.5 | 7.7 | 2 | 4 | 4 | 66.8 | 367.3 | Inactive c | - | - | - | - |
- Not tested.
a) Tice’s modified Rule of 5 for post emergence herbicides [28]
b) Lipinski’s Rule of 5 for pharmaceuticals [29]
c) Inactive at 435μg mL-1.
d) Potency score; 0: No effect as compared to formulation 0.5% DMSO and 0.08% Uptake™ in MeOH, 1: Colour fading, 2: Slight shriveling of leaf, 3: Leaf shriveled over area of application, 4: Leaf dead over area to which sample was applied, 5: Leaf tip dead, 6: Whole leaf dead [30].
Tice [28] used Ghose and Crippen’s [31] alog P to describe the bioavailability of herbicides which was shown to be comparable to Lipinski’s calculated log P values (alog P ≈ clog P). Based on this and the fact that clog P shows good correlation with experimental data [32], the clog P values of compounds 2 - 9 are reported here (Table 2). With the exception of clog P and PSA values, all eight compounds exhibited chemical properties desirable for herbicides (Table 2). There were several trends between the calculated chemical properties and the biological activities of this set of compounds. Tice et al. [28] determined that the average molecular mass for commercial herbicides is 329. The three active compounds, 2 - 4, are within 44 of this. The other compounds, 5 – 9, have a molecular mass above 329 and even though they do comply with the ‘Rule of 5’ by having a molecular mass lower than 500, they were nevertheless found to be inactive. Compounds 2 – 9 violate the clog P rule with values between 5.9 and 8.9. Even so, compounds 2 – 4, which have the lowest clog P values ranging from 5.9 to 6.4, were found to inhibit PPDK activity. Both 2 and 4 have a calculated PSA of 63.6 Å2 which is just outside the desired range, however, the PSA of 3 is significantly higher at 74.6 Å2 and clearly does not fit the criteria, correlating with a decrease in potency against PPDK. A similar trend exists for the number of hydrogen bonds. Of the herbicides currently in use, 93% have been shown to have two or fewer hydrogen-bond donors [28], again the two most active compounds, 2 and 4, have only one hydrogen-bond donor. As the number of rotatable bonds and the clog P increases there was a decrease in the activity observed. A conservative strategy is to stay within the boundaries of what is already known to work, however, some violations can be tolerated, as is the case for the PPDK active compounds 2 – 4.
The standard molecular volume (SMV), the volume which is enclosed by the molecular surface, can be used as a measure of molecular similarity and help in understanding the steric requirements of a receptor. The calculated molecular volumes show that compounds 2 – 4, 8 and 9 are of a similar size and that 5 – 7 are much larger suggesting that the larger compounds are unable to interact with PPDK. The SMV is not the only factor in the ligand-receptor interaction. The ligand molecule must be able to approach the receptor or target enzyme before the binding can take place. Compounds 8 and 9, although similar to 2 – 4 in terms of the SMV, differ in the substitution on the aromatic ring which obviously has a significant effect on their ability to interact with PPDK.
Tice et al. summarized the structural features present in commercial herbicides: aromatic heterocycles, acidic groups such as carboxylic acids and acylsulfonamides [28]. Compounds 2 - 9 do not have any of these characteristics, yet 2 - 4 inhibit PPDK activity and affect the C4 plant.
Anti-inflammatory activity
Phospholipase A2 (PLA2) specifically catalyses hydrolysis of esters of phospholipids to produce lysophospholipids and free fatty acids [18]. The fatty acid is the substrate for the biosynthesis of eicosanoids which are known to mediate inflammation. Based on this mode of action, compounds that inhibit PLA2 activity have been targeted as potential therapeutic agents in the treatment of inflammation.
The inhibition of bee venom PLA2 activity by ilimaquinone (2) was originally mentioned by Faulkner et al. [18]. Avarol (1) and bolinaquinone (10) (Table 1) have since been shown to inhibit human recombinant synovial PLA2, with IC50 values of 158 μM and 0.2 μM, respectively [33,26], however, to the best of our knowledge, no further studies have been reported. Using a colorimetric assay, we found that the hydroxyquinone, ethylsmenoquinone (4) and the aminoquinones, smeno-spongiarine (6) and smenospongidine (5) inhibited bee venom PLA2 with % inhibition of 73.2 ± 4.8 at 269 µM, 61.5 ± 6.1 at 242 µM and 41.0 ± 0.6 at 224 µM, respectively, revealing a higher potency than ilimaquinone (2) with % inhibition of 36.4 ± 8.2 at 279 µM (Table 2), but a lower potency that either 1 or 10. Caution should be taken when comparing human versus non-human PLA2 activity; for example, variabilin was identified as an inhibitor of human synovial secretory PLA2, with an IC50 value of 6.9 µM, but was found to be less potent on bee venom PLA2, with an IC50 value of 26.4 µM [34]. Interestingly, the order of decreasing PLA2 inhibitory potency: (4) ≈ (6) ≈ (5) > (2) > (7, 8) differs from that observed for PPDK.
Aoki et al. recently reported a structure-activity relationship study of ten sesquiterpene quinone/ hydroquinones with respect to their ability to induce differentiation of K562 cells into erythroblasts [35]. They found that the quinone and amine groups are essential for activity (low μM), but that the configuration at C-5 is not important. The profile observed for PLA2 inhibitory activity in our study was similar to that reported by Aoki et al. [35] where the two hydroxyquinones tested were active, two of the three aminoquinones were active and the one hydroquinone tested was inactive. Further studies are in progress in order to evaluate the potential anti-inflammatory activity of these compounds.
With the exception of clog P, all eight compounds exhibited chemical properties desirable for orally bioavailable pharmaceuticals based on Lipinski’s Rule of 5 [29]; molecular mass is less than or equal to 500; if the theoretical logarithm of the n-octanol/water partition coefficient (alog P), is less than or equal to 5.0; if the number of hydrogen bond donors is five or less; and if the number of hydrogen bond acceptors is ten or less (Table 2). Of the four active compounds (2, 4, 5 and 6), 2 was calculated to have a clog P closest to the desired range for pharmaceuticals yet was determined to be the least active against PLA2. Veber et al. [36] reported that a reduced PSA correlates better with increased oral bioavailability than does clog P. They surmise that either a PSA of ≤ 140 Å2 and the number of rotatable bonds ≤ 10, or the sum of H-bond donors and acceptors totals ≤ 12 and the number of rotatable bonds ≤ 10 will have a high probability of good oral bioavailability. Even applying these criteria to the activity profile of compounds 2 - 9 cannot explain why 4 – 6 are more potent that 2.
Conclusions
This study reports for the first time the herbicidal and anti-inflammatory activity of several sesquiterpenoid quinones related to ilimaquinone, 1. The different activity profiles observed for the eight sesquiterpene quinones/hydroquinones suggests that the structural elements responsible for PPDK inhibition are different to those responsible for PLA2 inhibition.
There has been limited development in novel modes of action for herbicides and very few new structural motifs are being used as herbicides [37]. Comparisons of the structures of ilimaquinone, 1, smenoquinone and ethylsmenoquinone (2 – 4) with commercial herbicides with known modes of action [28] show there is little similarity, suggesting that the hydroxyquinones’ phytotoxic response in whole C4 plants maybe in part due to their mode of action via inhibition of PPDK and are good candidates for further investigation.
PPDK inhibitory activity appears to rely on the presence of a hydroxyquinone functionality and a relatively short oxygenated side chain at C-20, and that an amine group at C-20 is not tolerated. Quinones are generally thought to act via redox reactions or Michael addition/elimination reactions [38,39] and it is the redox properties of the hydroquinone-quinone functionality that are believed to be responsible for the diverse activity profiles of the sesquiterpene quinone class of compound [40]. The results of this study are no exception. The hydroxyquinones, 2 – 4, contain hydroxide or alkoxide substituents at C-20 which are more labile and therefore are better leaving groups than the amines in the aminoquinones, 5 – 7, and would therefore be more likely to facilitate a Michael addition/ elimination reaction resulting in bioactivity. Neither of the hydroquinones, 8 or 9, were found to inhibit PPDK activity. One possible explanation is that the catechol moiety is not oxidising to the corresponding o-quinone, which would be a more reactive Michael acceptor, under the assay conditions. There is potential for synthetic modification of the drimane rearranged decalin ring and of the quinone scaffold, especially at position C-20, to improve potency and/or absorption properties therefore improving activity on the C4 plant.
The converse relationship was found to be true for the anti-inflammatory activity. PLA2 inhibitory activity of 2, 4, 5 and 6 appears to rely on the presence of a hydroxyquinone functionality with a larger side chain at C-20 and that an amine group is well tolerated. The greater resonance donor ability of the nitrogen substituents at C-20 is likely to result in a higher electron density at the C-18 oxygen making it susceptible to nucleophilic attack from a H2O molecule proximal to the HIS-48 imidazole of the catalytically active PLA2 [18]. Clearly a modification at C-20 plays a significant role in the type of activity exhibited by this class of compound. As for PPDK, improvement in PLA2 inhibitory activity might be possible through synthetic modification of the drimane rearranged decalin ring and of the quinone scaffold, especially the size of the C-20 nitrogen substituent. It may also be that the larger the substituent on C-20 (5 – 7), the greater the steric effect, which in turn impedes the interaction of the quinone with PPDK but facilitates its interaction with PLA2.
The C-9 position at which the quinone group is attached to the decalin ring in ilimaquinone, 2, may also prove to be a useful site for synthetic modification given that bolinaquinone, 10, in which the quinone attaches at C-8, has a reported potency against PLA2 greater than that found for either 2, 4, 5 or 6.
Experimental
General
All buffers and general chemicals were sourced from Sigma (St. Louis, MO, USA). NAD-MDH (EC 1.1.1.37) was purchased from Roche Diagnostics GmbH (Mannheim, Germany). Microtitre plates used for screening were flat-based polystyrene plates from Sarstedt (South Australia, Australia). Purified water was obtained from a MilliQ water purification system (Millipore, Billerica, MA, USA). PEPC (EC 4.1.1.31) was isolated and purified from maize leaves [41] and maize PPDK (EC 2.7.9.1; Genbank Accession number J03901) was expressed in E. coli with a His-tag and purified on a Ni-affinity column [20].
Naturally Occurring Compounds
Ilimaquinone (2) [3,4], smenospondiol (8) [24] and 18-hydroxy-5-epi-hyrtiophenol (9) [25] were isolated from the sponge Rhopaloeides sp., collected from Shelburne Bay off the north-east coast of Australia, as described previously [20]. Ilimaquinone (2) [3,4], smenoquinone (3) [22], ethylsmenoquinone (formerly smenorthoquinone) (4) [22,23], smenospongidine (5) [22], smenospongiarine (6) [22], smenospongorine (7) [22] and smenospondiol (8) [24] were isolated from the sponge Smenospongia sp., collected from the Red Sea, as described previously (Table 1).
PPDK, PEPC, NAD-MDH enzyme-coupled assay
PPDK was assayed using a coupled enzyme technique as described in a previous study [20]. Briefly, the reaction buffer consisted of PPDK (0.012 UmL-1), PEPC (0.4 UmL-1), NAD-MDH (6 UmL-1), 10 mM dithiothreitol, 10 mM MgSO4, 10 mM NaHCO3, 1 mM glucose-6-phosphate, 5 mM (NH4)2SO4, 2.5 mM NaH2PO4, 0.4 mM NADH and 1 mM ATP maintained at pH 8.0 using 50 mM HEPES-OH in a final volume of 100 μL. Compounds were dissolved in dimethyl sulfoxide (DMSO) to a stock concentration of 10 mgmL-1 of which 5 μL was used for initial testing, in duplicate. Reactions were initiated by the addition of 10 μL 24mM pyruvate (final concentration of 2 mM), giving a final concentration of 435 μgmL-1 of compound, and conducted at 22 - 25°C. Active compounds were then diluted for dose response titrations. The concentration causing 50% inhibition (IC50) is reported with 95% confidence intervals in parenthesis as determined using the sigmoid dose response curve fit
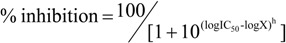 |
where X is the concentration of the test sample, h is the Hill slope and curve fitting is constrained between 0 and 100% inhibition (Graphpad Prism, San Diego, CA, USA).
Determination of C4 plant phytotoxicity
Whole plant phytotoxicity was performed as described in Haines et al. [30] with minor modifications. Compounds determined active in the PPDK assay were prepared in methanol (MeOH) containing 0.5% DMSO and 0.08% Uptake™ to give a final concentration of 10 mgmL-1 and applied to the top half of individual Digitaria ciliaris leaves on quadruplicate plants at a rate of 5 μL (50 μg compound) per leaf. Control plants were treated with formulation only. Plants were observed at 24, 48 and 72 h post application and the phytotoxic effect rated using the following potency scale previously reported: 0 = no effect as compared to formulation 0.5% DMSO and 0.08% Uptake™ in MeOH, 1 = colour fading, 2 = slight shrivelling of leaf, 3 = leaf shrivelled over area of application, 4 = leaf dead over area to which sample was applied, 5 = leaf tip dead, 6 = whole leaf dead.
Determination of PLA2 inhibition
Evaluation of the PLA2 inhibitory activity was based on a colorimetric assay using red phenol as a pH indicator according to the method of Lobo de Araujo and Radvanyi [42]. Briefly, the assay used bee venom (Apis mellifera from Sigma) as the source of enzyme and lecithin (L-α-phosphatidyl-choline from Sigma) as the substrate. Compounds (20 µg) were dissolved in 2 µL DMSO and incubated in 96-well plates for 1 h at room temperature with 0.02 µgµL-1 of bee venom PLA2. The reaction was started by addition of 200 µL of the reaction medium containing 3.5 mM lecithin, 7 mM Triton X-100, 100 mM NaCl, 10 mM CaCl2 and 0.055 mM red phenol adjusted to an optical density of 1.8 at 550 nm. Absorbances were read at 550 nm on a microplate reader (CERES 900 spectrophotometer). The enzymatic activity, as observed by the liberation of fatty acid, was calculated as the difference in absorbance between the control well and those wells containing the samples at time 0 and 5 min with 30 sec of shaking at room temperature. Values reported are means of three determinations and are given ± SE.
Theoretical chemical property calculations
The chemical structure of each compound was analysed using chemometric approaches. The theoretical logarithm of the n-octanol/water partition coefficient (clog P) was calculated using the online ClogP program provided by Daylight Chemical Information Systems which implements the method of Hansch and Leo [43]. The number of hydrogen bond donors and hydrogen bond acceptors were calculated as described by Lipinski et al. [29]. The number of rotatable bonds was calculated using the interactive software provided by Molinspiration Property Calculation Service (www.molinspiration.com). This same software was also used to calculate the molecule’s polar surface area (PSA), based on the method of Ertl et al. [44], and the standard molecular volume (SMV).
Acknowledgments
The authors are grateful to Professor James Burnell, James Cook University for providing the enzyme PPDK. Nufarm Pty Ltd provided the formulation Uptake™. Taxonomy of the Australian sponge was provided by the Queensland Museum, Brisbane, Queensland, Australia (voucher specimen QMG305403 located at the Queensland Museum). Collection of this sponge was conducted under permit number 88/345 from the Great Barrier Reef Marine Park Authority (Townsville, Australia). We also thank Jean Vacelet (Station Marine d’Endoume, France) for collection and identification of the Red Sea marine sponge Smenospongia sp.
Footnotes
Sample Availability: contact the authors.
References
- 1.Minale L., Riccio R., Sodano G. Avarol, a novel sesquiterpenoid hydroquinone with a rearranged drimane skeleton from the sponge Dysidea avara. Tetrahedron Lett. 1974;38:3401–3404. doi: 10.1016/S0040-4039(01)91918-5. [DOI] [Google Scholar]
- 2.de Rosa S., Minale L., Riccio R., Sodano G. The absolute configuration of avarol, a rearranged sesquiterpenoid hydroquinone from a marine sponge. J. Chem. Soc., Perkin Trans. I. 1976:1408–1414. [Google Scholar]
- 3.Luibrand R. T., Erdman T. R., Vollmer J. J., Scheuer P. J., Finer J., Clardy J. Ilimaquinone, a sesquiterpenoid quinone from a marine sponge. Tetrahedron. 1979;35:609–612. doi: 10.1016/0040-4020(79)87004-0. [DOI] [Google Scholar]
- 4.Capon R. J., MacLeod J. K. A revision of the absolute stereochemistry of ilimaquinone. J. Org. Chem. 1987;52:5059–5060. doi: 10.1021/jo00231a051. [DOI] [Google Scholar]
- 5.Loya S., Tal R., Kashman Y., Hizi A. Ilimaquinone, a selective inhibitor of the RNase H activity of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 1990;34:2009–2012. doi: 10.1128/aac.34.10.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourguet-Kondracki M.-L., Longeon A., Morel E., Guyot M. Sesquiterpene quinones as immunomodulating agents. Int. J. Immunopharm. 1991;13:393–399. doi: 10.1016/0192-0561(91)90009-V. [DOI] [PubMed] [Google Scholar]
- 7.Graillet C., Pesando D., Girard J. P. Cellular mechanisms of the cytotoxicity of natural substances derived from the sea, studied during fertilization and egg cleavage in the sea-urchin. Oceanus. 1991;17:229–230. [Google Scholar]
- 8.El Sayed K. A., Dunbar D. C., Goins D. K., Cordova C. R., Perry T. L., Wesson K. J., Sanders S. C., Janus S. A., Hamann M. T. The marine environment: a resource for prototype antimalarial agents. J. Nat. Toxins. 1996;5:261–285. [Google Scholar]
- 9.Popov A. M., Stekhova S. I., Utkina N. K., Rebachuk N. M. Antimicrobial and cytotoxic activity of sesquiterpenequinones and brominated diphenyl esters isolated from marine sponges. Pharm. Chem. J. 1999;33:71–73. doi: 10.1007/BF02508109. [DOI] [Google Scholar]
- 10.Radeke H. S., Digits C. A., Bruner S. D., Snapper M. L. New tools for studying vesicular-mediated protein trafficking: synthesis and evaluation of ilimaquinone analogs in a non-radioisotope-based antisecretory assay. J. Org. Chem. 1997;62:2823–2831. doi: 10.1021/jo962292l. [DOI] [PubMed] [Google Scholar]
- 11.Radeke H. S., Digits C. A., Casaubon R. L., Snapper M. L. Interactions of (-)-ilimaquinone with methylation enzymes: implications for vesicular-mediated secretion. Chem. Biol. 1999;6:639–647. doi: 10.1016/s1074-5521(99)80115-x. [DOI] [PubMed] [Google Scholar]
- 12.El Sayed K., Dunbar D. C., Bartyzel P., Zjawiony J. K., Day W., Hamann M. T. Marine natural products as leads to develop new drugs and insecticides in biologically active natural products. CRC Press; Boca Raton: 1999. pp. 233–252. [Google Scholar]
- 13.De Clercq E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med. Res. Rev. 2000;20:323–349. doi: 10.1002/1098-1128(200009)20:5<323::AID-MED1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Takizawa P. A., Yucel J. K., Veit B., Faulkner D. J., Deerinck T., Soto G., Ellisman M., Malhotra V. Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell. 1993;73:1079–1090. doi: 10.1016/0092-8674(93)90638-7. [DOI] [PubMed] [Google Scholar]
- 15.Tziveleka L. A., Vagias C., Roussis V. Natural products with anti-HIV activity from marine organisms. Curr. Top. Med. Chem. 2003;31:512–535. doi: 10.2174/1568026033451790. [DOI] [PubMed] [Google Scholar]
- 16.Veit B., Yucel J. K., Malhotra V. Microtubule independent vesiculation of Golgi membranes and the reassembly of vesicles into Golgi stacks. J. Cell. Biol. 1993;122:1197–1206. doi: 10.1083/jcb.122.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu P. H., Chueh S. C., Kung F. L., Pan S. L., Shen Y. C., Guh J. H. Ilimaquinone, a marine sponge metabolite, displays anticancer activity via GADD153-mediated pathway. Eur. J. Pharmacol. 2007;556:45–54. doi: 10.1016/j.ejphar.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 18.Potts B. C. M., Faulkner D. J. Phospholipase A2 inhibitors from marine organisms. J. Nat. Prod. 1992;55:1701–1717. doi: 10.1021/np50090a001. [DOI] [PubMed] [Google Scholar]
- 19.Ling T., Poupon E., Rueden E. J., Kim S. H., Theodorakis E. A. Unified synthesis of quinone sesquiterpenes based on a radical decarboxylation and quinone addition reaction. J. Am. Chem. Soc. 2002;124:12261–12267. doi: 10.1021/ja027517q. [DOI] [PubMed] [Google Scholar]
- 20.Doyle J. R., Burnell J. N., Haines D. S., Llewellyn L. E., Motti C. A., Tapiolas D. M. A rapid screening method to detect specific inhibitors of pyruvate, orthophosphate dikinase as leads for C4 plant-selective herbicides. J. Biomolec. Screen. 2005;10:67–75. doi: 10.1177/1087057104269978. [DOI] [PubMed] [Google Scholar]
- 21.Edwards G. E., Nakamoto H., Burnell J. N., Hatch M. D. Pyruvate,Pi dikinase and NADP-malate dehydrogenase in C4 photosynthesis: Properties and mechanism of light/dark regulation. Ann. Rev. Plant Physiol. 1985;36:255–286. doi: 10.1146/annurev.pp.36.060185.001351. [DOI] [Google Scholar]
- 22.Kondracki M.-L., Guyot M. Biologically active quinone and hydroquinone sesquiterpenoids from the sponge Smenospongia sp. Tetrahedron. 1989;45:1995–2004. doi: 10.1016/S0040-4020(01)80062-4. [DOI] [Google Scholar]
- 23.Urban S., Capon R. J. 5-epi-Isospongiaquinone, a new sesquiterpene/quinone antibiotic from an Australian marine sponge Spongia hispida. J. Nat. Prod. 1992;55:1638–1642. doi: 10.1021/np50089a012. [DOI] [PubMed] [Google Scholar]
- 24.Kondracki M.-L., Davoust D., Guyot M. Smenospondiol, a biologically active hydroquinone from the sponge Smenospongia sp. J. Chem. Res. Synop. 1989;3:74–75. [Google Scholar]
- 25.Salmoun M., Devijver C., Daloze D., Braekman J. C., Gomez R., de Kluijver M., Van Soest R. W. M. New sesquiterpene/quinones from two sponges of the genus Hyrtios. J. Nat. Prod. 2000;63:452–456. doi: 10.1021/np9903346. [DOI] [PubMed] [Google Scholar]
- 26.Lucas R., Giannini C., D’auria M. V., Pay´a M. Modulatory effect of bolinaquinone, a marine sesquiterpenoid, on acute and chronic inflammatory processes. J. Pharmacol. Exp. Ther. 2003;304:1172–1180. doi: 10.1124/jpet.102.045278. [DOI] [PubMed] [Google Scholar]
- 27.Kirkwood R. C. Recent developments in our understanding of the plant cuticle as a barrier to the foliar uptake of pesticides. Pestic. Sci. 1999;55:69–77. doi: 10.1002/(SICI)1096-9063(199901)55:1<69::AID-PS860>3.0.CO;2-H. [DOI] [Google Scholar]
- 28.Tice C. M. Selecting the right compounds for screening: does Lipinski's Rule of 5 for pharmaceuticals apply to agrochemicals. Pest Manag. Sci. 2001;57:3–16. doi: 10.1002/1526-4998(200101)57:1<3::AID-PS269>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997;23:3–25. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- 30.Haines D. S., Burnell J. N., Doyle J. R., Llewellyn L. E., Motti C. A., Tapiolas D. M. Translation of in vitro inhibition by marine natural products of the C4 acid cycle enzyme pyruvate P(i) dikinase to in vivo C4 plant tissue death. J. Agric. Food Chem. 2005;53:3856–3862. doi: 10.1021/jf048010x. [DOI] [PubMed] [Google Scholar]
- 31.Ghose A. K., Crippen G. M. Atomic physicochemical parameters for three dimensional structure-directed quantitative structure-activity relationships. I. Partition coefficients as a measure of hydrophobicity. J. Comput. Chem. 1986;7:565–577. doi: 10.1002/jcc.540070419. [DOI] [PubMed] [Google Scholar]
- 32.Medić-Šarić M., Mornar A., Jasprica I. Lipophilicity study of salicylamide. Acta Pharm. 2004;54:91–101. [PubMed] [Google Scholar]
- 33.Ferrándiz M. L., Sanz M. J., Bustos G., Payá M., Alcaraz M. J., De Rosa S. Avarol and avarone, two new anti-inflammatory agents of marine origin. Eur. J. Pharmacol. 1994;253:75–82. doi: 10.1016/0014-2999(94)90759-5. [DOI] [PubMed] [Google Scholar]
- 34.Escrig V., Ubeda A., Ferrandiz M. L., Darias J., Sanchez J. M., Alcaraz M. J., Paya M. Variabilin: A dual inhibitor of human secretory and cytosolic phospholipase A2 with anti-inflammatory activity. J. Pharmacol. Exp. Ther. 1997;282:123–131. [PubMed] [Google Scholar]
- 35.Aoki S., Kong D., Matsui K., Rachmat R., Kobayashi M. Sesquiterpene aminoquinones, from a marine sponge, induce erythroid differentiation in human chronic myelogenous leukaemia, K562 cells. Chem. Pharm. Bull. 2004;52:935–937. doi: 10.1248/cpb.52.935. [DOI] [PubMed] [Google Scholar]
- 36.Veber D. F, Johnson S. R, Cheng H. -Y., Smith B. R., Ward K. W., Kopple K. D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 37.Peng J., Shen X., El Sayed K., Dunbar D., Perry T., Wilkins S., Hamann M. Marine natural products as prototype agrochemical agents. J. Agric. Food Chem. 2003;51:2246–2252. doi: 10.1021/jf0207880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sladic D., Gasic M. J. Reactivity and biological activity of the marine sesquiterpene hydroquinone avarol and related compounds from sponges of the order Dictyoceratida. Molecules. 2006;11:1–33. doi: 10.3390/11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolton J. L., Trush M. A., Penning T. M., Dryhurst G., Monks T. J. Role of Quinones in Toxicology. Chem. Res. Tox. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 40.Molinari A., Oliva A., Aguilera N., Miguel del Corral J. M., Castro M. A., Gordaliza M., Garcia-Cravalos M. D., San Feliciano A. New antineoplastic prenylhydroquinones. Synthesis and evaluation. Bioorg. Med. Chem. 2000;8:1027–1032. doi: 10.1016/S0968-0896(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 41.Ashton R., Burnell J. N., Furbank R. T., Jenkins C. L. D., Hatch M. D. Enzymes of C4 photosynthesis. Meth. Plant Biochem. 1990;3:39–71. doi: 10.1016/B978-0-12-461013-2.50010-1. [DOI] [Google Scholar]
- 42.Lobo de Araujo A., Radvanyi F. Determination of phospholipase A2 activity by a colorimetric assay using a pH indicator. Toxicon. 1987;25:1181–1188. doi: 10.1016/0041-0101(87)90136-X. [DOI] [PubMed] [Google Scholar]
- 43.Leo A. J. Calculating log POCT from structures. Chem. Rev. 1993;93:1281–1304. doi: 10.1021/cr00020a001. [DOI] [Google Scholar]
- 44.Ertl P., Rohde B., Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000;43:3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]