ABSTRACT
Plants, being sessile in nature, are constantly exposed to various environmental stresses, such as solar UV radiations, soil salinity, drought and desiccation, rehydration, low and high temperatures and other vast array of air and soil borne chemicals, industrial waste products, metals and metalloids. These agents, either directly or indirectly via the induction of oxidative stress and overproduction of reactive oxygen species (ROS), frequently perturb the chemical or physical structures of DNA and induce both cytotoxic or genotoxic stresses. Such condition, in turn, leads to genome instability and thus eventually severely affecting plant health and crop yield. With the growing industrialization process and non-judicious use of chemical fertilizers, the heavy metal mediated chemical toxicity has become one of the major environmental threats for the plants around the globe. The heavy metal ions cause damage to the structural, enzymatic and non-enzymatic components of plant cell, often resulting in loss of cell viability, thus negatively impacting plant growth and development. Plants have also evolved with an extensive and highly efficient mechanism to respond and adapt under such heavy metal toxicity mediated stress conditions. In addition to morpho-anatomical, hormonal and biochemical responses, at the molecular level, plants respond to heavy metal stress induced oxidative and genotoxic damage via the rapid change in the expression of the responsive genes at the transcriptional level. Various families of transcription factors play crucial role in triggering such responses. Apart from transcriptional response, epigenetic modifications have also been found to be essential for maintenance of plant genome stability under genotoxic stress. This review represents a comprehensive survey of recent advances in our understanding of plant responses to heavy metal mediated toxicity in general with particular emphasis on the transcriptional and epigenetic responses and highlights the importance of understanding the potential targets in the associated pathways for improved stress tolerance in crops.
keywords: abiotic stress, environmental toxicity, heavy metals, oxidative and genotoxic stress, genome stability, transcripto-epigenetic regulations, arabidopsis thaliana, crop productivity
Abbreviations
CDK cyclin-dependent protein kinase
PS photosystem
RUBP ribulose 1,5 bis-phosphate
MAPK mitogen-activated protein kinase
HMA heavy metal associated
PCs Phytochelatins
MTs metallothioneins
ROS reactive oxygen species,
Introduction
Environmental chemical toxicity and genome instability in plants under heavy metal stress
Plants, being sessile in nature, are constantly exposed to myriads of environmental stresses which include both abiotic and biotic factors. UV light, infrared, cosmic and other extra-terrestrial radiations of variable wavelengths, high salinity, drought, flooding, chilling injury, nutrient imbalances, air and soil borne pollutants, exposure to bacterial and fungal pathogens and metabolic byproducts of endogenous processes represent some of the frequent stress factors for plants. In addition, high intensity solar radiation, continuous exposure to artificial light, high decible sound, emissions from the vehicles in busy roads and highways also negatively affect plant growth. These abiotic and biotic factors frequently induce oxidative and genotoxic stress and eventually result in the formation of various forms of lesions in DNA double helix. Unrepaied damages in the DNA stand in turn leads to genome instability and therefore, affecting plant health and productivity. Various abiotic stresses have both general and specific effects on plant growth and development. For example, photosynthetic declination, osmotic stress etc. compromises plant processes, interfere with nutrient availability and thus limit plant growth as frequently found in case of drought stress.1 Apart from the direct effect on plant growth, low and freezing temperatures have also been shown to induce osmotic stress.2 Plant exposure to drought, salinity and low temperature frequently generates osmotic, oxidative and finally genotoxic stress.
During the past couple of years, increased anthropogenic activities, rapid industrialization and modern agricultural practices have contributed to increased contamination of heavy metal elements in the environment and represent one of the major stresses experienced by the plants particularly in the developing countries. The heavy metals play essential role in each stage of the life cycle in plant. However, unbalanced doses may induce both cytotoxic and genotoxic effects and thus genome instability in plants. Soil, contaminated with heavy metals like cadmium and lead, generates one of the important stress conditions for the plants. Several studies have demonstrated heavy metal stress mediated plant growth inhibition, particularly pronounced during seed germination and at the early stages of seedling growth.3, 4 The heavy metal ions compete with the essential nutrient cations for binding and absorption in the root surface. After entering into plant cell, the heavy metals exert their cytotoxic and genotoxic effects because of the disruption of protein structure and function as the heavy metals directly attack the thiol groups of proteins and thus causing the conformation change in protein structure. In addition, the heavy metal induced ROS production causes oxidative damage of the cellular macromolecules and photosynthetic apparatus. These effects are eventually reflected at the physiological and biochemical levels with decreased membrane stability and photosynthetic yield, compromised pigment production, hormonal and nutrient imbalance, inhibition of DNA replication, gene expression and cell division.5 Depending on the concentration, metal type and developmental stages, heavy metal uptake generates variety of stress responses in plant cell. In response to this, plants have developed sophisticated modulating mechanisms to adapt and survive under heavy metal stress mediated chemical toxicity. However, under extreme conditions, heavy metal toxicity may severely affect plant health, eventually resulting in cell death.6-9 A coordinated and complex interlinked network facilitate plant cell with multiple metal-detoxifying mechanisms and repair of metal toxicity mediated damage in the genome to survive under heavy metal contaminated environments.10-12
The 53 d-block elements have been categorized and recognized as ‘heavy metals’ on the basis of their density (>5 g/cm3). Plant cells require only 19 elements, including C, O, H, Mg, S, N, Cd, P, K (macroelements) and Cu, Zn, Mn, Fe, Mo, B, Ni, Co, Cl, and Br (microelements) for their fundamental metabolic functioning. These macro and microelemts are essential for seveal physiological and biochemical processes in plants, such as chlorophyll biosynthesis, photosynthesis, nucleic acid metabolism, protein modification, intracompartmental redox reactions, carbohydrate metabolism and nitrogen fixation. Interestingly, although some heavy metal elements are used as microelements, other heavy metal elements, such as Al, Cd, Cr, Pb, Hg generate toxic effects for plants, such as low biomass production, chlorosis, reduced photosynthesis, altered water balance and nutrient assimilation. These factors eventually cause plant growth inhibition, senescence and finally yield loss.13 The roots are the first organ which first encounters the heavy metals and multiple studies have revealed root growth inhibition in plants following exposure to heavy metals. These effects are produced due to inhibition of mitotic activity in root meristem14 as reported with Cr (VI), which delays the progression through cell cycle, thus inhibits cell cycle.15 Similarly, Cd2+ ions suppress the expression of S-phase specific cyclin-dependent protein kinases (CDK), thereby delaying the progression through the S-phase of the cell cycle. Furthermore, the heavy metal mediated disruption of auxin transport in roots appears to be another important cause of root growth inhibition.16
The physico-chemical nature of plasma membranes has been shown to play important role in regulating the entry of heavy metal ions. Heavy metal tolerant properties have been shown to be associated with lower level of membrane depolarization with the rapid membrane voltage change as found in Arabidopsis halleri and A. arenosa.17 In view of this, heavy metal stress mediated reduction in photosynthetic and respiration rates may be linked to the changes in the ultrastructure of membrane in chloroplast and mitochondria. Previous studies have revealed decreased activity of PS (II) and quantum yield along with compromised chlorophyll content and intracellular CO2 in various plant species under heavy metal stress.18, 19 The heavy metal ions interfere with light reaction and cause significant decline in CO2 assimilation via either inhibition of RUBP carboxylase activity or by affecting the structural stability of RUBP carboxylase through interaction with the thiol groups. For example, Cu, which inhibits both carboxylase and oxygenase activities of RUBISCO, causes decreased RUBP carboxylase activity via interaction with the cysteine residues essential for the enzyme activity, finally leading to decreased quantum yield of PS (II), photosynthetic rate and CO2 assimilation.20 Apart from the photosynthetic machinery, heavy metals also affect the nitrogen metabolism process in plants. In general, heavy metals inhibit the activity of key enzymes involved in nitrate and ammonia metabolism and assimilation, such as nitrate reductase, nitrite reductase, glutamine synthetase, glutamine oxoglutarate aminotransferase and glutamine dehydrogenase, respectively.21 Plants under Cd stress frequently suffer from primary nitrogen assimilation process because of inhibition in nitrogen uptake and transport along with decreased activities of nitrate reductase and glutamine synthetase.
Plant cells respond to heavy metal mediated toxicity through complicated interlinked mechanisms which are functional at various levels and include both short-term and long-term processes. The short-term or immediate responses include the rapid changes in the transcriptional rates of hundreds or even thousands of responsive genes with the concomitant changes at the physiological and metabolic levels. The long-term responses, on the other hand, are associated with the genetic modifications and epigenetic changes.22 Regulation of gene expression, which functions as an integral part of plant stress response, generally involves both universal and unique changes of transcript levels of the stress responsive genes.23 Therefore, it seems logical to expect that depending on the situation, plant respond to heavy metal toxicity, which induces both oxidative and genotoxic effects, through the coordination and integration of various components of stress perception and signaling networks with possible cross talks at various steps.
Sources of heavy metals and plant response to heavy metal stress
The heavy metal elements in general are not rapidly metabolized and found to gradually accumulate in biological system. Such heavy metal components eventually mount up in ecological food chain through uptake via the primary producers, the plants and then at the consumer level through consumption. In plants, with their intrinsic sessile nature, the roots are primarily the contact and binding sites for the metal ions. In aquatic systems, almost whole plant body gets exposed to polluted water. In addition, metal ions may also be directly absorbed by the leaves due to particle deposition on leaf surfaces.24 Variety of sources may contribute to the accumulation of heavy metal elements in the environment. Some of the major sources may include natural, agricultural, industrial, domestic effluents and atmospheric sources. In nature, the heavy metals originate within the earth's crust and become available in the soil during the course of weathering process. The geologic plant materials have been shown to contain an elevated concentrations of Cr, Mn, Co, Ni, Cu, Zn, Cd, Sn, Hg and Pb, respectively.25, 26 The igneous rocks, including the olivine, augite and hornblende generally contribute considerable amounts of Mn, Co, Ni, Cu, and Zn to soil. Among the sedimentary rocks, shale crust has been shown to possess considerably higher concentrations of Cr, Mn, Co, Ni, Cu, Zn, Cd, Sn, Hg, and Pb. In addition, sandstone and limestone have also been considered as important source of diverse heavy metal elements. Along with toxic and other harmful gases, volcanic eruptions and forest fires also emit high levels of heavy metal elements, which eventually accumulate in the soil. The major sources of heavy metal elements in the agricultural soil mainly include the inorganic and organic fertilizers, sewage sludge, contaminated irrigation water, pesticides and fungicides. In the industrial and adjoining regions, heavy metals enter into the environment in particulate and vapour forms due to processing of metals at high temperature, such as smelting and casting. The vapour forms of heavy metals, including As, Cd, Cu, Pb, Sn and Zn combine with water in the atmosphere, forming aerosols and subsequently dispersed by wind (dry deposition) or precipitated with rainfall (wet deposition), further contaminating the soil and water bodies. Mining, erosion of mine wastes, corrosion of metals and leaching of heavy metals may also contaminate soil and groundwater system. Moreover, other industrial sources, such as processing of plastics, textiles, microelectronics, wood preservation and paper processing may contribute heavy metal elements to soil. The domestic effluents are also regularly contributing an elevated level of heavy metal elements and other chemical components in the rivers and lakes27 (Fig.1).
Figure 1.
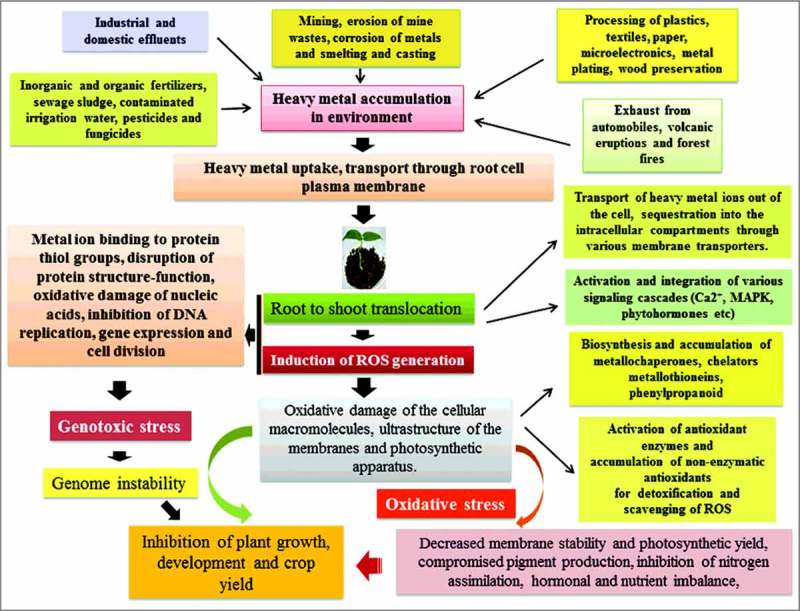
Diagrammatic representation showing the various sources of heavy metals and plant responses to heavy metal stress mediated toxicity. The initial stress response includes inhibition of seed germination and seedling growth. As part of initial defence plants utilize various morphological barriers and detoxification of heavy metals via metal ion binding and chelations. At the physiological and biochemical levels, the stress response is subsequetly reflected with decreased photosynthetic and respiration rates because of the changes in the ultrastructure of membrane in chloroplast and mitochondria, compromised pigment production, hormonal and nutrient imbalance, inhibition of DNA replication, gene expression and cell division, finally resulting in plant growth inhibition.
Abiotic stresses are the prominent causes, reducing an average of more than 50% loss of crop yield worldwide for most crops.27-31 Being sessile in nature, plants cannot escape from the exposure to heavy metals elements, which stimulate wide range of physiological and metabolic responses. After entering into the plant cell through specific transporters, the heavy metal elements generally bind to the functional sites of the fundamental biomolecules, including structural and functional proteins and membrane lipids, leading to change in their structural conformation. This, in turn, disrupts cellular structural integrity, interferes with the production of essential metabolites, creating osmotic imbalance and finally normal functioning of the cell.32 The heavy metal elements also cause damage to the nucleic acids either via direct binding and cleavage or indirectly through oxidative stress and generation of reactive oxygen species (ROS).33-35 Some of the common effects of heavy metal toxicity in plants include decreased rate of seed germination, reduced seedling growth, leaf chlorosis, insufficient photosynthesis, loss of turgour, necrosis and early senescence.36
Heavy metal stress activates various signaling cascades in plants
Similar to other stress responses, plants respond to heavy metal toxicity via the typical signaling pathways, which include – sensing of external stress signal, followed by transmission of signal to the downstream components and activation of appropriate measures to neutralize the harmful effects of heavy metal mediated stress. This leads to regulation of cellular function at the physiological, biochemical, and molecular levels. As part of early stress response, monitoring the induction of oxidative stress, transcriptional and proteomic pattern and accumulation of stress responsive secondary metabolites like flavonoids provide meaningful information on heavy metal toxicity associated stress response in plants.13, 37, 38 Plant response to heavy metal stress involves a complicated signal transduction network that is activated immediately after sensing the existence of heavy metal element in the extracellular environment, followed by stimulation of several signaling networks, such as the Ca-calmodulin pathway, phytohormonal response, ROS mediated signaling, and also the mitogen-activated protein kinase (MAPK) mediated phosphorylation cascade (Fig.2).
Figure 2.
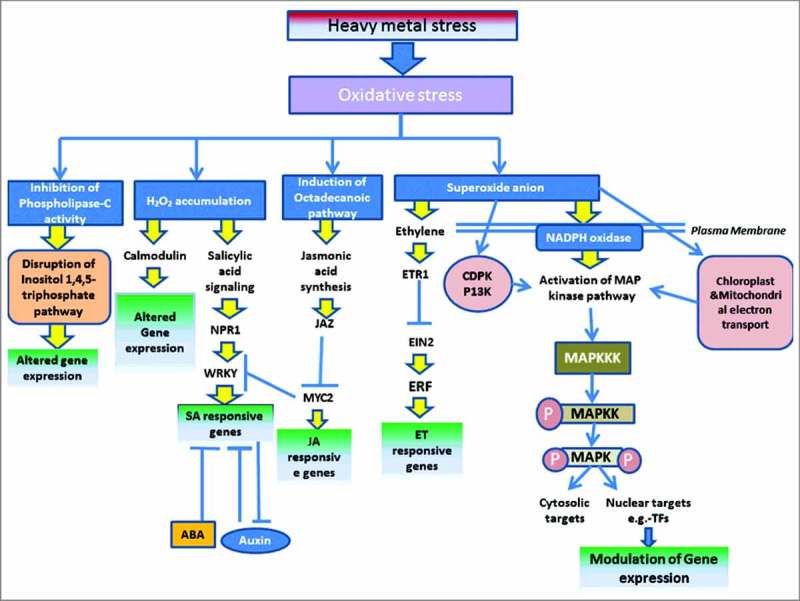
Induction of various signaling cascades and their interactions under heavy metal stress. Schematic diagram showing the heavy metal stress and subsequent oxidative damage mediated activation of different interlinked signaling networks, including the calmodulin pathway, hormonal and MAP kinase signaling cascades for regulation of expression the target stress responsive genes associated with plant response for heavy metal stress adaptation. EIN2 – ethylene-insensitive protein 2, ETR1 – ethylene receptor 1, ERF – ethylene response factor, SA – salicylic acid, JA – jasmonic acid, JAZ – JASMONATE-ZIM DOMAIN, NPR1 – natriuretic peptide receptor 1. Blunt end arrows indicate inhibition.
Ca2+ signaling is intrinsically associated with several abiotic stress conditions, such as high salinity, high and low temperature, osmotic and oxidative stress, anoxia and mechanical changes.39-41 Excess heavy metals have been shown to modify the stability of Ca2+-channels, thus increasing calcium flux into the cell. The intracellular free calcium acts as common second messenger in the signaling of heavy metal mediated abiotic stress responses, thus regulating the expressions downstream genes involved in heavy metal transport, metabolism, and tolerance. Previous studies have extensively investigated the involvement of Ca2+ in yeast cells (Saccharomyces cerevisiae) following exposure to elevated levels of various heavy metal elements, such as Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, and Hg2+, respectively. Interestingly, yeast cells showed differential response to various heavy metal elements tested. A prominant rise in the cytosolic Ca2+ was observed in response to exposure to Cd2+, while the response was less for Cu2+ and almost undetectable for Mn2+, Co2+, Ni2+, Zn2+, and Hg2+. The rise in the cytosolic Ca2+ in response to Cd2+ has been shown to be due to transportation of external Ca2+ through the Cch1p/Mid1p channel.42 Based on the dose and the ability for the generation of hydroxyl radicals, the heavy metals regulate calcium in roots.
The impact of heavy metals, particularly Cd on the activation of MAP kinase cascades, generation of reactive oxygen species (ROS) and nitric oxide (NO) in diverse plant species have been extensively reviewed in previous work.43 Cadmium has received special importance because of its size similarity with Ca and therefore, appears to interfere with Ca2+-mediated processes. Cd has been shown to affect plant cell in various ways, such as depolarization of root epidermal plasma membrane, thus impairing Ca2+ influx and returding root growth.44 In Arabidopsis, exposure to excess level of Cd inhibits root hair growth, disrupting Ca2+ influx and eventually the terminal cytosolic Ca2+ gradient essential for growth.45 However, the effects of extracellular trasport of transition heavy metal elemts, such as Cu and Pb on plasma membrane bound Ca2+ channels and the cytosolic free Ca2+ mediated signaling cascades are rather elusive. Electrophysiological studies involving Arabidopsis root epidermal plasma membrane have demonstrated role of Cu in the activation of Ca2+ channels via oxidative stress mediated ROS production.46
Variations in the level of phytohormones and the hormonal balance are also crucial for initiating the signaling cascades in response to heavy metal stress and may include the crosstalks of phytohomone signaling with other cascades operating in plants, such as ROS and NO signaling47, 48 and the MAP kinase signaling pathway. Earlier studies have demonstrated importance of exogenous application of phytohormones in providing improved protection against heavy metal mediated toxicity.49, 50 Enhanced ethylene biosynthesis following Cd treatment has been observed in various plant species, including Arabidopsis, mustard, pea and soybean.51, 52 Biochemical and molecular approaches in onion and tomato have indicated role of ethylene in the accumulation of H2O2 in response to Cd treatment.53 In all such cases, heavy metal stress mediated ethylene production appears to provide cellular protection through the activation of other phytohormone synthesis like auxins and H2O2 mediated stimulation of antioxidant production for improved tolerance. GA and cytokinins have also been shown to play important roles in plants for improved protection against heavy metal stress.50, 54 Several studies have shown increased accumulation of stress related phytohormones like ABA, ethylene, jasmonic acid and salicylic acid after exposure to heavy metal stress. These phytohormones regulate the expression of the responsive genes via the activation of several transcription factors for improved stress response. Cadmium (Cd) treatment clearly induces increased accumulation of endogenous ABA levels in roots of various plant species.55 In rice (Oryza sativa), gene expression studies following arsenic stress have shown prominent expression of OsNCED2 and OsNCED3, involved in ABA biosynthesis. In addition, four other genes in ABA signaling cascades have shown up-regulated expression.56 Together, these observations have suggested that apart from ethylene, ABA also plays a crucial role in the protection mechanisms against heavy metal induced toxicity.57
The MAP kinase (MAPK) signaling cascades regulate plant response to various stress conditions, including response to heavy metal stress. In Medicago sativa (alfalfa), exposure of seedlings to elevated levels of Cu and Cd ions has been found to activate distinct cellular signaling cascades in roots, including the activation of four MAPKs, such as SIMK, MMK2, MMK3, and SAMK.58 In soybean, exposure of seedlings to Cd during the early developmental stages induces ethylene biosynthesis, along with up-regulated expression of genes involved in polyamine metabolism, NO generation, and MAPK cascades,59 indicating the integration of phytohormone and MAPK signaling under heavy metal stress. In rice root cells, free cytosolic Ca2+ accumulates following exposure Cu, which also activates NADPH oxidases and CIPK activity, eventually resulting in the stimulation of MAP kinase activity.60 In Arabidopsis, physiological concentrations of Cu and Cd, along with ROS accumulation, induce MPK3 and MPK6 transcripts in time-dependent manner,61 suggesting cross talk between redox signaling and MAPK cascades under heavy metal induced toxicity. In rice, specific expression of OsMKK4/OSMPK3 MAPK was detected following exposure to 50 μM arsenite.62 In japonica rice cultivar, a novel MAPK, OsMSRMK2 has been shown to be activated in response to excess levels of Cd, Cu and Hg.63 In Brassica juncea, specific involvement of MAP kinase mediated signaling cascade has been demonstrated following exposure to As (III).64 Heavy metal stress mediated activation of MAP kinase signaling has also been reported in maize.65 Together, these observations have indicated activation and involvement of complicated and coordinated network, which includes and integrates various signaling pathways, such as the phytohormone, calcium and MAP kinase cascades in plants in response to heavy metal mediated toxicity.
Heavy metal uptake, transport through root cell plasma membrane, root to shoot translocation and sequestration
Heavy metal uptake requires suitable transport system for their entry into plants. Higher plants have developed remarkably efficient systems for the uptake of inorganic nutrients and heavy metal ions from the soil and shown to be modulated by root exudates and the rhizospheric microorganisms. It is suggested that no specific transporters exist for the toxic heavy metals, such as Cd and Pb, since these elements do not have any direct biological function. For instance, As, which behaves as chemical analogue of phosphate, enters into plant cell via the phosphate transporters.66 The root cell membrane in Pteris vittata has been shown to contain high density of phosphate/arsenate transporters.67 However, various concentrations of heavy metals are known to be absorbed from the soil by some hyperaccumulators of heavy metals.68 Several factors like soil pH, availability of water and presence of other organic compounds in the rhizosphere critically affect heavy metal uptake by the hyperaccumulating plant species. Earlier studies have demonstrated important role of soil pH for dissolution of heavy metals and promoting growth of heavy metal accumulating plant species by affecting the proton secretion by the roots for further acidification of the rhizosphere.69 Several line of evidences have also indicated that the organic substances released from the rhizosphere of hyperaccumulating plants facilitate in dissolving the heavy metals by complex formation and thus enhancing absorption by roots.70
Plant cell possesses various families of plasma membrane based transporters, which play key role in metal uptake and homeostasis. Different heavy metal ions are generally co-transported along with other soil nutrients across the plasma membrane in the roots and show wide range of substrate specificity. Detail information on the mechanisms developed in plants for the acquisition of essential transition heavy metal micronutrients is still limited. However, several genes, which encode potential heavy metal transporters, have recently been identified in plants. Earlier studies in Arabidopsis and some other metal hyperaccumulator plants have indicated important role of P1B-type heavy metal ATPases (HMAs) in the translocation of specific heavy metal ions in plants. Based on their metal-substrate specificity, the HMA transporters have been categorized into two subgroups, such as the copper/silver (Cu/Ag) group and the zinc/cobalt/cadmium/lead (Zn/Co/Cd/Pb) group. Rice genome encodes for nine HMA genes. Among these, OsHMA1-OsHMA3 are the members of Zn/Co/Cd/Pb subgroup, while OsHMA2 has also been shown to play important role in root to shoot translocation of Zn and Cd and transport of Zn and Cd to developing seeds.71
The metal transporters on the plasma membrane and tonoplast play important role in the regulation of physiological concentrations of heavy metals and probably involved in heavy metal induced stress response in plant cell. Comparative studies on heavy metal hyperaccumulating plants species, such as Arabidopsis halleri, Thlapsi caerulescens and other non-hyperaccumulating species have revealed role of ZIP family proteins (Zinc and iron regulated transporter proteins) as membrane based transporters of heavy metal ions. ZIP6 and ZIP9 in A. halleri and ZTN1 and ZTN2 in T. caerulescens are the important members of ZIP family transporters involved in heavy metal uptake, regulating preferential transport of Zn over Cd with decreased Cd uptake under higher concentration of Zn.72 The ZIP family of transporters represent one of the major classes of heavy metal transporter and have been identified in various plant species. In Arabidopsis thaliana, IRT1 represents one of first characterized heavy metal transporter, mainly expressed in root cells and involved in the transport of transport Cd2+, Fe2+, Mn2+, Ni2+ and Zn2+, respectively.73 The NRAMP metal transporters are known to transport various heavy metal ions, such as such as Cd2+, Ni2+, Co2+, Cu2+, Mn2+, Zn2+, and Fe2+ across the plasma membranes.74, 75 The copper transporters (CTR) family initially identified in yeast and mammalian and then in plants, plays key role in transporting Cu2+ across the membrane.76 In plants, the CTR-like transporters constitute the COPT family with six members.77 In Arabidopsis, the tonoplast based copper transporter COPT5 plays important role as Cu exporter. COPT5 has been shown to regulate plant response under Cu deficient conditions in vitro.78
After the uptake via the transporters in root cells, the heavy metal ions are subsequently loaded onto the xylem elements and transported to the shoots in the form of complexes along with various metal ion chelators. Variety of transporter proteins has been shown to operate for the heavy metal ion transport from root to shoot. The HMAs (Heavy metal associated), one of the P-type ATPases, functions as efflux pumps for removal of heavy metal ions from the cell. However, HMAs also act as internal transporters for loading Cd and Zn metals into the xylem elements from the adjoining regions. In Arabidopsis thaliana, AtHMA4 encodes for the plasma membrane based P-type ATPase for transporting divalent metal ions essential for maintaining homeostasis of Zn2+ ions and also plays key role in detoxification of Cd, which otherwise disrupts cytosolic free Ca2+.79
Plants employ various strategies to alleviate the harmful effects of heavy metal ion mediated cytotoxicity. One such approach involves transporting the heavy metal ions out of the cell or sequestering into the intracellular compartments through various membrane transporters. The ABC transporters represent one of the predominant families of transporters involved in transporting the heavy metals into the vacuole. In Arabidopsis, AtMRP1 and AtMRP2 are two important members of the ABC family and shown to be involved in the transport of phytochelatin-Cd complexes into the vacuole. Function of ABC transporters has also been implicated in metal ion efflux from the plasma membrane.80, 81 In Arabidopsis, AtPDR8, another member of ABC family transporter, has been shown to confer tolerance to heavy metal by functioning as an efficient efflux pump of Cd in the plasma membrane of root hairs and epidermal cells.82 The role of plasma membrane localized ZIP (ZRT, IRT-like protein) family transporters have been implicated in Cd detoxification in Arabidopsis and shown to be activated under Zn-limiting conditions.83-85 The HMAs, member of the P-type metal ATPase are involved in the efflux of metal ions from the cytoplasm. In Arabidopsis, AtHMA3 functions as Zn/Cd transporter and has been shown to be involved in maintaining Cd and Zn homeostasis and detoxification of various other heavy metals via sequestration into the vacuole.79, 86, 87 The natural resistance-associated macrophage proteins (NRAMPs) represent another family of metal transporter. In Arabidopsis, AtNRAMP3 and AtNRAMP4 participate in the mobilization of vacuolar iron and Cd.88, 89
Protection against heavy metal stress – chelation of heavy metal ions
Plant cells have evolved with array of mechanisms to avoid and tolerate the harmful effects of heavy metal mediated toxicity. Some morphological structures, such as thick epidermal cuticle layer, leaf surface trichomes, lignification of cell walls and mycorrhizal associations serve as the initial layer of defence against heavy metal stress.90 Trichomes play important role as the immediate storage site of heavy metals for the subsequent detoxification process.91 The trichomes are also known to release various secondary metabolites to mitigate the harmful effects of heavy metals,92 however, after sensing the existence of heavy metals in the intracellular environments, plant cells activate various interlinked biochemical pathways, leading to the biosynthesis and accumulation of diverse classes of metabolites for neutralizing the heavy metal induced toxicity. These compounds generally include low-molecular weight proteins, the metallochaperones or chelators (spermine, spermidine, putrescine, nicotianamine, glutathione, phytochelatins, other organic acids etc), metallothioneins, phenylpropanoid compounds (flavonoids, anthocyanins), amino acids (proline, histidine etc), stress responsive phytohormones and even heat shock proteins.93, 94
The heavy metal ions which are not utilized for metabolic processes may accumulate at elevated level inside plant cells and usually induce cytotoxic effects, depending on the plant species, growth stage and the level of heavy metal ion tolerance level.95 Plant cells respond to the conditions of elevated concentrations of heavy metal ions via activation of production of chelating compounds for removing excess heavy metals from the cytosol and their subsequent sequestration in specific sub-cellular compartments. Various classes of small molecules, cytosolic low-molecular weight proteins, organic acids, and derivatives of phosphate are involved in metal chelation inside plant cells.
Phytochelatins (PCs) are thiol-rich low molecular weight peptides, produced from by the activity of phytochelatin synthase from glutathione (GSH) and represent one of the extensively characterized heavy metal chelators in plants.96 PCs are family of metal-binding peptides with high affinity of binding to heavy metal to initiate the pathway for metal homeostasis and detoxification. Besides plants, PCs have been identified and characterized in diverse groups of organisms, such as fungi and several species of animals.97 PCs are synthesized in the cytosol and then transported as high molecular weight metal-phytochelatin complexes to the vacuole via the ATP-binding cassette (ABC) transporter or Mg ATP-dependent carrier.81, 98 The synthesis and accumulation of PCs have been shown to be strongly activated under Cd2+ ion mediated stress as found in different plant species and accumulate initially in roots, as shown in sunflower, exposed to higher concentrations of Cd.99 However, in Brassica juncea, prolonged exposure to Cd has shown to cause about 3-fold higher accumulation of PCs in leaves than roots.100 Phytochelatins and enzymatic antioxidant activity have been suggested to function in additive fashion to safe guard plants under heavy metal stress, leading to improved resistance. Genes encoding phytochelatin synthase have been isolated and characterized from several plant species, including Arabidopsis, wheat (Triticum aestivum), rice (Oryza sativa) and Brassica juncea, respectively.101, 102 Overexpression of garlic arsenic-phytochelatin synthase 1 (AsPCS1) and yeast cadmium factor 1 (YCF1) in Arabidopsis has shown to improve tolerance to Cd and As. In addition, expression of phytochelatin genes has been shown to cause enhanced resistance to various heavy metals in transgenic Arabidopsis and tobacco plants.103, 104
The metallothioneins (MTs) represent another important family of small, low molecular weight, cysteine rich polypeptides, involved in the detoxification of wide range of metal ions like Cu, Zn, Cd, and As in diverse forms of life, including prokaryotes, invertebrates, fungi, mammals and plants.105, 106 However, MTs in plant differ considerably from those found in mammals and fungi. In plants, MTs play crucial role in mitigating the harmful effects of heavy metals by maintaining homeostasis of intracellular metal ions, through cellular sequestration and transport. In addition, MTs have also been shown to actively participate in maintaining redox level by scavenging ROS107, 108 and repair of plasma membrane.109 Based on the arrangement of cysteine residues, four different types of MTs have been identified in plants. The four sub groups of MTs have been suggested to possess distinct but overlapping functions for detoxification of heavy metal induced cytotoxicity. Previous studies have indicated role of MT isoforms 1, 2 (1a, 2a, and 2b) and 3 in chelation of Cu2+ ions in Arabidopsis, while in rice (Oryza sativa), OsMT1a (type 1 metallothionein) has been shown to play crucial role in zinc homeostasis in roots.110 In soybean (Glycine max), role of MT1, MT2, and MT3 have been implicated in the detoxification of Cd, while MT4 has been shown to be associated in homoeostasis and detoxification of Zn2+.111, 112
Organic acids and amino acids have also been shown to bind heavy metals with considerable proficiency. Along with several other functions in the cell, organic acids, such as malate, citrate, and oxalate may confer metal tolerance via transporting metals through the xylem and sequestrating ions in the vacuole. Amino acids and their derivatives are also capable of chelating metals for conferring resistance to plants for toxic levels of metal ions. Histidine has been considered as the most important free amino acid in heavy metal metabolism.9 Several reports have also indicated role of proline in increasing resistance to heavy metal stress in plants. Heavy metal stress has been shown to induce accumulation of proline indirectly as part of plant response to water stress. Heavy metal mediated ROS generation has been suggested to be scavenged by proline mainly through detoxification of hydroxyl radicals and quenching of singlet oxygen species.113
Heavy metal mediated chemical toxicity generates oxidative and genotoxic stress
In modern era, the heavy metals represent one of the important categories of environmental pollutants particularly in the industrial and adjoining areas in the developing countries. Chemical toxicity in agricultural fields occurs mainly due to excessive use of chemical fertilizers, such as pesticides, insecticides and fungicides, leading to contamination of soil, ground water and associated water sources with various heavy metals like cadmium (Cd), copper (Cu), lead (Pb), chromium (Cr) and mercury (Hg). The heavy metals, in excessive doses induce both oxidative and genotoxic stress response, leading to cytotoxicity and damage to different cellular components, including proteins, membranes, and nucleic acids, therefore, generating typical abiotic stress response in plants. Extreme heavy metal toxicity frequently disrupts the preliminary protection strategies and the cellular redox system, causes enhanced production of reactive oxygen species (ROS) via oxidative stress.35, 114 Plant cells have developed two very essential antioxidant defence mechanisms. One such component involves the activation of antioxidant enzymes, such as superoxide dismutase (SOD), catalase, (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) for the direct inactivation of the reactive radicals. The other mechanism includes the accumulation of non-enzymatic antioxidant compounds like phenylpropanoids (flavonoids, tannins, and lignin) carotenoids, ascorbate (AsA), glutathione (GSH), alkaloids and proline for the detoxification of heavy metal induced ROS and subsequent removal of free radicals by scavenging to neutralize the heavy metal mediated toxicity14, 115-117 (Fig. 3). In barley (Hordeum vulgare), exposure of the seedlings to various heavy metals has shown to enhance lipid peroxidation with the concomitant decrease in dry biomass of the seedlings.118, 119 The inorganic arsenic, which occurs in nature and produced due to anthropogenic activities, is highly toxic for plants and has been shown to be reduced into As (III) by glutathione (GSH) after uptake. The As (III) form directly reacts with the thiol-groups of the proteins, resulting in cellular cytotoxicity. Arsenic induced oxidative stress has shown to enhance considerable level of low molecular weight thiols (LMWTs) in plants, such as cysteine, glutathione, γ-glutamylcysteine and phytochelatins which eventually detoxify the effects via binding to As (III).120, 121 In vitro studies in Vicia faba have shown enhanced activities of SOD and CAT in both roots and leaves following arsenate mediated oxidative stress.122 Among the various heavy metals, Cd has been considered as one of the major factor for the induction of oxidative stress via ROS generation and impairment of non-enzymatic antioxidant defence.123 Cd-mediated oxidative stress has been shown to frequently disrupt the biomembrane functioning in plants through membrane lipid peroxidation.124 Chromium mediated oxidative stress also involves membrane lipid peroxidation in plants, damaging cell membrane, ultrastructure of chloroplasts, photosynthetic pigments and eventually disrupts antioxidant metabolism, resulting in severe growth retardation in plants.125 Lead (Pb), which accumulates in soil because of mining and smelting activities, has been shown to induce oxidative stress via ROS generation.116
Figure 3.

Oxidative and genotoxic stress in plants in response to heavy metal mediated toxicity. Heavy metal induced oxidative and genotoxic stress response in plants for maintaing the intracellular balance in redox system and genome stability to mitigate the harmful affects of heavy metal mediated oxidative and genotoxic damages. Plant cells activate the antioxidant enzymes and non-enzymatic anti-oxidants like phenylpropanoids for the inactivation and detoxification of heavy metal induced reactive oxygen species to minimixe the oxidative damages of cellular components. The heavy metal induced ROS also causes oxidative damage of bases in the DNA double helix and disrupts the phyco-chemical structure of the DNA, thus influences genome instability, leading the activation of DNA damage response, signaling and repair processes. Role of SOG1 (Suppressor of gamma response1), an unique NAC domain transcription factor in plants, has been shown in coupling the oxidative stress with the genotoxic stress mediated responses via another essential signaling component BRCA1 (breast cancer susceptibility 1) for the activation of various downstream responses, such as DNA damage repair, transcriptional response, cell cycle checkpoint functions and programmed cell death. An interesting switch from cell division to endoreduplication under stress has been indicated as part of stress adaptation mechanism in plant genome.
ROS are traditionally known to be produced as by-products of endogenous metabolic processes and involved in the induction of oxidative damages in living cells. However, recent studies have implicated dual functions of ROS in plants. Apart from their harmful effects, ROS have been shown to play key role as signaling molecule to regulate various fundamental processes such as cell growth and differentiation, programmed cell death, responses to abiotic and biotic stress and induction of systemic signaling. In addition, cell death under oxidative stress has now been considered as the outcome of ROS mediated activation of signaling pathway resulting in cell death.126 However, excessive production of ROS and the subsequent disruption of cellular redox environment have been found as the predominant cause of heavy metal induced oxidative stress response in plants.34 Oxidative damage and differential antioxidant responses have been observed in spinach (Spinacea oleracea) following exposure to various heavy metal ions.35 Elevated level of Cu cause damage to macromolecules and disrupts metabolic pathways via ROS generation.127 Excess concentration of Hg2+ causes membrane lipid peroxidation and disruption of mitochondrial functioning and finally cellular metabolism in plants through oxidative stress mediated ROS generation.128, 129 In rice (Oryza sativa) shoots, Ni2+ has been shown to affect the structural integrity of membrane lipids and H(+)-ATPase activity of the plasma membrane.130 In wheat, excess level of Ni2+ results in enhanced accumulation of malonaldehyde (MDA) content,131 which then affects biomembrane function and ion balance in the cytoplasm.132
Several lines of evidences have established a strong correlation between heavy metal induced oxidative stress and genotoxicity via the ROS mediated DNA damage and genome instability (Fig.3). The reactive oxygen species (ROS), generated in plant cells after exposure to abiotic stress like heavy metals or as by-products of endogenous metabolic processes, cause oxidative damage to most macromolecules, including lipids, proteins, and nucleic acids. Therefore, oxidative stress induced by ROS acts as an important causative agent of DNA damage, mutagenesis and genome instability, resulting in deterioration plant growth and development with loss of crop productivity. ROS induce various forms of DNA damages among which 7, 8-dihydro-8-oxoguanine (8-oxo-G) is the predominant one. Because of its prevalence and high mutagenic potential, 8-oxo-G is recognized as one of the most abundant mutagenic oxidative DNA lesions.133 Oxidative damages in the DNA, including 8-oxo-G and 1,2-dihydro-2-oxoadenine (2-OH-A) are repaired via base excision repair (BER) pathway. However, prolonged replication stress due to inefficient BER activity induces the formation of additional potentially very harmful DNA lesions such as DNA single stand breaks (SSBs) and double strand breaks (DSBs).134 Unrepaired SSBs and DSBs often results in structural abnormalities in chromosomes and thus severely affect plant growth and development during the early stages of germination due to inhibition of DNA replication and transcription, resulting in loss of cell viability and germination efficiency.
Seed germination under heavy metal stress often associated with oxidative stress and ROS generation, resulting in the accumulation of various forms of DNA damage, such as oxidative damage of adenine or guanine, single and double strand breaks and chromosome aberrations in the genome of seed embryo, thus establishing a link between DNA damage and reduced germination potential of seeds under heavy metal stress.135-139 Consistent with this observation, freshly harvested unaged Arabidopsis seeds have been found to display transcriptional up-regulation of DNA damage responsive genes during the earliest stages of germination, coincident with DNA repair synthesis.140 Together, these observations have indicated the importance of DNA damage repair function for maintaining the genome integrity of in seed embryo during imbibition and before the initiation of cell division to reduce growth inhibition and mutagenesis during the subsequent stages of seedling development in presence of heavy metals.
Assessment of the heavy metal contamination in the environment by utilizing some indicator plants has become very effective parameter in environmental biogeochemistry. Biochemical and molecular studies on heavy metal mediated genotoxicity involving Nettle (Urtica dioica) have revealed heavy metal induced nuclear DNA damage as found in the RAPD profiles of heavy metal exposed plants.141 The commonly identified heavy metals, such Hg and Pb, which are mainly released in the environment through anthropogenic activities, very effectively generate highly toxic oxygen species, such as superoxide radical (O2−), hydroxyl radical (OH•−) and hydrogen peroxide (H2O2).142 Apart from the direct effect of such oxygen species on membrane lipids and proteins, such as site-specific amino acid modifications and fragmentation of peptide chains, the oxygen species frequently induce damage to the genome via base degradation, generation of single and double strand breaks and induction of DNA-protein cross links.93, 143 The damages in the DNA have been shown to directly influence the structural integrity of the chromosomes.137, 144 Hg and Pb have been shown to induce both clastogenic and mutagenic effects in plant genome. The various forms of DNA lesions generated via the heavy metal induced ROS activity disrupts DNA replication and transcription and thus affecting genome stability,145 leading to different physiological effects, including reduced protein synthesis, damage of cell membrane and photosynthetic proteins, which eventually adversely affect plant growth and development.
The heavy metals, such as Cd, Hg and Pb have been shown to exert strong genotoxic effects. The mercuric ions appears to form covalent bonds with DNA and shown to induce sister chromatid exchange,146, 147 decreased mitotic index and increased frequency of chromosomal aberrations in dose dependent manner.148 Several studies have been carried out in recent years using cytological (chromosome aberrations and micronuclei formation assay), molecular (comet assay) and molecular genetic approaches (RAPD, AP-PCR, AFLP, SSR etc) for the assessment of genotoxicity in plants following exposure to various heavy metals, such as mercury, lead, copper, manganese and cadmium.143, 149, 150 Chickpea (Cicer arietinum) plants cultivated in soil polluted with various heavy metals like Cd, Pb, Cr and Zn have shown significant reduction in germination rate with compromised radicle length. Along with morpho-anatomical defects, the seedlings showed increased level of chromosomal abnormalities, including, bridges, laggards, stickiness, and chromosome fragmentation.151 In Vicia faba, along with ROS mediated oxidative damage of membrane lipids, an elevated level of Cd accumulation has been shown to induce significant percentage of DNA double strand breaks and genome instability.152
Transcriptional response of heavy metal toxicity in plants
Several transcription factors, belonging to diverse families, play pivotal role in modulating plant response to heavy metal induced stress through positive and negative regulations of the stress responsive genes. Since heavy metal mediated toxicity is known to induce immediate and short-term response, transcriptional regulation of plant response under heavy metal stress acts as an essential and integral part of stress signaling network.153 Transcriptional response under Cd stress has been investigated in detail in various plant species154-158 and indicated the involvement of array of transcription factors.71, 159 160 The MYB and WRKY family transcription factors have been shown to be activated in plants under heavy metal stress.161, 162 In Arabidopsis, the MYB4 transcription factor, one of the members of R2R3 family of MYB protein, showed strong induction following exposure to Cd and Zn, while other members, including MYB43, MYB48 and MYB124 were found to be specifically induced in roots in response to Cd stress.163, 164 In addition, role of MYB72 and bHLH100, a member of the helix-loop-helix transcription factor family, have been implicated in heavy metal homeostasis, specifically under Cd stress.165-167 Again, in Arabidopsis, WRKY17, the WRKY-binding protein, MYB39, MYB45, MYB63, MYB93 and MYB94 play important role in regulating transcriptional response under heavy metal stress. Such response has been shown to be often rapid and transient. Early expression of WRKY and MYB family transcription factors has been shown to regulate the expression of the downstream targets involved in protection against heavy metal mediated toxicity.168, 169 In Alpine Penny-cress (Thlaspi caerulescens), MYB28 and WRKY53 showed strong induction after cd-stress. The members of transcription factor families like WRKY, basic leucine Zipper (bZIP), bHLH, MYB and ethylene-responsive factor (ERF) play key role in regulating the specific response of plants under Cd stress via modulating the expression of specific responsive genes.170, 171 However, the Cd-stress related transcriptional response share the same signaling pathways associated other abiotic stresses, such as salinity, drought and dehydration and low temperature, suggesting the functional overlap of Cd-responsive transcription factors and involvement in complex, interlinked abiotic stress responsive network.172 Cd stress has been shown to regulate the expression of ethylene response factor 1 and 2 (ERF1, ERF2), belonging to the ethylene-responsive element-binding protein (EREBP) family/APETALA2 (AP2). ERF1and ERF2 generally binds to DRE (dehydration responsive elements) and also to several pathogenesis-related promoters.168 The dehydration responsive element binding protein 2A (DREB2A), after being induced by Cd stress, binds specifically to the DRE motif of Rd29A (desiccation responsive), resulting in Cd stress mediated expression of Rd29A protein.173 TGA3 protein has also been shown to play important role in regulation of gene expression under Cd stress. In Brassica juncea, BjCdR15, an orthologue of TGA3, has been shown to be induced immediately after brief exposure to Cd and modulate the expression of various metal transporter genes involved in long distance root-to-shoot Cd transport. Transgenic Arabidopsis thaliana and tobacco plants, overexpressing BjCdR15, showed improved resistance against Cd stress.174 OBF5, a member of the bZIP group of transcription factors, participates in regulating the expression of glutathione S-transferase under Cd stress.168 In Arabidopsis, the members of the bHLH family of transcription factors, such as AtbHLH38, AtbHLH39, AtbHLH100, and AtbHLH101 have been shown to be induced in roots and leaves under Fe deficiency.175-177 In addition, the metal responsive transcription factors regulate the expression of metallothioneins through binding the metal calmodulin regulatory elements (MREs) in promoter regions of the MT genes.178 Recent studies in Arabidopsis have indicated role of AtbHLH38 or AtbHLH39 in promoting the expression of various transporters, such as iron regulated transporter 2 (IRT2), HMA3 and MTP3 for maintaining homoeostasis of Fe ions under Cd stress.171 However, detail information on the transcriptional regulation of plant response to heavy metal stress is still limited and additional research is essential to shed more light on this field to accumulate more information on transcriptional response in plants under some commonly encountered heavy metals like As, Cd, Pb and Hg in the developing countries.
Epigenetic regulation and changes in chromatic structure under heavy metal stress
Epigenetics modifications also play very essential role in modulating gene expression in plants under various stress conditions. Several epigenetic mechanisms, such as DNA methylation, histone modifications, and microRNA (miRNA) expression influence to regulate genome function under stress conditions.179 Various processes have been shown to be regulated by DNA methylation pattern, such as chromatin structure and remodelling activities, chromosome stability and transcription180, 181 (Fig. 4). DNA methylation associated epigenetic modifications and stress tolerance in plants has been reviewed in detail in previous work.182 Several lines of evidences have revealed that after exposure to stress, the entire plant population may acquire certain level of tolerance through the adaptive processes which are mainly governed by epigenetic modifications.183-185 The progeny of the stressed plant population has been shown to exhibit improved stress tolerance through the processes of transgenerational adaptation186 and display changes in DNA methylation pattern and genome stability,187, 188 governed by the regulatory small RNA molecules which play key role as trans-acting epigenetic signals for the reversible and sequence specific modifications of gene expression at the transcription level.189 Following exposure to stress, the small RNA molecule may regulate differential DNA methylation patterns (hypo- and hypermethylation) in various regions of the genome of stressed tissues. In hemp and clover, exposure of heavy metals induces hypomethylation at several genomic loci.190 A more recent study has shown that transgenerational changes in homologous recombination frequency in the progeny population may lead to improved tolerance to heavy metal stress than the parental population.186 A genome wide study of DNA methylation pattern in maize roots in response to Pb stress have revealed increased methylation in CpG islands. Further investigation has identified 140 genes which showed altered DNA methylation pattern, including the stress-responsive transcription factor genes, such as AP2/ERF, bZIP, MYB, serine-threonine/tyrosine-proteins and F-box proteins.191 In rice, transgenerational inheritance of altered DNA methylation pattern has shown to confer enhanced tolerance to heavy metal stress in the progeny population under heavy metal stress.192 The components of the DNA methylation machinery, including MET1, CMT3, DRM2, ROS1, NRPD1, and NRPE1 have been shown to respond strongly under abiotic stress like heavy metal toxicity through quick change in the transcription of the responsive genes, leading to altered DNA methylation pattern at the genome wide level or sequence specific manner, eventually creating enhanced stress tolerance in the progeny population.193 Heavy metal mediated abiotic stress compromises plant growth and development due to inhibition of cell proliferation and expansion.194, 195 The progression through cell cycle is primarily regulated by the cyclin dependent protein kinases.196 Plethora of studies has revealed importance of chromatin modification in the regulation of expression of stress responsive genes in plant genome. However, information is still inadequate regarding the relationship between epigenetic modification and cell cycle gene expression under heavy stress in plants.
Figure 4.
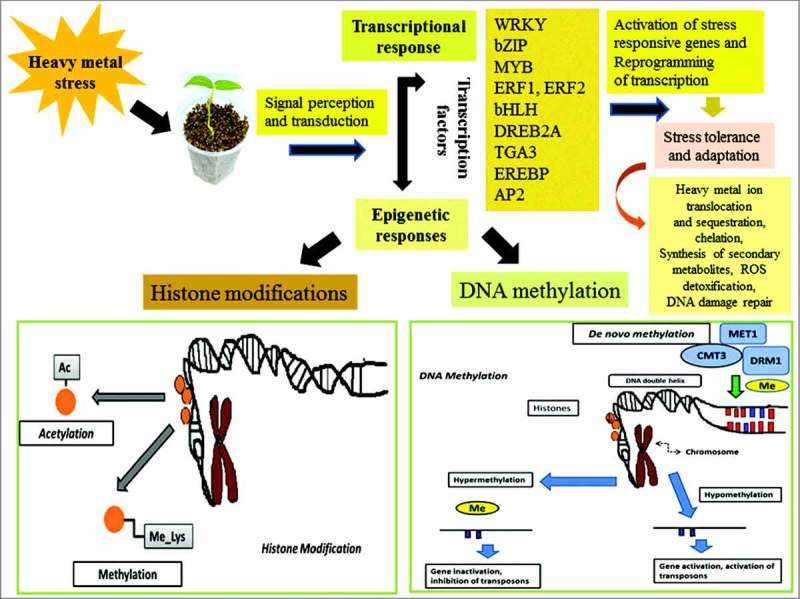
Transcriptional and epigenetic responses in plants under heavy metal stress. Schematic diagram showing the activation of transcriptional and epigenetic mechanisms in plants under heavy metal stress. The transcriptional mechanism involves participation of various transcription factors, which regulate the expression of the stress responsive genes involved in various signaling network. The epigenetic modifications under heavy metal stress mainly involve methylation of DNA and histone modifications, thus eventually regulating the chromatin structure and gene expression. In general, hypomethylation of DNA leads to transcriptional activation, while hypermethylation has opposite effects. Histone modification via methylation and acetylation also plays crucial role in the regulation of gene expression under heavy metal stress.
As like other eukaryotes, plant genome is organized into chromatin which is the functional template for variety of fundamental biological processes, like DNA replication, transcription, repair, and recombination.137 Chromatin structure is crucial for stability of genome and is constituted by the association of histone complexes with DNA to form nucleosomes. Two major pathways regulate this step,197 one of which is dependent on histone gene repressor (HIRA) whereas the other pathway requires chromatin assembly factor-1 (CAF-1), which is tightly linked with DNA replication.198 In Arabidopsis, the CAF-1 chaperone, a heterotrimeric complex, comprising of FASCIATA 1 (FAS1), FAS2, and MULTICOPY SUPPRESSOR OF IRA1 (MSI1) subunits (Hennigetal.,2003), targets acetylated histone H3/H4 onto nascent DNA strand, allowing de novo assembly of nucleosomes.197 Various types of histone modifications have been identified, including acetylation, methylation, phosphorylation, and ubiquitination199-203 (Fig. 4). These histone modifications affect gene expression by altering chromatin structure and accessibility of transcription factors under various abiotic stress conditions.204, 205 Histone hyperacetylation is generally associated with transcriptionally active chromatin, while the deacetylated histone is always located on inactive chromatin regions.206, 207 Recent studies revealed that the changes of histone modification occur under abiotic stresses in plants. Earlier studies have indicated increased level of demethylation in histone H3K4 after exposure to nickel, leading to variation in chromatin structure and transgene silencing.208-210 Nickel has also been shown to cause increased demethylation of H3K9, thus increasing the ubiquitination histone H2A and H2B. Investigations on growth and developmental pattern in maize seedlings under heavy metal stress have revealed combination of hyperacetylation and hypoacetylation pattern of specific lysine residues on H3 and H4 histones tails in the promoter regions of cell cycle regulatory genes plays crucial role in regulating the expression of specific cell cycle gene expression under heavy metal stress, leading to expanded cell cycle duration and growth inhibition.211
Outlook and conclusion
Established facts and data have clearly shown that enhancement in the agricultural practices for last couple of decades in various parts of the world, along with the support from extensive research, institutions and appropriate policies resulted in significant increment in global food grain production from about 850 million tons to 2350 million tons during the period from 1960 to 2007.212 In the developing countries, approximately 3.1 billion people mainly live the rural areas and among them around 2.5 billion population are dependent on agriculture for their livelihood. Based on the domestic products regularly obtained from agriculture, this has shown to account for approximately 30% to the overall economic growth.213 Over the past 40 years or so, although the global food production rate has been consistent with the population growth, more than a billion of population in the rural regions of the developing countries still remain mostly undernourished.214 Some recent evaluations have recommended for about 70% increase in global food crop production by 2050 for providing adequate food to the growing global population with increasing incomes and consumption. Along with this, the growing competition for land, food, water, energy, labour and capital have also created insecurity for food in various regions of the developing countries of the world and an increasing magnitude of pressure for improved crop production per unit area of land.215
The change in global climate along with increased anthropogenic activities and growing industrialization are also continuously contributing for the unfavourable changes in the environment, including soil, water and air in various ways with several factors. Consistent with this, the heavy metal stress mediated effects on plant growth and crop productivity have become one of the major concerns for the past couple of decades. Along with the pressure of increasing population, the negative impact of environmental stress on plant health offering constraints on global food crop production. These facts are again intimately linked with the increasing demand for development and improvement of abiotic stress tolerance in crop plants. Exposure of plants to various abiotic and biotic stresses adversely affects plant health and imposes genome instability. Plants respond to abiotic factors like heavy metal stress via immediate change in expression of the stress responsive genes at the transcriptional level. Apart from the transcriptional regulation, accumulating level of evidences have also revealed key role of chromatin structure alterations in the regulation of abiotic stress responsive genes. The dynamic epigenetic changes, which frequently occur in DNA and histone proteins in response to abiotic stress and the epigenetic memory have become important field of research for improvement of abiotic stress tolerance in plants. Earlier studies have shown strong link between the changes in gene expression pattern and chromatin modifications in plants in response to stress. Epigenetic processes have been shown to provide crucial adaptive mechanisms for transgenerational inheritance of stress responsive potentials215. The genetic engineering approaches for development of heavy metal stress tolerance in crop plants relies on the modulation of expression of stress responsive genes which encode proteins involved in stress signaling or response and synthesis of metabolites to confer heavy metal stress tolerance. Genetic management of heavy metal-responsive genes, in particular the transcription factors, metabolites and additional proteins have shown improvement in stress tolerance capacity. However, the transfer of technology to field conditions to its full potential needs further extensive research. However, interestingly, several natural plant species have been identified as the natural accumulator of heavy metals as these plants display better growth potential in soil containing elevated levels of heavy metals than their close or distant relatives. In this context, role of anti-oxidant components like glutathione, phytochelatin, cysteine synthase and glyoxylate pathway genes has been implicated in heavy metal stress tolerance. Apart from transgenic approaches, these components have provided important target for genetic manipulation of crop plants in the context of heavy metal stress tolerance. Abiotic stresses are undoubtedly complex in nature. However, understanding the full potentiality of using biotechnological approaches may provide important avenue for improved crop production. The recent fast growing sophisticated technologies, such as chromosome engineering, transcriptome profiling, targeted gene replacement using zinc-finger nucleases and nanotechnology provide promising future prospect for the development of designer crops with higher efficiency of utilization of natural resources and improved productivity under stress conditions.
Funding Statement
Bangladesh Council of Scientific and Industrial Research CSIR, Govt. of India to SR (Scheme number: 38(1417)/16/EMR-II, dated: 17/05/2016). This work has been financially supported by the research grant from CSIR, Govt. of India to SR (Scheme number: 38(1417)/16/EMR-II, dated: 17/05/2016).
Acknowledgements
The authors sincerely thank Prof. K. P. Das, Department of Chemistry, Bose Institute, Kolkata, India and Dr. Swarup Roy Choudhury, Donald Danforth Plant ScienceCenter, St. Louis, Missouri, for the critical reading and corrections of the Manuscript. We apologize to all authors whosework was not cited due to the length limitations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zhu JK. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002;53:247–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomashow MF. Molecular genetics of cold acclimation in higher plants. Adv. Genet. 1990;28:99–131. [Google Scholar]
- 3.Zhang Y, Yu Z, Fu X, Liang C. Noc3p, a bHLH protein, plays an integral role in the initiation of DNA replication in budding yeast. Cell 2002;109:849–60.doi: 10.1016/S0092-8674(02)00805-X [DOI] [PubMed] [Google Scholar]
- 4.Li WX, Chen TB, Huang ZC, Lei M, Liao XY. Effect of arsenic on chloroplast ultrastructure and calcium distribution in arsenic hyperaccumulator Pteris vittata L.. Chemosphere. 2006;62:803–9.doi: 10.1016/j.chemosphere.2005.04.055 [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Ma LQ, Vu JC, Raj A. Effects of arsenic on nitrate metabolism in arsenic hyperaccumulating and non-hyperaccumulating ferns. Environ. Pollut. 2009;157:2300–5. 10.1016/j.envpol.2009.03.036 [DOI] [PubMed] [Google Scholar]
- 6.Gwozdz EA, Przymusinski R, Rucinska R, Deckert J. Plant cell responses to heavy metals: Molecular and physiological aspects. Acta Physiol. Plant. 1997;19:459–65. [Google Scholar]
- 7.Das P, Samantaray S, Rout GR. Studies on cadmium toxicity in plants: A review. Env. Pollut. 1997;98:29–36. [DOI] [PubMed] [Google Scholar]
- 8.Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, del Rio LA. Cadmium induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001;52:2115–26. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Dubey RS. Involvement of oxidative stress and role of antioxidative defense systemin growing rice seedlings exposed to toxic concentrations of aluminium. Plant Cell Rep. 2007;26(11):2027–38. [DOI] [PubMed] [Google Scholar]
- 10.Clemens S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta. 2001;212:475–86. [DOI] [PubMed] [Google Scholar]
- 11.Cobbett C, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002;53:159–82. [DOI] [PubMed] [Google Scholar]
- 12.Shingu Y, Kudo T, Ohsato S, Kimura M, Ono Y, Yamahuchi I, Hamamoto H. Characterization of genes encoding metal tolerance proteins isolated from Nicotiana glauca and Nicotiana tabacum. Biochem. Biophys. Res. Comm. 2005;331:675–80. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Parihar P, Singh R, Singh VP, Prasad SM. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics review. front. Plant Sci. 2016. 10.3389/fpls.2015.01143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hossain MA, Piyatida P, da Silva JAT, Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012(37) doi: 10.1155/2012/872875 [DOI] [Google Scholar]
- 15.Sundaramoorthy P, Chidambaram A, Ganesh KS, Unnikannan P, Baskaran L. Chromium stress in paddy:(i) nutrient status of paddy under chromium stress; (ii) phytoremediation of chromium by aquatic and terrestrial weeds. C. R. Biol. 2010;333:597–607.doi: 10.1016/j.crvi.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 16.Zobel RW, Kinraide TB, Baligar VC. Fine root diameters can change in response to changes in nutrient concentrations. Plant Soil. 2007;297:243–54.doi: 10.1007/s11104-007-9341-2 [DOI] [Google Scholar]
- 17.Kenderešová L, Staˇnová A, Pavlovkin J, ˇDurišová E, Nadubinská M, Ciamporová M. Early Zn2+- induced effects on membrane potential account for primary heavy metal susceptibility in tolerant and sensitive Arabidopsis species. Ann. Bot. 2012;110:445–59.doi: 10.1093/aob/mcs111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong J, Wu FB, Zhang GP. Effect of cadmium on growth and photosynthesis of tomato seedlings. J. Zhejiang Univ.Sci. 2005;B 6:974–80.doi: 10.1631/jzus.2005.B0974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Huang X, Borthakur D, Ni H. Photosynthetic activity and antioxidative response of seagrass Thalassia hemprichii to trace metal stress. Acta Oceanol. Sin. 2012;31:98–108.doi: 10.1007/s13131-012-0210-3 [DOI] [Google Scholar]
- 20.Schafer C, Simper H, Hofmann B. Glucose feeding results in coordinated changes of chlorophyll content, ribulose-1,5-biphosphate carboxylase-oxygenase activity and photosynthetic potential photoautotrophic suspension cultured cells of Chenopodium ruburum. Plant Cell Environ. 1992;15:343–50. [Google Scholar]
- 21.Chaffei C, Gouia H, Ghorbel MH. Nitrogen metabolism in tomato plants under cadmium stress. J. Plant Nutr. 2003;26:1617–34. doi: 10.1081/PLN-120022372 [DOI] [Google Scholar]
- 22.Zhang W, Bone JR, Edomondson DG, Turner BM, Roth SY. Essential and redundant functions of histone acetylation revealed mutation of target lysines and loss of the Gcn5 p acetyl transferase. EMBO J. 1998;17:3155–67. doi: 10.1093/emboj/17.11.3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 2000;3:217–23. [PubMed] [Google Scholar]
- 24.Gill M. Heavy metal stress in plants: A review. Int. J. Adv. Res. 2014;2(6):1043–55. [Google Scholar]
- 25.Kavamura VN, Esposito E. Biotechnological strategies applied to the decontamination of soils polluted with heavy metal. Biotechnol. Adv. 2010;28:61–9. doi: 10.1016/j.biotechadv.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 26.Wuana RA, Okieimen FE. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology. 2011:20. doi: 10.5402/2011/402647 [DOI] [Google Scholar]
- 27.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metals toxicity and the environment. EXS. 2012;101:133–64. doi: 10.1007/978-3-7643-8340-4_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bray EA, Bailey-Serres J, Weretilnyk E. Responses to abiotic stress. In: Buchanan BB, Gruissen W, Jones RL, Biochemistry and molecular biology of plants, 1st edn. Rockville: Wiley; 2000, p. 1158–249. [Google Scholar]
- 29.Barnabas B, Jagner K, Feher A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008;31:11–38. [DOI] [PubMed] [Google Scholar]
- 30.Rodziewicz P, Swarcewicz B, Chmielewska K, et al. . Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiol Plant. 2014;36(1):1–19. 10.1007/s11738-013-1402-y [DOI] [Google Scholar]
- 31.Pandey P, Irulappan V, Bagavathiannan MV, Senthil-Kumar M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front Plant Sci. 2017;8:537. doi: 10.3389/fpls.2017.00537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aslam R, Ansari MYK, Choudhary S, Bhat TM, Jahan N. Genotoxic effects of heavy metal cadmium on growth, biochemical, cyto-physiological parameters and detection of DNA polymorphism by RAPD in Capsicum annuum L.- an important spice crop of India. Saudi J. Plant Sci. 2014;21:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erdei S, Hegedus A, Hauptmann G, Szali J, Horvath G. Heavy metal induced physiological changes in the antioxidative response system. Acta Biol. Szeged. 2002;46:89–90. [Google Scholar]
- 34.Nada E, Ferjani BA, Ali R, Bechir BR, Imed M, Makki B. Cadmium induced growth inhibition and alteration of biochemical parameters in almond seedlings grown in solution culture. Acta Physiol. Plant. 2007;29:57–62. [Google Scholar]
- 35.Pandey N, Pathak GC, Pandey DK, Pandey R. Heavy metals, Co, Ni, Cu, Zn and Cd, produce oxidative damage and evoke differential antioxidant responses in spinach. Braz. J. Plant Physiol. 2009;21(2):103–11. [Google Scholar]
- 36.Gamalero E, Lingua G, Berta G, G BRGlick. Beneficial role of plant growth promoting bacteria and arbuscular mycorrhizal fungi on plant responses to heavy metal stress. Canadian J. Microbiol. 2009;55(5):501–14. [DOI] [PubMed] [Google Scholar]
- 37.Zhao L, Wang P, Hou H, Zhang H, Wang Y, Yan S, Huang Y, Li H, Tan J, Hu A. Transcriptional regulation of cell cycle genes in response to abiotic stresses correlates with dynamic changes in histone modifications in maize. PLoS ONE. 2014;9(8):e106070. doi: 10.1371/journal.pone.0106070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarattini M, Forlani G. Toward unveiling the mechanisms for transcriptional regulation of proline biosynthesis in the plantcell response to biotic and abiotic stress conditions. Front. Plant Sci. 2017;8:927. doi: 10.3389/fpls.2017.00927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carroll AD, Moyen C, Van Kesteren P, Tooke F, Battey NH, Brownlee C. Ca2+, annexins, and GTP modulate exocytosis from maize root cap protoplasts. Plant Cell. 1998;10:1267–76.doi: 10.1105/tpc.10.8.1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002;32:539–48.doi: 10.1046/j.1365-313X.2002.01442.x [DOI] [PubMed] [Google Scholar]
- 41.Wilkins KA, Matthus E, Swarbreck SM SM, Davies JM. Calcium-mediated abiotic stress signaling in roots. Front. Plant Sci. 2016;7:1296. doi: 10.3389/fpls.2016.01296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruta LL, Popa VC, Nicolau I, Danet AF, Iordache V, Neagoe AD, Farcasanu IC. Calcium signaling mediates the response to cadmium toxicity in Saccharomyces cerevisiae cells. FEBS Lett. 2014;588(17:3202–12. [DOI] [PubMed] [Google Scholar]
- 43.Chmielowska-Bak J, Gryl J, Rucinska-Sobkowiak R, Arasimowicz-Jelonek M, Deckert J. The new insights into cadmium sensing. Front. Plant Sci. 2014;5:245. doi: 10.3389/fpls.2014.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S, Yu J, Zhu M, Zhao F, Luan S. Cadmium impairsion homeostasis by altering KC and Ca2+ channel activities in rice root hairs. Plant Cell Environ. 2012;35:1998–2013.doi: 10.1111/j.1365-3040.2012.02532.x [DOI] [PubMed] [Google Scholar]
- 45.Fan JL, Wei XZ, Wan LC, Zhang LY, Zhao XQ, Liu WZ, et al. . Disarrangement of actin filaments and Ca2+ gradient by CdCl2 alters cell wall construction in Arabidopsis thaliana root hairs by inhibiting vesicular trafficking. J. Plant Physiol. 2011;168:1157–67.doi: 10.1016/j.jplph.2011.01.031 [DOI] [PubMed] [Google Scholar]
- 46.Laohavisit A, Shang ZL, Rubio L, Cuin TA, Véry AA, Wang AH, et al. . Arabidopsis annexin 1 mediates the radical-activated plasma membrane Ca2+ and K+-permeable conductance in root cells. Plant Cell. 2012;24:1522–33. doi: 10.1105/tpc.112.097881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liptáková L, Bočová B, Huttová J, Mistrík I, Tamás L. Superoxide production induced by short-term exposure of barley roots to cadmium, auxin, alloxan and sodium dodecyl sulphate. Plant Cell Rep. 2012;31:2189–97.doi: 10.1007/s00299-012-1329-6 [DOI] [PubMed] [Google Scholar]
- 48.Wang Q, Liang X, Dong Y, Xu L, Zhang X, Kong J, et al. . Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J. Plant Growth Regul. 2013;32:721–31. doi: 10.1007/s00344-013-9339-3 [DOI] [Google Scholar]
- 49.Agami RA, Mohamed GF. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium correlates with stomatal responses to ABA. J. Exp. Bot. 51 (2013) 197–205. [DOI] [PubMed] [Google Scholar]
- 50.Masood A, Khan MIR, Fatma M, Asgher M, Per TS, Khan NA. Involvement of ethylene in gibberellic acid-induced sulfur assimilation, photosynthetic responses, and alleviation of cadmium stress in mustard. Plant Physiol. Biochem. 2016;104:1–10. [DOI] [PubMed] [Google Scholar]
- 51.Arteca RN, Arteca JM. Heavy-metal induced ethylene production in Arabidopsis thaliana. J. Plant Physiol. 2007;164:1480–8. doi: 10.1016/j.jplph.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 52.Rodríguez-Serrano M, Romero-Puertas MC, Pazmino DM, Testillano PS, Risueno MC, del Río LA, Sandalio LM. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009;150: 229–43. doi: 10.1104/pp.108.131524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu K, Shen L, Sheng J. Improvement in cadmium tolerance of tomato seedlings with an antisense DNA for 1-aminocyclopropane-1-carboxylate synthase. J. Plant Nutr. 2008;31:809–27. doi: 10.1080/01904160802043080 [DOI] [Google Scholar]
- 54.Bücker-Neto L, Paiva ALS, Machado RD, Arenhart RA, Margis-Pinheiro M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017;40:373–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stroinski A, Chadzinikolau T, Gizewska K, Zielezinska M. ABA or cadmium induced phytochelatin synthesis in potato tubers. Biol. Plant. 2010;54:117–20. [Google Scholar]
- 56.Huang TL, Nguyen QTT, Fu SF, Lin CY, Chen YC, Huang HJ. Transcriptomic changes and signaling pathways induced by arsenic stress in rice roots. Plant Mol. Biol. 2012;80:587–608. [DOI] [PubMed] [Google Scholar]
- 57.Hollenbach B, Schreiber L, HartungWand Dietz KJ. Cadmium leads to stimulated expression of the lipid transfer protein genes in barley: Implications for the involvement of lipid transfer proteins in wax assembly. Planta. 1997;203:9–19. [DOI] [PubMed] [Google Scholar]
- 58.Jonak C, Nakagami H. H. hirt heavy metal stress. activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 2014;136:3276–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chmielowska-Bąak J, Lefèvre I, Lutts S, Deckert J. Short term signaling responses in roots of young soybean seedlings exposed to cadmium stress. J. Plant Physiol. 2013;170:1585–94. doi: 10.1016/j.jplph.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 60.Yeh CM, Chien PS, Huang HJ. Distinct signaling pathways for induction of MAP kinase activities by cadmium and copper in rice roots. J. Expt. Bot. 2007;58:659–71. doi: 10.1093/jxb/erl240 [DOI] [PubMed] [Google Scholar]
- 61.Liu XM, Kim KE, Kim KC, Nguyen XC, Han HJ, Jung MS, Kim HS, Kim SH, Park HC, Yun DJ, Chung WS. Cadmium activates arabidopsis MPK3 and MPK6 via accumulation of reactive oxygen species. Phytochemistry. 2010;71:614–8. [DOI] [PubMed] [Google Scholar]
- 62.Rao KP, Vani G, Kumar K, Wankhede DP, Misra M, Gupta M, Sinha AK. Arsenic stress activates MAP kinase in rice roots and leaves. Arch. Biochem. Biophys. 2011;506:73–82. [DOI] [PubMed] [Google Scholar]
- 63.Agrawal GK, Rakwal R, Iwahashi H. Isolation of novel rice (Oryza sativa L.) multiple stress responsive MAP kinase gene, OsMSRMK2, whose mRNA accumulates rapidly in response to environmental cues. Biochem. Biophys. Res. Commun. 2002;294:1009–16. [DOI] [PubMed] [Google Scholar]
- 64.Gupta M, Sharma P, Sarin NB, Sinha AK. Differential response of arsenic stress in two varieties of Brassica juncea L.. Chemosphere. 2009;74:1201–8. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Ding H, Zhang A, Ma F, Cao J, Jiang M. A novel mitogen-activated protein kinase gene in maize (Zea mays), ZmMPK3, is involved in response to diverse environmental cues. J. Integr. Plant Biol. 2010;52:442–52. [DOI] [PubMed] [Google Scholar]
- 66.Meharg AA, Hartley-Whitaker J. Arsenic up take and metabolism in arsenic resistant and non-resistant plant species. New Phytol. 2002;154:29–43.doi: 10.1046/j.1469-8137.2002.00363.x [DOI] [Google Scholar]
- 67.Caille N, Zhao FJ, McGrath SP. Comparison of root absorption, translocation and tolerance of arsenicin the hyper accumulator Pterisvittata and then on-hyper accumulator Pteristremula. New Phytol. 2005;165:755–61.doi: 10.1111/j.1469-8137.2004.01239.x [DOI] [PubMed] [Google Scholar]
- 68.Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelly ED. Afernthat hyperaccumulates arsenic. Nature. 2001;409:579.doi: 10.1038/35054664 [DOI] [PubMed] [Google Scholar]
- 69.Peng HY, Yang XE, Jiang LY. Copper phytoavailability and uptake by Elsholtzia splendens from contaminated soilas affected by soil amendments. J. Environ. Sci. Health. 2005;40:839–56.doi: 10.1081/ESE-200048283 [DOI] [PubMed] [Google Scholar]
- 70.Krishnamurti GSR, Cieslinski G, Huang PM, Van Rees KCJ. Kinetics of cadmium release from soils as influenced by organic acids: Implication in cadmium availability. J. Environ. Qual. 1997;26:271–7. 10.2134/jeq1997.00472425002600010038x [DOI] [Google Scholar]
- 71.Takahashi R, Bashir K, Ishimaru Y, Nishizawa NK, Nakanishi1 H. The role of heavy-metal ATPases, HMAs, in zinc and cadmium transport in rice. Plant Signal Behav. 2012;7(12):1605–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weber M, Trampczynska A, Clemens S. Comparative transcriptome Analysis of toxic metal responses in Arabidopsis thaliana and the Cd2+-Hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ. 2006;29(9):50–963. doi: 10.1111/j.1365-3040.2005.01479.x [DOI] [PubMed] [Google Scholar]
- 73.Rogers EE, Eide DJ, Guerinot ML. Altered selectivity in an Arabidopsis metal transporter, Proc. Natl. Acad. Sci. L.E. Williams, J.K. Pittman, J.L. Hall, Emerging mechanisms for heavy metal transports in plants. Biochim. Biophys. Acta. 2000;1465:104–26.10748249 [Google Scholar]
- 74.Tandy S, Williams M, Leggett A, Lopez-Jimenez M, Dedes M, Ramesh B, Srai SK, P Sharp Nramp2 expression is associated with pH-dependent iron uptake across the apical membrane of human intestinal Caco-2 cells. J. Biol. Chem. 2000;275:1023–9 [DOI] [PubMed] [Google Scholar]
- 75.Nevo Y, Nelson N. The NRAMP family of metal-ion transporters. Biochim. Biophys. Acta - Mol. Cell Res. 2005;1763:609–20. [DOI] [PubMed] [Google Scholar]
- 76.Kampfenkel K, Kushnir S, Babiychuk E, Inze D, Van MM. Molecular characterization of a putative Arabidopsis thaliana copper transporter and its yeast homologue. J. Biol. Chem. 1995;270:28479–86. [DOI] [PubMed] [Google Scholar]
- 77.Sancenon V, Puig S, Mira H, Thiele DJ, Penarrubia L. Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol. Biol. 2003;51:577–87. [DOI] [PubMed] [Google Scholar]
- 78.Klaumann S, Nickolaus SD, Furst SH, Starck S, Schneider S, Neuhaus HE, Trentmann O. The tonoplast copper transporter COPT5 acts as an exporter and is required for interorgan allocation of copper in Arabidopsis thaliana. New Phytol. 2011;192:393–404. [DOI] [PubMed] [Google Scholar]
- 79.Mills RF, Francini A, da Rocha PSCF, Baccarini PJ, Aylett M, Krijger GC, Williams LE. The plant P1B-type ATPase AtHMA4 transports Zn and Cd and plays a role in detoxification of transition metals supplied at elevated levels. FEBS Lett. 2005;579:783–91. [DOI] [PubMed] [Google Scholar]
- 80.Mendoza-Cózatl DG, Jobe TO, Hauser F, Schroeder JI. Long-distance transport, vacuolar sequestration and transcriptional responses induced by cadmium and arsenic. Curr. Opin. Plant Biol. 2011;14(5):554–62. doi: 10.1016/j.pbi.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song WY, Mendoza-Cózatl DG, Lee Y, Schroeder JI, Ahn SN, Lee HS, Wicker T, Martinoia E Phytochelatin–metal(loid) transport into vacuoles shows different substrate preferences in barley and Arabidopsis. Plant Cell Environ. 2014;37(5):1192–201. doi: 10.1111/pce.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007;50:207–18. [DOI] [PubMed] [Google Scholar]
- 83.Eng BH, Guerinot ML, Eide D, Saier MH, J. Membr. Biol. 1998;166:1–7. [DOI] [PubMed] [Google Scholar]
- 84.Grotz N, Guerinot ML. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim. Biophys. Acta. 2006;1763:595–608. [DOI] [PubMed] [Google Scholar]
- 85.Komal T, Mustafa M, Ali Z, Kazi AG. Heavy metal uptake and transport in plants. In: Sherameti I, Varma A, Heavy metal contamination of soils, soil biol.44. Springer, Cham: 2015; p. 184–91. [Google Scholar]
- 86.Krämer U, Talke IN, Hanikenne M. Transitionmetal transport. FEBS Lett. 2007;581:2263–72. doi: 10.1016/j.febslet.2007.04.010 [DOI] [PubMed] [Google Scholar]
- 87.Morel M, Crouzet J, Gravot A, Auroy P, Leonhardt N, Vavasseur A, Richaud P. AtHMA3, a P1B-ATPase Allowing Cd/Zn/Co/Pbvacuolar storage in Arabidopsis. Plant Physiol. 2009;149(2):894–904. doi: 10.1104/pp.108.130294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lanquar V, Lelièvre F, Bolte S, Hamès C, Alcon C, Neumann D, Vansuyt G, Curie C, Schröder A, Krämer U, BarbierBrygoo H, Thomine S. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005;24:4041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Molins H, Michelet L, Lanquar V, Agorio A, Giraudat J, Roach T, Krieger-Liszkay A, Thomine S. Mutants impaired in vacuolar metal mobilization identify chloroplasts as a target for cadmium hypersensitivity in Arabidopsis thaliana. Plant Cell Environ. 2013;36:804–17. [DOI] [PubMed] [Google Scholar]
- 90.Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 53 2002;366:1–11. [PubMed] [Google Scholar]
- 91.Harada E, Kim JA, Meyer AJ, Hell R, Clemens S, Choi YE. Expression profiling of tobacco leaf trichomes identifies genes for biotic and abiotic stresses. Plant Cell Physiol. 2010;51(10):1627–163. [DOI] [PubMed] [Google Scholar]
- 92.Hauser MT. Molecular basis of natural variation and environmental control of trichome patterning. Front. Plant Sci. 2014;5(320):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharma SS, Dietz KJ. The significance of amino acidsand amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006;57(4):711–26. [DOI] [PubMed] [Google Scholar]
- 94.Dalvi AA, Bhalerao SA. Response of plants towards heavy metal toxicity: An overview of avoidance, tolerance and uptake mechanism. Ann. Plant Sci. 2013;2(9):362–8. [Google Scholar]
- 95.Solanki R, Dhankhar R. Biochemical changes and adaptive strategies of plants under heavy metal stress. Biologia. 2011;66(2):195–204. [Google Scholar]
- 96.Vatamaniuk OK, Bucher EA, Ward JT, Rea PA. A new pathway for heavy metal detoxification in animals, phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans. J. Biol. Chem. 2001;276(24):20817–20. [DOI] [PubMed] [Google Scholar]
- 97.Bundy JG, Kille P, Liebeke M, Spurgeon DJ. Metallothioneins may not be enough- the role of phytochelatins in invertebrate metal detoxification. Environ. Sci. Technol. 2014;48(2):885–6. [DOI] [PubMed] [Google Scholar]
- 98.Manara A. Plant responses to heavy metal toxicity in Plants and Heavy Metals. In: Springer briefs in molecular science. Furini A, Springer, Dordrecht, Netherlands; 2012, p. 27–53. [Google Scholar]
- 99.Thangavel P, Long S, Minocha R. Changes in phytochelatins and their biosynthetic intermediates in red spruce (Picea rubens Sarg.) cell suspension cultures under cadmium and zinc stress. Plant Cell Tissue Organ Cult. 2007;88(2):201–16. [Google Scholar]
- 100.Heiss S, Wachter A, Bogs J, Cobbett C, Rausch T. Phytochelatinsynthase (PCS) protein is induced in Brassica juncea leaves after prolonged Cd exposure. J. Exp. Bot. 2003;54(389):1833–39. [DOI] [PubMed] [Google Scholar]
- 101.Cobbett CS. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000;123(3):825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shen GM, Zhu C, Du QZ. Genome-wide identification of Phytochelatin and Phytoch Synth domaincontaining phytochelatin family from rice. Electron. J. Biol. 2010;6(3):73–9. [Google Scholar]
- 103.Guo J, Xu W, Ma M. The assembly of metals chelation bythiols and vacuolar compartmentalization conferred increased tolerance to and accumulation of cadmium and arsenic in transgenic Arabidopsis thaliana. J. Hazard. Mater. 2012;199-200:309–13. [DOI] [PubMed] [Google Scholar]
- 104.Postrigan BN, Knyazev AB, Kuluev BR, Yakhin OI, Chemeris AV. Expression of the synthetic phytochelatin gene in tobacco. Russ. J. Plant Physiol. 2012;59(2):275–80. [Google Scholar]
- 105.Du J, Yang JL, Li CH. Advances in metallotione in studies in forest trees. Plant OMICS. 2012;5(1):46–51. [Google Scholar]
- 106.Cai Y, Ma LQ. Metal tolerance accumulation and detoxication in plants with emphasis on arsenic in terrestrial plants. In: Proceedings of the ACS symposium series 835 on biogeochemistry of environmentally important trace elements. Cai Y, Btaids OC, American Chemical Society, 2003, p. 95–114. [Google Scholar]
- 107.Wong HL, Sakamoto T, Kawasaki T, Umemura K, Shimamoto K. Down-regulation of metallothionein, a reactive oxygen scavenger, by the small GTPase OsRac1 in rice. Plant Physiol. 2004;135(3):1447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kohler A, Blaudez D, Chalot M, Martin F. Cloning andexpression of multiple metallothioneins from hybrid poplar. New Phytol. 2004;164(1):83–93. [DOI] [PubMed] [Google Scholar]
- 109.Mishra S, Dubey RS. Heavy metal uptake and detoxification mechanisms in plants. Int. J. Agric. Res. 2006;1(2):122–41. [Google Scholar]
- 110.Yang Z, Wu Y, Li Y, Ling HQ, Chu C. OsMT1a, a type 1 metallothionein, plays the pivotal role in zinc homeostasis and drought tolerance in rice. Plant Mol Biol. 2009;70:219–29. [DOI] [PubMed] [Google Scholar]
- 111.Grennan AK. Metallothioneins, a diverse protein family. Plant Physiol. 2011;155(4):1750–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pagani MA, Tomas M, Carrillo J, Bofill R, Capdevila M, Atrian S, Andreo CS. The response of the different soybean metallothionein isoforms to cadmiumin toxication. J. Inorg. Biochem. 2012;117:306–15. [DOI] [PubMed] [Google Scholar]
- 113.Emamverdian A, Ding Y, Mokhberdoran F, Xie Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015;2015 10.1155/2015/756120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mourato M, Reis R, Martins LL. Characterization of plant antioxidative system in response to abiotic stresses: A focus on heavy metal toxicity. In: Montanaro G, Dichio B, Advances in selected plant physiology aspects. Tech, Vienna, Austria: 2012; p. 23–44. [Google Scholar]
- 115.Michalak A. Phenolic compounds and their antioxidant activityin plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006;15(4):523–30. [Google Scholar]
- 116.Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012:26. [Google Scholar]
- 117.Štolfa I, Ž. Pfeiffer T, Špoljarić D, Teklić T, Lončarić Z, Lončarić Z, Teklić T, Špoljarić D, Ž. Pfeiffer T, Štolfa I. Heavy metal-induced oxidative stress in plants: Response of the Antioxidative System. In: Gupta D, Palma J, Corpas F, Francisco J, Reactive oxygen species and oxidative damage in plants under Stress. Springer; 2015. [Google Scholar]
- 118.Juknys R, Vitkauskaitė G, Račaitė M, et al. . The impacts of heavy metals on oxidative stress and growth of spring barely. Cent Eur J Biol. 2012;7:299–306. [Google Scholar]
- 119.Kacienė G, Žaltauskaitė J, Milčė E, Juknys R. Role of oxidative stress on growth responses of spring barley exposed to different environmental stressors. J. Plant Ecol. 2015;8:605–16. [Google Scholar]
- 120.M.H Zenk heavy metal detoxification in higher plants: a review. Ene. 1996;179:21–30. [DOI] [PubMed] [Google Scholar]
- 121.Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Trivedi PK, P.K Tandon phytochelatins and antioxidant systems respond differentially during arsenite and arsenate stress in Hydrillaverticillata (L.f.) Royle. Environ Sci Technol. 2007;41(8):2930–6. [DOI] [PubMed] [Google Scholar]
- 122.Lin A, Zhang X, Zhu YG, Zhao FJ. Arsenate-induced toxicity: Effects on antioxidative enzymes and DNA damage in Vicia faba. Environ. Toxicol. Chem. 2008;27(2):413–9. [DOI] [PubMed] [Google Scholar]
- 123.Cho UH, Seo NH. Oxidative stress in arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci. 2005;168:113–20. [Google Scholar]
- 124.Gratao PL, Polle A, Lea PJ, Azedevo RA. Making the life of heavy metal- stressed plants a little easier. Funct. Plant Biol. 2005;32:481–94. [DOI] [PubMed] [Google Scholar]
- 125.Panda SK, Choudhury S. Changes in nitrate reductase activity and oxidative stress response in the moss Polytrichum commune subjected to chromium, copper and zinc phytotoxicity. Braz. J. Plant Physiol. 2005;17:191–7. [Google Scholar]
- 126.Ron M. Ros are good. Trends Plant Sci. 2017;22(1): 10.1016/j.tplants.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 127.Erdei S, Hegedus A, Hauptmann G, Szali J, Harvath G. Heavy metal induced physiological changes in the antioxidative response system. Acta Biol. Szeged. 2002;46:89–90 [Google Scholar]
- 128.Messer RL, Lockwood PE, Tseng WY, Edwards K, Shaw M, Caughman GB, Lewis JB, Wataha JC. Mercury (II) alters mitochondrial activity of monocytes at sublethal doses via oxidative stress mechanisms. J. Biomed. Mater. Res. B. 2005;75:257–63. [DOI] [PubMed] [Google Scholar]
- 129.Cargnelutti LATabaldi, Spanevello RM, Jucoski GO, Battisti V, Redin M, Linares CEB, Dressler VL, Flores MM, Nicoloso FT, Morsch VM, et al. . Mercury toxicity induces oxidative stress in growing cucumber seedlings. Chemosphere. 2006;65:999–1006. [DOI] [PubMed] [Google Scholar]
- 130.Ros R, Cooke DT, Burden RS, Jones CS. Effects of the herbicide MCPA and the heavymetals cadmium and nickel on the lipid composition, Mg2+-ATPase activity andfluidity of the plasma membrane from rice, Oryzasativa (cv. Bahía) shoots. J Exp Bot. 1992;41:457–62. [Google Scholar]
- 131.Pandolfini T, Gabbrielli R, Comparini C. Nickel toxicity and peroxidase activity inseedlings of Triticumaestivum L. Plant Cell Environ. 1992;15:719–25. [Google Scholar]
- 132.Gonnelli C, Galardi F, Gabbrielli R. Nickel and copper tolerance in three Tuscan populations of Sileneparadoxa. Physiol. Plant. 2001;113:507–14. [Google Scholar]
- 133.Markkanen E E, van Loon B, Ferrari E, Parsons JL, Dianov GL, Hübscher U. Regulation of oxidative DNA damage repair by DNA polymerase λ and MutYH by cross-talk of phosphorylation and ubiquitination. Proc. Natl. Acad. Sci. USA. 2011;109(2):437–42. doi: 10.1073/pnas.1110449109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zucca E, Bertoletti F, Wimmer U, Ferrari E, Mazzini G, Khoronenkova S, Grosse N, van Loon B, Dianov G, Hubscher U, et al. . Silencing of human DNA polymerase λ causes replication stress and issynthetically lethal with an impaired S phase checkpoint, Nucl. Acids Res. 2013;41:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morales ME, Derbes RS, Ade CM, Ortego JC, Stark J, Deininger PL, Roy-Engel AM. Heavy metal exposure influences double strand break dna repair outcomes. PLoS ONE. 2016;11(3):e0151367 10.1371/journal.pone.0151367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moura DJ, Péres VF, Jacques RA, Saffi J. Heavy metal toxicity: Oxidative stress parameters and DNA repair. In: Gupta D, Sandalio L, Metal toxicity in plants: perception, signaling and remediation. Berlin, Heidelberg: Springer; 2012. [Google Scholar]
- 137.Roy S. Maintenance of genome stability in plants: Repairing DNA double strand breaks and chromatin structure stability, Front. Plant Sci. 2014;5:487. doi: 10.3389/fpls.2014.00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Waterworth WM, Drury GE, Bray CM, West CE. Repairing breaks in the plant genome: The importance of keeping it together. New Phytol. 2011;192:805–22. 10.1105/tpc.108.060525 [DOI] [PubMed] [Google Scholar]
- 139.Osborne DJ, Dell'Aquila A, Elder RH. DNA repair in plant cells, An essential event of early embryo germination in seeds. Folia Biol. (Praha). 1984;30:155–69. [PubMed] [Google Scholar]
- 140.Waterworth WM, Masnavi G, Bhardwaj RM, Jiang Q, Bray CM, West CE. A plant DNA ligase is an important determinant of seed longevity. Plant J. 2010;63:848–60. [DOI] [PubMed] [Google Scholar]
- 141.Gjorgieva D, Panovska TK, Ruskovska T, Bačeva K, Stafilov T. Influence of heavy metal stress on antioxidant status and DNA damage in urticadioica. Bio. Med. Res. Int. 2013;6. doi: 10.1155/2013/276417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gao Y, Guo YK, Lin SH, Fang YY, Bai JG. Hydrogen peroxide pretreatment alters the activity of antioxidant enzymes and protects chloroplast ultrastructure in heat-stressed cucumber leaves. Sci. Hortic. 2010;126:20–6. 10.1016/j.scienta.2010.06.006 [DOI] [Google Scholar]
- 143.Liu W, Li PJ, Qi XM, Zhou QX, Zheng L, Sun TH, Yang YS. DNA changes in barley (Hordeum vulgare) seedlings induced by cadmium pollution using RAPD analysis, .hemosphere. 2005;61:158–67. doi: 10.1016/j.chemosphere.2005.02.078 [DOI] [PubMed] [Google Scholar]
- 144.Tuteja N, Ahmad P, Panda BB, Tuteja R. Genotoxic stress in plants: Shedding light on DNA damage, repair and DNA repair helicases, Mutat. Res. 2009;681:134–49. [DOI] [PubMed] [Google Scholar]
- 145.Britt AB. Molecular genetics of DNA repair in higher plants. Trends Plant Sci. 1999;4:20–5. [DOI] [PubMed] [Google Scholar]
- 146.Chaoui A, Mazhoudi S, Ghorbal MH, Ferjani E. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci. 1997;127:139–47. 10.1016/S0168-9452(97)00115-5 [DOI] [Google Scholar]
- 147.Beauford W, Barber J, Barringer RA. Uptake and distribution of mercury within higher plants. Physiol. Plant. 2006;39:261–5. 10.1111/j.1399-3054.1977.tb01880.x. [DOI] [Google Scholar]
- 148.Patra M, Bhowmik N, Bandopadhyay B, Sharma A. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ. Exp. Bot. 2004;52:199–223. 10.1016/j.envexpbot.2004.02.009 [DOI] [Google Scholar]
- 149.Atienzar FA, Jha AN. The random amplified polymorphic DNA (RAPD) assay and related techniques applied togenotoxicity and carcinogenesis studies: A critical review. Mutat. Res. 2006;613:76–102. [DOI] [PubMed] [Google Scholar]
- 150.Enan MR. Application of random amplified polymorphic DNA (RAPD) to detect the genotoxic effect of heavy metals. Biotechnol. Appl. Biochem. 2006;43:147–54. doi: 10.1042/BA20050172 [DOI] [PubMed] [Google Scholar]
- 151.Siddiqui S. DNA damage in Cicer </i>; plant grown on soil polluted with heavy metals. JKSUS. 2015;27:217–23. [Google Scholar]
- 152.Lin AJ, Zhang XH, Chen MM, Cao Q. Oxidative stress and DNA damages induced by cadmium accumulation. J. Environ. Sci. 2007;19:596–602. [DOI] [PubMed] [Google Scholar]
- 153.Roy S. Function of MYB domain transcription factors in abiotic stress and epigenetic control of stress response in plant genome. Plant Signal Behav. 2016;11(1):e1117723. doi: 10.1080/15592324.2015.1117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nakashima K, Ito Y, Yamaguchi-Shinozaki K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 2009;149:88–95. doi: 10.1104/pp.108.129791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dal Corso G, Farinati S, Furini A. Regulatory networks of cadmium stress in plants. Plant Signal Behav. 2010;5(6):663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roy SK, Cho SW, Kwon SJ, Kamal AH, Kim SW, Oh MW, Lee MS, Chung KY, Xin Z, Woo SH. Morpho-Physiological and proteome level responses to cadmium stress in sorghum. Plos One. 2016;11(2):e0150431. doi: 10.1371/journal.pone.0150431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zou J, Wang G, Ji J, Wang J, Wu H, Ou Y, Li B. Transcriptional, physiological and cytological analysis validated the roles of some key genes linked Cd stress in Salix matsudana Koidz. Environ. Exper. Bot. 2017;134:116–29. 10.1016/j.envexpbot.2016.11.005. [DOI] [Google Scholar]
- 158.Ramos J, Clemente MR, Naya L, Loscos J, Perez-Rontome C, Sato S. Phytochelatin synthases of themodellegume lotusjaponicus. a small multigene family with different responses to cadmium and alternatively spiced variants. Plant Physiol. 2007;143:110–8. doi: 10.1104/pp.106.090894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006;140:411–32. doi: 10.1104/pp.105.073783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Herbette S, Taconnat L, Hugouvieux V, Piette L, Magniette ML, Cuine S, Auroy P, Richaud P, Forestier C, Bourguignon J, et al. . Genome-wide transcriptome profiling of the early cadmium Response of Arabidopsis roots and shoots. Biochie. 2006;88:1751–65. doi: 10.1016/j.biochi.2006.04.018 [DOI] [PubMed] [Google Scholar]
- 161.Opdenakker K, Remans T, Keunen E, Vangronsveld J, Cuypers A. Exposure of Arabidopsis thaliana to Cd or Cu excess leads to oxidative stress Mediated alterations in MAP kinase transcript levels. Environ. Exp. Bot. 2012;83:53–61. doi: 10.1016/j.envexpbot.2012.04.003 [DOI] [Google Scholar]
- 162.Castrillo G, Sánchez-Bermejo E, deLorenzo L, Crevillén P, Fraile-Escanciano A, Tc M, Mouriz A, Catarecha P, Sobrino-Plata J, Olsson S, et al. . WRKY6 Transcription factor restrict sarsenate uptake and transposon activation in Arabidopsis. Plant Cell. 2013;25:2944–57. doi: 10.1105/tpc.113.114009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.van de Mortel JE, Schat H, Moerland PD, Ver Loren van Themaat E, van der Ent S, Blankestijn H, Ghandilyan A, Tsiatsiani S, Aarts MG. Expression differences for genes involved in lignin, glutathione and sulphate metabolism in response to cadmium in Arabidopsis thaliana and the related Zn/Cd-hyperaccumulator Thlaspicaerulescens. Plant Cell Environ. 2008;31(3):301–24. [DOI] [PubMed] [Google Scholar]
- 164.Oono Y, Yazawa T, Kawahara Y, Kanamori H, Kobayashi F, Sasaki H H, Mori S, Wu J, Handa H, Itoh T, Matsumoto T, et al. . Genome-wide transcriptome analysis reveals that cadmium stress signaling controls the expression of genes in drought stress signal pathways in rice. PLoS ONE. 2014;9(5):e96946 10.1371/journal.pone.0096946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sivitz AB, Hermand V, Curie C, Vert G. Arabidopsis bHLH100 and bHLH101 control iron homeostasis via a FIT-independent pathway. PLoS ONE. 2012;7(9):e44843 10.1371/journal.pone.0044843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Palmer CM, Hindt MN, Schmidt H, Clemens S, Guerinot ML. MYB10 and MYB72 are required for growth under iron-limiting conditions. PLoS Genet. 2013;9(11):e1003953 10.1371/journal.pgen.1003953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li X, Zhang H, Ai Q, Liang G, Yu D. Two bHLH transcription factors, bHLH34 and bHLH104, regulate iron homeostasis in arabidopsis thaliana. Plant Physiol. 2016;170(4):2478–93. doi: 10.1104/pp.15.01827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singh K, Foley RC, Oñate-Sánchez L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002;5:430–6. doi: 10.1016/S1369-5266(02)00289-3 [DOI] [PubMed] [Google Scholar]
- 169.Shiu SH, Shih MC, Li WH. Transcription factor families have Much higher expansion rates in plants than in animals. Plant Physiol. 2005;139:18–26. doi: 10.1104/pp.105.065110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wei W, Zhang Y, Han L, Guan Z, Chai T. A novel WRKY transcriptional factor from Thlaspicaerulescens negatively regulates the osmotic stress tolerance of transgenic tobacco. Plant Cell Rep. 2008;27(4):795–803. [DOI] [PubMed] [Google Scholar]
- 171.Wu H, Chen C, Du J, Liu H, Cui Y, Zhang Y, He Y, Wang Y, Chu C, Feng Z, et al. . Co-overexpression FIT with AtbHLH38 or AtbHLH39 in Arabidopsis-enhance Cadmium tolerance via increased cadmium sequestration in roots and improved iron homeostasis of shoots. Plant Physiol. 2012;158:790–800. doi: 10.1104/pp.111.190983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fusco N, Micheletto L, Dal Corso G, Borgato L, Furini A. Identification of cadmium-regulated genes by cDNA-AFLP in the heavy metal accumulator Brassica juncea L.. J. Exp. Bot. 2005;56:3017–27. [DOI] [PubMed] [Google Scholar]
- 173.Suzuki N, Koizumi N, Sano H. Screening of cadmiumresponsive genes in Arabidopsis thaliana. Plant Cell Environ. 2001;2:1177–88. [Google Scholar]
- 174.Farinati S, DalCorso G, Varotto S, Furini A. The brassica juncea BjCdR15, an ortholog of arabidopsis TGA3, is a regulator of cadmium uptake, transport and accumulation in shoots and confers cadmium tolerance in transgenic plants. New Phytol. 2010;185:964–78. [DOI] [PubMed] [Google Scholar]
- 175.Wang H, Klatte M, Jakoby M, Bäumlein H, Weisshaar B, Bauer P. Iron deficiency-mediated stress regulation of four subgroup Ib BHLH genes in Arabidopsis thaliana. Planta. 2007;226:897–908. [DOI] [PubMed] [Google Scholar]
- 176.Yuan YX, Zhang J, Wang DW, Ling HQ. AtbHLH29 of Arabidopsis thaliana is a functional ortholog of tomato FER involved in controlling iron acquisition in strategy I plants. Cell Res. 2005;15:613–21 [DOI] [PubMed] [Google Scholar]
- 177.Yuan Y, Wu H, Wang N, Li J, Zhao W, Du J, Wang D, Ling H. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res. 2008;18:385–97. [DOI] [PubMed] [Google Scholar]
- 178.Olsson PE, Kling P, Erkell LJ, Kille P. Structural and functional analysis of the rainbow trout (Oncorhyncusmykiss) metallothionein-A gene. Eur. J. Biochem. 1995;230:344–9. [DOI] [PubMed] [Google Scholar]
- 179.Chuang JC, Jones PA. Epigenetics and micro RNAs. Pediatr. Res. 2007;61:24–9. [DOI] [PubMed] [Google Scholar]
- 180.Grewal SI, Moazed D. Heterochromatin and epigenetic control of gene expression. Science. 2003;301:798–802. [DOI] [PubMed] [Google Scholar]
- 181.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–93. [DOI] [PubMed] [Google Scholar]
- 182.Yaish MW. DNA methylation-associated epigenetic changes in stress tolerance of plants. Molecular stress physiology of plants. India: Springer; 2013. p. 427–40 doi: 10.1007/978-81-322-0807-5_17 [DOI] [Google Scholar]
- 183.Boyko A, Kovalchuk I. Genetic and epigenetic effects of plant-pathogeninteractions: An evolutionary perspective, Mol. Plant. 2011;4:1014–102310. 1093/mp/ssr022 [DOI] [PubMed] [Google Scholar]
- 184.Boyko A, Kovalchuk I. Genome instability and epigenetic modification-heritable responses to environmental stress? Curr. Opin. Plant Biol. 2011;14:260–6. 10.1016/j.pbi.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 185.Mirouze M, Paszkowski J. Epigenetic contribution to stress adaptation in Plants. Curr. Opin. Plant Biol. 2011;14:267–74. doi: 10.1016/j.pbi.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 186.Rahavi MR, Migicovsky Z, Titov V, Kovalchuk I. Transgenerational adaptation to heavy metal salts in Arabidopsis. Front. Plant Sci. 2011;2:91 10.3389/fpls.2011.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Molinier J, Oakeley EJ, Niederhauser O, Kovalchuk I, Hohn B. Dynamic response of plant genome to ultraviolet radiation and other genotoxic stresses. Mutat. Res. 2005;571:235–47. [DOI] [PubMed] [Google Scholar]
- 188.Boyko A, Kovalchuk I. Epigenetic control of plant stress response. Environ. Mol. Mutagen. 2008;49:61–72. [DOI] [PubMed] [Google Scholar]
- 189.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Aina R, Sgorbati S, Santagostino A, Labra M, Ghiani A, Citterio S. Specific hypomethylation of DNA is induced by heavy metals in white clover and industrial hemp. Physiol. Plant. 2004;121:472–80. [Google Scholar]
- 191.Ding H, Gao J, Qin C, Ma H, Huang H, Song P, Luo X, Lin H, Shen Y, Pan G, et al. . The dynamics of DNA methylation in maize roots under Pb stress. Int. J. Mol. Sci. 2014;15:23537–54. 10.3390/ijms151223537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ou X, Zhang Y, Xu C, Lin X, Zang Q, Zhuang T, Jiang L, von Wettstein D, Liu B. Transgenerational inheritance of modified DNA methylation patterns and enhanced tolerance induced by heavy metal stress in rice (Oryza sativaL.). PLoS One. 2012;7:e41143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bilichak A, Kovalchuk I. Transgenerational response to stress in plants and its application for breeding. J. Exp. Bot. 2016;67:2081–92. [DOI] [PubMed] [Google Scholar]
- 194.Molinier J, Ried G, Zipfel C, Hohn B. Transgeneration memory of stressin plants. Nature. 2006;442:1046–9 [DOI] [PubMed] [Google Scholar]
- 195.Turner BM. Epigenetic responses to environmental change and their evolutionary implications. Phil. Trans. R. Soc. B. 2009;364:3403–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lukens LN, Zhan SH. The plant genome's methylation status andresponse to stress: Implications for plant improvement, Curr. Opin. Plant Bio. 2007;10:317–22. [DOI] [PubMed] [Google Scholar]
- 197.Polo SE, Almouzni G. Chromatin assembly, a basic recipe with various flavours. Curr. Opin. Genet. Dev. 2006;16:104–11. 10.1016/j.gde.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 198.Ramirez-Parra E, Gutierrez C. E2F regulates FASCIATA1, a chromatinassembly gene whose loss switches on the endocycle and activates gene expression by changing the epigenetic status. Plant Physiol. 2007;144:105–20. doi: 10.1104/pp.106.094979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang Y, Reinberg D. Transcription regulation by histone methylation interplay between different covalent modifications of the core histone tails. Gene Dev. 2001;15:2343–60. doi: 10.1101/gad.927301 [DOI] [PubMed] [Google Scholar]
- 200.Bartee L, Malagnac F, Bender J. Arabidopsis cmt3 chromomethylase mutations block non-CG methylation and silencing of an endogenous gene. Gene Dev. 2001;15:1753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pandey R, Muller A, Napoli CA, Selinger DA, Pikaard CS, Richards EJ, Bender J, Mount DW, Jorgensen RA. Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 30 (2002) 5036–55. doi: 10.1093/nar/gkf660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pokholok DK, Harbison CT, Levine S, Cole M, Hannett NM, Lee TI, Bell GW, Walker K, Rolfe PA, Herbolsheimer E, et al. . Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517–27. doi: 10.1016/j.cell.2005.06.026 [DOI] [PubMed] [Google Scholar]
- 203.Cao X, Jacobsen SE. Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proc. Natl. Acad. Sci. USA. 2002;99(SUPPL. 4):16491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kim JM, To TK, Ishida J, Morosawa T, Kawashima M, Matsui A., Toyoda T, Kimura H, Shinozaki K, Seki M. Alterations of lysine modifications on the histone H3N-tail under drought stress conditions in Arabidopsis thaliana. Plant Cell Physiol. 2008;49:1580–8. doi: 10.1093/pcp/pcn133 [DOI] [PubMed] [Google Scholar]
- 205.Lindroth AM, Cao X, Jackson JP, Zilberman D, McCallum CM, Henikoff S, Jacobsen SE. Requirement of CHROMOMETHYLASE3 for maintenance of CpXpGmethylation. Science. 2001;292:2077–80. [DOI] [PubMed] [Google Scholar]
- 206.Jullien PE, Berger F. DNA methylation reprogramming during plantsexual reproduction? Trends Genet. 2010;26:394–9. [DOI] [PubMed] [Google Scholar]
- 207.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010;11:204–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chen H, Ke Q, Kluz T, Yan Y, Costa M. Nickel ions increase histone H3 lysine 9 dimethylation and induce transgene silencing. Mol. Cell Biol. 2006;26:3728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tchou-Wong KM, Kiok K, Tang Z, Kluz T, Arita A, Smith PR, Brown S, Costa M. Effects of nickel treatment on H3K4 trimethylation and gene expression. PLoS ONE. 2011;6(3):e17728 10.1371/journal.pone.0017728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sun H, Shamy M, Costa M. Nickel and epigenetic gene silencing. Genes. 2013;4:583–95. doi: 10.3390/genes4040583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhao L, Wang P, Hou H, Zhang H, Wang Y, Yan S, Huang Y, Li H, Tan J, Hu A, et al. . Transcriptional regulation of cell cycle genes in response to abiotic stresses correlates with dynamic changes in histone modifications in maize. PLoS ONE. 2014;9(8):e106070. doi: 10.1371/journal.pone.0106070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C. Food security: The challenge of feeding 9 billion people. Science. 2010;327:812–8. [DOI] [PubMed] [Google Scholar]
- 213.FAO Statistical year book viale delle terme di caracalla. Rome 2012. [Google Scholar]
- 214.Hazell P, Wood S. Drivers of change in global agriculture. Philos. Trans. R. Soc. B. 2008;363:495–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chinnusamy V, Zhu JK. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009;12(2):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


