ABSTRACT
Plants are constantly exposed to numerous diverse microbes and pests. They lack an adaptive immune system and rely on innate immunity to perceive and ward off potential pathogens. The plant immune system enables plants to overcome invading microorganisms, and can be defined as highly successful in this regard. Nevertheless, specialized pathogens are able to overcome structural barriers, preformed defenses, innate immunity and are a persistent threat to crop and food supplies worldwide. The rapidly growing world population results in massive demands for agricultural products and reliable crop yields. Therefore, the ability to precisely manipulate plant immunity to resist diverse diseases holds significant promise for enhancing crop production.
Keywords: plant immunity, nucleotide binding leucine rich repeat proteins, pattern-triggered immunity, pattern recognition receptors
Plant immunity is conventionally depicted as a two-tiered system. The first line of plant defense is formed by pattern recognition receptors (PRRs), located at the cell surface, that recognize microbe-associated molecular patterns (MAMPs) and trigger pattern-triggered immunity (PTI).1 Successful pathogens are able to overcome plant PTI using secreted effectors that suppress PTI, leading to plant susceptibility. Concomitantly, plants evolved the second line of defense based on cytoplasmic immune receptors that recognize effectors (Avrs) and deploy effector-triggered immunity (ETI).2,3 However, accumulating evidence from the last decade blurs the distinction between the two systems and reveals significant overlap and diversity in immune signaling networks.4–6
Blurred distinction
MAMPs are traditionally defined as highly conserved moieties within a class of microbes, essential for microbial fitness,7,8 while effectors are considered to be species, race or strain specific and confer virulence.9,10 However, several MAMPs and effectors refuse to follow these rules, creating exceptions to this man-made distinction. For instance, the necrosis-induced peptide 1 (NEP1) and NEP1-like proteins are effectors required for virulence, but they are conserved among bacteria, fungi and oomycetes.4,5 Other examples of widespread effectors required for pathogen virulence are extracellular protein 6 (Ecp6) and Ecp6 conserved orthologs – LysM effectors, harpin effectors and crinkler effectors that are widely conserved in fungi, Gram-negative bacteria and oomycetes, respectively.4,5 On the other hand, we find examples of MAMPs with a very narrow known distribution such as Ax21 and Pep-13 that are present in only a few Xanthomonas strains and Phytophthora species, respectively.4,5 Additionally, MAMPs such as flagellin, lipopolysaccharide, peptidoglycan, chitin, EIX and PWL (pathogenicity toward weeping lovegrass) contribute to pathogen virulence.4,5
The distinction between the outcomes of PTI and ETI can also be confusing. ETI is frequently noted as leading to a more rapid, sustainable and stronger immune response, culminating in cell death known as the hypersensitive response (HR).2,11 Nonetheless, we know of several MAMPs that are able to induce HR, among them flagellin, CBEL and EIX.12–15 Meanwhile, we can find weak ETI responses like the ones mediated by RPS4 and Ve1, which recognize Pseudomonas and Verticillium effectors, respectively.16,17
PTI is mediated by cell surface PRRs that can be classified, based on their domains, into different categories. The largest category is that of extracellular leucine-rich repeat (eLRR) containing receptors, and can be further divided into receptor like proteins and kinases (eLRR-RLP/K).18 ETI is frequently mediated by cytoplasmic receptors, which are primarily nucleotide binding leucine rich repeat (NLR) proteins.19 However, there are several examples of eLRR-RLP type cell surface receptors that can recognize effectors and mediate ETI, such as Cf2, Cf4, Hcr9-4E, Cf5, Cf9, Ve1, l and LepR3/RLM2,20–27 and even an eLRR-RLK – I-3.28 Given the gradient of responses that can be induced by either MAMP or effector recognition, it is now becoming clear that the responses governed by diverse plant immune receptors are integrated in diverse manners.6
Convergent immune signaling
Despite the diversity of pathogen components recognized and strength of immune responses, there are commonalities in defense signaling outputs, indicating that recognition of both MAMPs and effectors utilize similar signaling networks. PTI induces rapid activation of MAPK cascades which leads to the activation of downstream signaling pathways.29 Unsurprisingly, several effectors target MAPK cascade components in order to disrupt PTI.30–34 More interestingly, there are cases of ETI leading to activation of the same PTI-MAPK cascades.35 In addition, PTI/ETI share other signaling pathways including ROS signaling, Ca2+ signaling, hormone signaling and substantial overlapping transcriptional regulation, resulting in plant immune responses- as recently reviewed by Peng et al.36 These evidences demonstrate a certain level of convergence in PTI/ETI signaling.
Helper NRCs as signaling convertors
In the classical gene-for-gene model proposed by Harold Flor in 1942, an R gene typically encodes an NLR receptor that detects and responds to an effector (Avr) gene product.37,38 More recently, a diversity of recognition mechanisms have been revealed.3 One additional model demonstrates that NLRs can function in pairs, with one NLR functioning as a sensor- for effector detection (s-NLR), and the other as a helper- initiating immune signaling (h-NLR),39,40 (Figure 1,. One specific NLR family in Solanaceae, termed NLR required for cell death (NRC), emerges as a key family of h-NLR required signaling downstream of multiple s-NLRs.41 Recent publications indicates NRCs function not only as h-NLRs mediating s-NLR signaling, but also as h-NLRs mediating MAMP and effector signaling sensed by diverse eLRR-receptors. NRC2 and NRC3 from N. benthamiana were shown to be involved in defense responses mediated by the eLRR-RLP Cf4, which recognizes the Avr4 effector from Cladosporium fulvum.42 We have recently described tomato NRC4 as associated with an eLRR-RLP required for perception of a fungal MAMP – EIX (LeEIX2) and with an eLRR-RLK required for perception of bacterial flagellin (FLS2), enhancing defense responses mediated by them.43 We have further shown that NRC4 is required for LeEIX2 and likely also FLS2-mediated defense responses. Furthermore, NRC4’s N-terminal coiled-coil domain is sufficient to mediate the association with LeEIX2 and can enhance EIX and flagellin- elicited defense responses as efficiently as the full length protein.43 Earlier works have indicated that tomato NRC1 may be involved in defense responses sensed by the eLRR-RLPs Cf4, Cf9, Ve1 and LeEIX2 as well.44 Keeping with the sensor-helper model, the eLRR receptors – Cf4, Cf9, Ve1, LeEIX2 and FLS2 can be defined as sensors, detecting effectors and MAMPs. In this context, eLRR-RLP/K act as sensor (s-RLP/K), while NRCs keep their role as an h-NLR (Figure 1). We propose that taken together, these data essentially position NRCs as a key signaling node required for the initiation of signaling sensed in both PTI and ETI immune pathways (Figure 1).
Figure 1.
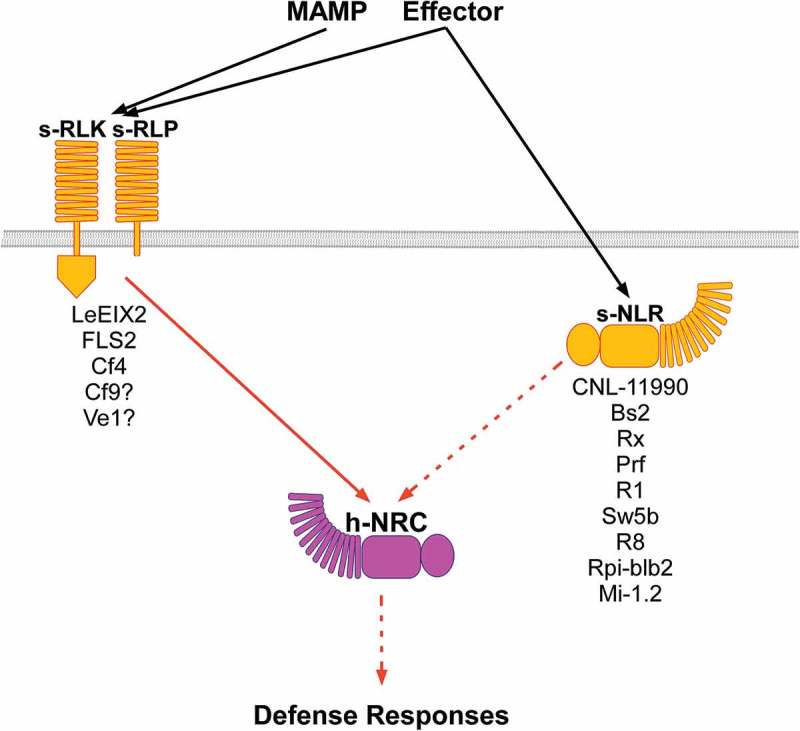
Schematic overview of plant defense signaling mediated by the NRC helper NLR (h-NRC). Perception of MAMPs or effectors by extracellular or intracellular immune receptors act as pathogen sensors (s-RLK, s-RLP, s-NLR) and lead to initiation of immune-signaling by a family of helper NLRs (h-NRC).
In accordance with this concept, we have recently shown that DNA manipulation (using CRISPR-cas9 editing) of tomato NRC4 leads to significant enhancement of immunity. NRC4 CRISPRed plants, encoding a 67 aa truncated variant, displayed intensified defense responses when challenged with EIX, and presented a higher resistance to B.cinerea.43 Our work, together with previous publications,41–44 positions NRC as a signaling funnel for multiple PTI and ETI sensor-receptors, demonstrating that CRISPR editing of NRCs could potentially result in agriculturally improved Solanaceae varieties possessing resistance to a broad spectrum of pathogens.
Funding Statement
This work was supported by the Chief Scientist of the Israel Ministry of Agriculture and Rural Development [13-37-0001];BARD, The United States — Israel Binational Agriculture Research and Development Fund [IS-4842-15];United States - Israel Binational Science Foundation [2013227];
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Boutrot F, Zipfel C.. Function, discovery, and exploitation of plant pattern recognition receptors for broad-spectrum disease resistance. Annu Rev Phytopathol. 2017;55:257–286. doi: 10.1146/annurev-phyto-080614-120106. [DOI] [PubMed] [Google Scholar]
- 2.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 3.Cui H, Tsuda K, Parker JE. Effector-triggered immunity: from pathogen perception to robust defense. Annu Rev Plant Biol. 2015;66:487–511. doi: 10.1146/annurev-arplant-050213-040012. [DOI] [PubMed] [Google Scholar]
- 4.Thomma BP, Nurnberger T, Joosten MH. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell. 2011;23:4–15. doi: 10.1105/tpc.110.082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidhyasekaran P, editor. PAMP signaling in plant innate immunity In: PAMP signals in plant innate immunity. signaling and communication in plants. Dordrecht: Springer; 2014. p. 17–161. [Google Scholar]
- 6.Wu CH, Derevnina L, Kamoun S. Receptor networks underpin plant immunity. Science. 2018;360:1300–1301. doi: 10.1126/science.aat2623. [DOI] [PubMed] [Google Scholar]
- 7.Nurnberger T, Brunner F. Innate immunity in plants and animals: emerging parallels between the recognition of general elicitors and pathogen-associated molecular patterns. Curr Opin Plant Biol. 2002;5:318–324. [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R, Janeway CA Jr.. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. [DOI] [PubMed] [Google Scholar]
- 9.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Bent AF, Mackey D. Elicitors, effectors, and R genes: the new paradigm and a lifetime supply of questions. Annu Rev Phytopathol. 2007;45:399–436. doi: 10.1146/annurev.phyto.45.062806.094427. [DOI] [PubMed] [Google Scholar]
- 11.Tsuda K, Katagiri F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol. 2010;13:459–465. doi: 10.1016/j.pbi.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Naito K, Taguchi F, Suzuki T, Inagaki Y, Toyoda K, Shiraishi T, Ichinose Y. Amino acid sequence of bacterial microbe-associated molecular pattern flg22 is required for virulence. Mol Plant Microbe Interact. 2008;21:1165–1174. doi: 10.1094/MPMI-21-9-1165. [DOI] [PubMed] [Google Scholar]
- 13.Hann DR, Rathjen JP. Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana. Plant J. 2007;49:607–618. doi: 10.1111/j.1365-313X.2006.02981.x. [DOI] [PubMed] [Google Scholar]
- 14.Khatib M. LC, Esquerré-Tugayé M.-T., Bottin A., Rickauer M. The CBEL elicitor of Phytophthora parasitica var. nicotianae activates defence in Arabidopsis thaliana via three different signalling pathways. New Phytol. 2004;162:501–510. doi: 10.1111/j.1469-8137.2004.01043.x. [DOI] [Google Scholar]
- 15.Ron M, Avni A. The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell. 2004;16:1604–1615. doi: 10.1105/tpc.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Esse HP, Fradin EF, De Groot PJ, De Wit PJ, Thomma BP. Tomato transcriptional responses to a foliar and a vascular fungal pathogen are distinct. Mol Plant Microbe Interact. 2009;22:245–258. doi: 10.1094/MPMI-22-3-0245. [DOI] [PubMed] [Google Scholar]
- 17.Wirthmueller L, Zhang Y, Jones JD, Parker JE. Nuclear accumulation of the Arabidopsis immune receptor RPS4 is necessary for triggering EDS1-dependent defense. Curr Biol. 2007;17:2023–2029. doi: 10.1016/j.cub.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 18.Liang X, Zhou JM. Receptor-Like Cytoplasmic Kinases: central Players in Plant Receptor Kinase-Mediated Signaling. Annu Rev Plant Biol. 2018;69:267–299. doi: 10.1146/annurev-arplant-042817-040540. [DOI] [PubMed] [Google Scholar]
- 19.Dodds PN, Rathjen JP. Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet. 2010;11:539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 20.Catanzariti AM, Do HT, Bru P, De Sain M, Thatcher LF, Rep M, Jones DA. The tomato I gene for Fusarium wilt resistance encodes an atypical leucine-rich repeat receptor-like protein whose function is nevertheless dependent on SOBIR1 and SERK3/BAK1. Plant J. 2017;89:1195–1209. doi: 10.1111/tpj.13458. [DOI] [PubMed] [Google Scholar]
- 21.Dixon MS, Jones DA, Keddie JS, Thomas CM, Harrison K, Jones JD. The tomato Cf-2 disease resistance locus comprises two functional genes encoding leucine-rich repeat proteins. Cell. 1996;84:451–459. [DOI] [PubMed] [Google Scholar]
- 22.Thomas CM, Jones DA, Parniske M, Harrison K, Balint-Kurti PJ, Hatzixanthis K, Jones JD. Characterization of the tomato Cf-4 gene for resistance to Cladosporium fulvum identifies sequences that determine recognitional specificity in Cf-4 and Cf-9. Plant Cell. 1997;9:2209–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westerink N, Brandwagt BF, De Wit PJ, Joosten MH. Cladosporium fulvum circumvents the second functional resistance gene homologue at the Cf-4 locus (Hcr9-4E) by secretion of a stable avr4E isoform. Mol Microbiol. 2004;54:533–545. doi: 10.1111/j.1365-2958.2004.04288.x. [DOI] [PubMed] [Google Scholar]
- 24.Dixon MS, Hatzixanthis K, Jones DA, Harrison K, Jones JD. The tomato Cf-5 disease resistance gene and six homologs show pronounced allelic variation in leucine-rich repeat copy number. Plant Cell. 1998;10:1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones DA, Thomas CM, Hammond-Kosack KE, Balint-Kurti PJ, Jones JD. Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science. 1994;266:789–793. [DOI] [PubMed] [Google Scholar]
- 26.Larkan NJ, Lydiate DJ, Parkin IA, Nelson MN, Epp DJ, Cowling WA, Rimmer SR, Borhan MH. The Brassica napus blackleg resistance gene LepR3 encodes a receptor-like protein triggered by the Leptosphaeria maculans effector AVRLM1. New Phytol. 2013;197:595–605. doi: 10.1111/nph.12043. [DOI] [PubMed] [Google Scholar]
- 27.Fradin EF, Zhang Z, Juarez Ayala JC, Castroverde CD, Nazar RN, Robb J, Liu CM, Thomma BP. Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol. 2009;150:320–332. doi: 10.1104/pp.109.136762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catanzariti AM, Lim GT, Jones DA. The tomato I-3 gene: a novel gene for resistance to Fusarium wilt disease. New Phytol. 2015;207:106–118. doi: 10.1111/nph.13348. [DOI] [PubMed] [Google Scholar]
- 29.Meng X, Zhang S. MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol. 2013;51:245–266. doi: 10.1146/annurev-phyto-082712-102314. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Wu Y, Gao M, Zhang J, Kong Q, Liu Y, Ba H, Zhou J, Zhang Y. Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe. 2012;11:253–263. doi: 10.1016/j.chom.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Liu Y, Huang H, Gao M, Wu D, Kong Q, Zhang Y. The NLR protein SUMM2 senses the disruption of an immune signaling MAP kinase cascade via CRCK3. EMBO Rep. 2017;18:292–302. doi: 10.15252/embr.201642704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J, et al. A pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe. 2007;1:175–185. doi: 10.1016/j.chom.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Li J, Hou S, Wang X, Li Y, Ren D, Chen S, Tang X, Zhou JM. A pseudomonas syringae ADP-ribosyltransferase inhibits arabidopsis mitogen-activated protein kinase kinases. Plant Cell. 2010;22:2033–2044. doi: 10.1105/tpc.110.075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eschen-Lippold L, Jiang X, Elmore JM, Mackey D, Shan L, Coaker G, Scheel D, Lee J. Bacterial AvrRpt2-like cysteine proteases block activation of the arabidopsis mitogen-activated protein kinases, MPK4 and MPK11. Plant Physiol. 2016;171:2223–2238. doi: 10.1104/pp.16.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thulasi Devendrakumar K, Li X, Zhang Y. MAP kinase signalling: interplays between plant PAMP- and effector-triggered immunity. Cell Mol Life Sci. 2018. doi: 10.1007/s00018-018-2839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng Y, Van Wersch R, Zhang Y. Convergent and Divergent Signaling in PAMP-Triggered Immunity and Effector-Triggered Immunity. Mol Plant Microbe Interact. 2018;31:403–409. doi: 10.1094/MPMI-06-17-0145-CR. [DOI] [PubMed] [Google Scholar]
- 37.Flor HH. Inheritance of pathogenicity in Melampsora lini. Phytopathology. 1942;32:653–669. [Google Scholar]
- 38.Flor HH. Current status of gene-for-gene concept. Annu Rev Phytopathol. 1971;9:275-+. doi: 10.1146/annurev.py.09.090171.001423. [DOI] [Google Scholar]
- 39.Bonardi V, Tang S, Stallmann A, Roberts M, Cherkis K, Dangl JL. Expanded functions for a family of plant intracellular immune receptors beyond specific recognition of pathogen effectors. Proc Natl Acad Sci U S A. 2011;108:16463–16468. doi: 10.1073/pnas.1113726108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collier SM, Hamel LP, Moffett P. Cell death mediated by the N-terminal domains of a unique and highly conserved class of NB-LRR protein. Mol Plant Microbe Interact. 2011;24:918–931. doi: 10.1094/MPMI-03-11-0050. [DOI] [PubMed] [Google Scholar]
- 41.Wu CH, Abd-El-Haliem A, Bozkurt TO, Belhaj K, Terauchi R, Vossen JH, Kamoun S. NLR network mediates immunity to diverse plant pathogens. Proc Natl Acad Sci U S A. 2017;114:8113–8118. doi: 10.1073/pnas.1702041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu CH, Belhaj K, Bozkurt TO, Birk MS, Kamoun S. Helper NLR proteins NRC2a/b and NRC3 but not NRC1 are required for Pto-mediated cell death and resistance in Nicotiana benthamiana. New Phytol. 2016;209:1344–1352. doi: 10.1111/nph.13764. [DOI] [PubMed] [Google Scholar]
- 43.Leibman-Markus M, Pizarro L, Schuster S, Lin ZJD, Gershony O, Bar M, Coaker G, Avni A. The intracellular nucleotide binding leucine-rich repeat receptor - SlNRC4a enhances immune signaling elicited by extracellular perception. Plant Cell Environ. 2018. doi: 10.1111/pce.13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gabriels SH, Vossen JH, Ekengren SK, Van Ooijen G, Abd-El-Haliem AM, Van Den Berg GC, Rainey DY, Martin GB, Takken FL, De Wit PJ, et al. An NB-LRR protein required for HR signalling mediated by both extra- and intracellular resistance proteins. Plant J. 2007;50:14–28. doi: 10.1111/j.1365-313X.2007.03027.x. [DOI] [PubMed] [Google Scholar]


