Abstract
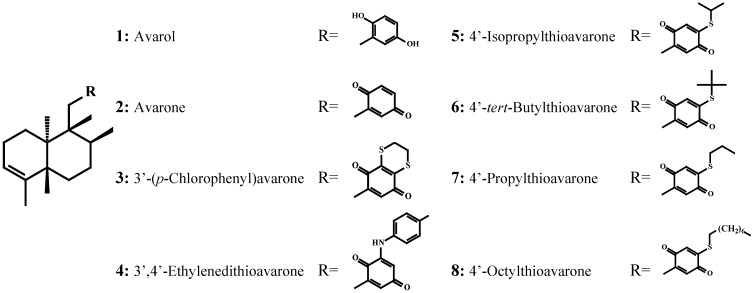
The sesquiterpene hydroquinone avarol (1) was isolated from the marine sponge Dysidea avara, whereas the corresponding quinone, avarone (2), was obtained by oxidation of avarol, and the significantly more lipophilic compounds [3’-(p-chloro-phenyl)avarone (3), 3’,4’-ethylenedithioavarone (4), 4’-isopropylthioavarone (5), 4’-tert-butylthioavarone (6), 4’-propylthioavarone (7), 4’-octylthioavarone (8)] were obtained by nucleophilic addition of thiols or p-chloroaniline to avarone. All these compounds were tested, at concentrations ranging from 0.5 to 50 μg/mL, for their effect on the settlement of the cyprid stage of Balanus amphitrite, for toxicity to both nauplii and cyprids and for their growth inhibitory activity on marine bacteria (Cobetia marina, Marinobacterium stanieri, Vibrio fischeri and Pseudoalteromonas haloplanktis) and marine fungi (Halosphaeriopsis mediosetigera, Asteromyces cruciatus, Lulworthia uniseptata and Monodictys pelagica).
Keywords: Antifouling activity, settlement inhibition, antimicrobial activity, avarol, avarone
Introduction
One of the most serious problems that marine technology is currently facing is the control of biofouling on man-made structures [1,2]. Efficient antifouling paints are based on copper compounds and booster biocides that when submerged, release toxic compounds causing adverse environmental effects [3,4,5,6,7,8]. Organotin-based paints have been linked to pollution of food webs and are of particular concern to human consumers [9,10]. Environmental protection is now a major concern in the search for new active compounds. New environmental regulations generate further restrictions on the use of biocides in industrial formulations [11] for the protection of rivers, lakes, estuaries, coastal waters and groundwater from further deterioration and for protection of biodiversity. There is clearly a need to develop new non-toxic or environmentally benign antifouling alternatives that would be efficient against the most severe fouling organisms such as barnacles, blue mussels, bryozoans and algae [12]. On one hand, there are solutions based on interference with biological adhesion to surfaces; the so-called foul-release coatings. On the other hand, there is an increasing interest in exploring the antifouling potential of natural compounds. In the marine environment, where all surfaces are constantly exposed to the threat of surface colonization, many sessile organisms remain relatively clean and control epibiont growth using effective antifouling mechanisms. In addition to physical defense mechanisms comprising structural elements made of lignin, CaCO3, silica, etc., sessile organisms such as sponges, soft corals, and seaweeds are known to elaborate chemical defense mechanisms against predation and epibiont growth. Many sponges have been shown to synthesize toxic metabolites to prevent predation, and because of this, frequently other organisms attach sponges to themselves for their protection [13].
In continuation of our studies towards the discovery of natural products that are effective against marine biofouling [14,15], we investigated barnacle settlement inhibition and marine bacterial growth inhibition with the sponge metabolites avarol (1), avarone (2) and the synthetic derivatives 3-8. Barnacles were selected as test organisms because they are one of the most significant forms of animal fouling [14]. We also assayed the same set of compounds on fouling bacteria since it is widely accepted that biofilm formation is problematic on even the most effective antifouling and foul-release coatings [16].
A great number of marine quinones and hydroquinones are of considerable interest with regard to their diverse biological activities. Furthermore, sesquiterpenes in which a decaline type unit and a quinoid moiety are structurally associated, often exhibit pronouced cytotoxicity [17,18,19,20]. Another reason that led us to evaluate the barnacle settlement inhibition of D. avara metabolites is that a significant number of other sponge metabolites have exhibited promising activities in an array of antifouling assays [21,22,23,24].
Results and Discussion
The redox couple avarol (1) and avarone (2), isolated for the first time from the sponge Dysidea avara by Minale and coworkers [25], has shown strong activity against mouse lymphoma cells [26,27,28], antiviral activity [29,30,31], antiinflammatory activity [32], antipsoriatic properties [33], as well as moderate antifungal and antibacterial activity against Gram-positive strains [34,35]. Intrigued by the wide range of biological activities of these metabolites and the fact that avarol (1) is biosynthesized in large amounts by the sponge, implying an important ecological role [36], we decided to investigate their antifouling activity and prepare a number of lipophilic derivatives that would reduce their solubility in sea water and improve their incorporation in a potential antifouling preparation.
Antimicrobial activity against Cobetia marina, Marinobacterium stanieri, Vibrio fischeri and Pseudoalteromonas haloplanktis
Compounds 1-8 were tested against the marine bacteria C. marina, M. stanieri, V. fischeri and P. haloplanktis (Table 1). Among the eight compounds tested, only compound 4 did not exhibit antimicrobial activity with a minimum inhibitory concentration (MIC) above 50µg/mL. High levels of inhibition against C. marina were observed with compounds 5 and 7 (MIC = 0.5 μg/mL), as well as with avarone (2) and compounds 3, 8 (MIC =1 μg/mL). Avarol (1) and 6 were noticeably less active with MIC of 2.5µg/mL and 5µg/mL, respectively.
Table 1.
Evaluation of antimicrobial activity (MIC, μg/mL).
| No | Compound | Cobetia marina | Marinobacterium stanieri | Vibrio fischeri | Pseudoalteromonas haloplanktis |
|---|---|---|---|---|---|
| 1 | Avarol | 2.5 | 5.0 | 10.0 | 10.0 |
| 2 | Avarone | 1.0 | 1.0 | 2.5 | 2.5 |
| 3 | 3’-(p-Chlorophenyl)avarone | 1.0 | 5.0 | 2.5 | 5.0 |
| 4 | 3’,4’-Ethylenedithioavarone | >50.0 | >50.0 | >50.0 | >50.0 |
| 5 | 4’-Isopropylthioavarone | 0.5 | 0.5 | 1.0 | 1.0 |
| 6 | 4’-tert-Butylthioavarone | 5.0 | 5.0 | 5.0 | 1.0 |
| 7 | 4’-Propylthioavarone | 0.5 | 0.5 | 1.0 | 1.0 |
| 8 | 4’-Octylthioavarone | 1.0 | 2.5 | 2.5 | 2.5 |
M. stanieri growth was strongly inhibited in the presence of compounds 5 and 7 (MIC = 0.5 μg/mL), and avarone (2) (MIC = 1 μg/mL). Compound 8 gave a MIC of 2.5µg/mL. MIC values of 5µg/mL were recorded when bacteria were incubated with avarol (1) and compounds 3 and 8.
Compounds 5 and 7 were the most efficient growth inhibitors of V. fischeri (MIC = 1.0 μg/mL). Moderate levels of activity with MIC ranging between 2.5 and 5 μg/mL were found for avarone (2), compounds 3, 8 and 6. The less active compound was found to be avarol (1) with a MIC = 10 μg/mL.
Growth of P. haloplanktis was strongly inhibited by compounds 5, 6 and 7, with MICs = 1μg/mL. Moderate levels of activity with MIC ranging between 2.5 and 5 μg/mL were found for avarone (2), 8 and 3. Finally, avarol (1) showed weak activity (MIC = 10 μg/mL).
Antifungal activity against Halosphaeriopsis mediosetigera, Asteromyces cruciatus, Lulworthia uniseptata and Monodictys pelagica
The inhibition of marine fungal growth by compounds 1-8 is presented in Table 2. Compounds 4 and 6 were inactive towards the four strains of marine fungi tested.
Table 2.
Evaluation of antifungal activity (MIC, μg/mL).
| No | Compound | Halosphaeriopsis mediosetigera | Asteromyces cruciatus | Lulworthia uniseptata | Monodictys pelagica |
|---|---|---|---|---|---|
| 1 | Avarol | 10.0 | 10.0 | 25.0 | 25.0 |
| 2 | Avarone | 1.0 | 2.5 | 1.0 | 1.0 |
| 3 | 3’-(p-Chlorophenyl)avarone | 25.0 | 25.0 | 25.0 | 50.0 |
| 4 | 3’,4’-Ethylenedithioavarone | >50.0 | >50.0 | >50.0 | >50.0 |
| 5 | 4’-Isopropylthioavarone | 5.0 | 10.0 | 10.0 | 5.0 |
| 6 | 4’-tert-Butylthioavarone | >50.0 | >50.0 | >50.0 | >50.0 |
| 7 | 4’-Propylthioavarone | 1.0 | 1.0 | 2.5 | 1.0 |
| 8 | 4’-Octylthioavarone | 10.0 | 10.0 | 5.0 | 5.0 |
The two most active compounds were avarone (2) and compound 7, which exhibited a MIC < 2.5 μg/mL against the four strains. Moderate levels of inhibition (MIC < 10.0 μg/mL) were obtained against all strains with compounds 5 and 8. Low levels of inhibition (MIC < 25.0 μg/mL) were recorded for avarol (1) and compound 3.
Cyprid settlement and mortality
The effects of compounds 1-8 on cyprid settlement and mortality are presented in Table 3. Only avarol (1) and avarone (2) were toxic at high concentrations. Avarol was the most toxic, with a concentration inducing 50% mortality in comparison to the control (LC50) of 13.28 µg/mL, while avarone had an LC50 of 27.12 µg/mL. All compounds had concentrations inhibiting settlement by 50% in comparison to the control (EC50) lower than the corresponding LC50. The three most active compounds were avarol (1), 3 and 7 with EC50 < 1 µg/mL. Avarone (2), 5, 6 and 8 had EC50 between 1 and 5 µg/mL. Compound 4 was the least active, with EC50 = 26.22 µg/mL.
Table 3.
Evaluation of anti-settlement and toxicity of compounds 1-8 on B. amphitrite.
| No | Compound | Balanus amphitrite | ||
|---|---|---|---|---|
| EC50 (μg/mL) | Cyprid LC50 (μg/mL) | Nauplii LC50 (μg/mL) | ||
| 1 | Avarol | 0.65 ± 0.03 | 13.28 ± 0.70 | 1.58 ± 0.05 |
| 2 | Avarone | 3.41 ± 0.12 | 27.12 ± 1.51 | 25.12 ± 0.93 |
| 3 | 3’-(p-Chlorophenyl)avarone | 0.65 ± 0.02 | >50.0 | >50.0 |
| 4 | 3’,4’-Ethylenedithioavarone | 26.22 ± 0.27 | >50.0 | >50.0 |
| 5 | 4’-Isopropylthioavarone | 1.33 ± 0.06 | >50.0 | >50.0 |
| 6 | 4’-tert-Butylthioavarone | 4.23 ± 0.12 | >50.0 | >50.0 |
| 7 | 4’-Propylthioavarone | 0.45 ± 0.02 | >50.0 | >50.0 |
| 8 | 4’-Octylthioavarone | 1.46 ± 0.05 | >50.0 | >50.0 |
Toxicity of compounds 1–8 to nauplii
The toxicity test results are presented in Table 3. Six compounds (3–8) showed no toxicity at the tested concentrations (LC50 >50 μg/mL). Mortality was noted only for avarol (1) and avarone (2) which had LC50 1.58 µg/mL and 25.12 µg/mL respectively.
Conclusions
Biofouling is one of the most serious problems the maritime domain currently faces. It has been estimated that the growth of marine fouling organisms costs the shipping and other marine industries over $6.5 billion per year [37]. Biofouling is considered to have four distinct stages, the first one starting from the moment a man-made object is immersed in water. The surfaces of these objects quickly accumulate dissolved organic matter and molecules, such as polysaccharides and protein fragments. Gradually, bacteria and single-cell diatoms sense the surface and start settling on it, forming a microbial film [38]. Subsequently, the adhesive substances and rough irregular microbial colonies trap more particles and organisms. Spores of algae, e.g. species of Enteromorpha intestinalis, Ulothrix zonata, marine fungi and ciliate protozoa soon appear on the film [39]. In the final stage, other marine organisms, such as barnacles, tunicates, mussels, bryozoans, polychaetes and tubeworms, settle on the submerged surfaces [40].
Many marine sponges, as well as other benthic organisms, are relatively free of settlement by fouling organisms [41] due to the production of biogenic compounds that possess antibacterial, antialgal, antifungal, antiprotozoan and antimacrofouling properties. Therefore, the isolation and production of these natural products from marine organisms could be used for the prevention of biofouling.
To gain a better understanding of chemical antifouling mechanisms of marine organisms, it is necessary: a) to identify the settling preferences of common fouling species, b) to determine the concentrations of secondary metabolites that settlers would experience in the field and c) to develop assay methodologies that deploy compounds in ecologically realistic ways [42].
For marine antifouling research, bioactive substances of particular interest should be ones that show deterrence properties and can be used for the development of antifouling coatings. Natural products rarely are available in sufficient quantity to be commercially harvested from marine macroorganisms. Moreover, most potent natural product compounds are structurally too complex to be commercially synthesized.
The observation that the sponge D. avara maintains an epibiont-free surface, in conjunction to the fact that the main metabolite avarol (1), which as already mentioned exhibits a wide range of biological activities, can be relatively easily synthesized in eight steps with a 28% overall yield [43], prompted us to evaluate its antifouling properties.
Avarol (1) showed promising antisettlement activity, but showed significant toxicity towards barnacle larvae. Therefore, unless rapidly biodegradable, it seemed unlikely to be a good candidate for new antifouling agents. For that reason, we proceeded with the preparation of simple synthetic derivatives aiming at an analogue that would maintain the levels of activity but without toxicity.
Avarone (2) and 3 showed significant antisettlement activity, but were almost equally toxic to barnacle larvae as avarol (1). Conversely, compound 4 possessed insufficient antimicrobial and antisettlement activity to be a promising antifoulant. The most active antisettlement compounds were 1, 3 and 7 with EC50 values < 1 µg/mL.
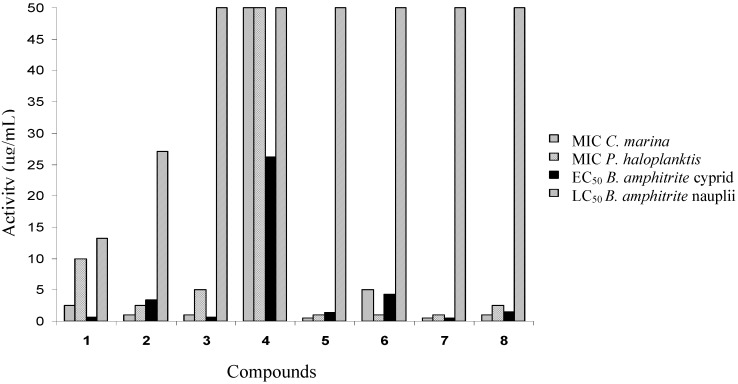
With the exception of compound 4, all tested compounds showed significant antibacterial activity. Compounds 5 and 7 were found to be the most active with MIC < 1 µg/mL. A comparison of activity in relation to structure suggests that steric hindrance exerted by bulky substituents on the quinone ring significantly reduced antimicrobial activity. Blockage of both positions 3’ and 4’ also deprived the compounds of their antimicrobial properties, as was the case for compound 4. The low activity of compounds 1 and 2 compared to some derivatives (3–8) can be explained by their higher polarity and therefore lower membrane permeability. A summary of all results obtained in this study is shown in Figure 2.
Figure 2.
Effect of compounds 1-8 on the growth of marine bacteria, settlement (EC50 in μg/mL) and mortality (LC50 in μg/mL) of Balanus amphitrite larvae.
The therapeutic ratio, LC50/EC50 is a way of expressing the effectiveness of the compound in relation to its toxicity. From the perspective of potency for use in an antifouling coating, the desired target ratio should be much greater than 1.0 [44].
Compounds 5 and 7 are of particular interest because they showed good antisettlement and antimicrobial activity at concentrations that were not acutely toxic to barnacle larvae, with an LC50/EC50 > 40, thus satisfying the above mentioned criterion. In the future, field assays are planned to determine how specific or broadly active these bioactive compounds are.
Experimental
Isolation of avarol (1) and avarone (2)
Dysidea avara specimens (5.1 kg wet tissue) were collected from the Gulf of Kotor, Monte Negro, Yugoslavia, at 5-15 m by SCUBA diving. The freshly collected sponge was initially freeze dried and then exhaustively extracted at room temperature with CH2Cl2/MeOH (2/1, v/v) mixtures. The residues were subjected to vacuum chromatography using silica gel and a step-wised gradient solvent system ranging from 100% cyclohexane to 100% ethyl acetate. The medium polarity fractions, containing avarone (2) and avarol (1) (identified by TLC comparison with authentic material) were further purified either by vacuum/column chromatography and HPLC until pure metabolites 1 (0.15% wet weight) and 2 (0.02% wet weight) were isolated. Structural elucidation of the natural products was based on their spectral data (NMR, MS, IR, UV) and comparison with literature values [25] (Figure 1).
Figure 1.
Structures of assayed compounds 1-8.
Preparation of compounds 3-8
Alkylthio and p-chlorophenylamino derivatives of avarone 3-8 were synthesized by nucleophilic addition of thiols and p-chloroaniline, respectively, to avarone, as previously described [45]. In a typical experiment, the nucleophile (1.6 mmol) was added to a solution of avarone (2, 1.6 mmol) in ethanol-water (1:1, 50 mL). To improve the reactivity of aliphatic thiols, the solution was made weakly alkaline with NaHCO3 and the reaction was carried out under an inert nitrogen atmosphere to prevent polymerization of the quinone moiety. Following purification by chromatographic methods, the structure of the obtained quinones was determined using 1H-NMR and 13C-NMR spectroscopy (Figure 1).
Antibacterial assays
Compounds were tested for inhibitory activity against four strains of marine bacteria: Cobetia marina (ATTC 25374), Marinobacterium stanieri (ATCC 27130), Vibrio fischeri (ATCC 7744) and Pseudoalteromonas haloplanktis (ATCC 14393). Experiments were performed as previously described [46]. Compounds (at concentrations of 0.5, 1, 2.5, 5, 10, 25 and 50μg/mL) were incubated with the bacteria (2x108 cells/mL) in 96-well plates (MERCK) in MHB medium (Mueller Hinton Broth, SIGMA), supplemented with NaCl (15g/L), at 25°C for 24 h. Each treatment and the seawater control were replicated six times. MICs compared to the seawater control, were determined by the microtitre broth dilution method [47].
Antifungal assays
Compounds were tested for inhibitory activity against four strains of marine fungi obtained from the culture collection of the School of Biological Sciences, University of Portsmouth, UK: Halosphaeriopsis mediosetigera, Asteromyces cruciatus, Lulworthia uniseptata and Monodictys pelagica. Experiments were performed as previously described [48]. Compounds (at final concentrations of 0.5, 1, 2.5, 5, 10, 25 and 50μg/mL) were incorporated into maize agar 12% (SIGMA) into Pyrex Petri dishes (15mm x 30mm, Fisher, UK). Plates were inoculated aseptically at their centre with an 8 mm diameter agar plug of mycelia. After incubation for 4 weeks at 25°C, MIC were determined. Assays were performed in duplicate.
Balanus amphitrite adult broodstock maintenance
Adult barnacles, Balanus amphitrite Darwin, were supplied courtesy of Prof. Dan Rittschof (Duke University Marine Laboratory, Beaufort, North Carolina). After removal of epibionts, adults were maintained in 20-L plastic aquaria containing 10 µm filtered and UV-irradiated seawater. Tanks were aerated and kept at 22oC on a 14:10 L:D cycle. The adults were fed daily with Artemia sp. nauplii and the water was changed every other day, at which time the adults were cleaned again as previously described [49].
Larval culture
Egg hatching was synchronized by drying adult barnacles overnight followed by re-immersing them in fresh seawater. Nauplii were attracted to a point source of light over a 1.5 h period and collected using a Pasteur pipette. Larvae were then transferred to a 2-L beaker containing filtered seawater. They were then reared to the cyprid stage by conventional methodology [40] in aerated 0.7 µm filtered seawater laced with antibiotics (streptomycin at 36.5 mg/mL and penicillin at 21.9 mg/mL) at 28°C on a 14:10 L:D cycle. Larvae were fed daily on a diet of Skeletonema costatum (1 L of 2x105 cells/mL) [50]. When the majority of larvae had metamorphosed into cyprids (4 days), the culture was filtered through a series of plankton mesh filters (300 µm, 250 µm and 160 µm). Cyprids were retained on the 250µm filter and subsequently transferred to fresh 0.45 µm filtered seawater [51] and stored at 6°C [52].
Algal culture
Seed cultures of S. costatum were purchased from Seasalter Shellfish Ltd (Whitstable, U.K.) and cultured in f/2 medium [53] at 18 °C under constant illumination. Cultures were enriched by gassing with CO2 (20 mL/min for 30 sec day-1).
Balanus amphitrite cyprid settlement assay
Settlement assays were conducted by adding 10-15 cyprids (3 days old) to the wells of a 24-well microplate (Iwaki) containing 2 mL of the compounds in seawater. The compounds were tested at concentrations of 0.5, 1, 2.5, 5, 10, 25 and 50 μg/mL. Test plates were incubated at 28 ºC in the dark and results were recorded after 24 hours incubation. Each larva was examined under a dissecting microscope and its condition recorded. Cyprids that did not move, had extended appendages and did not respond after a light touch by a metal probe were regarded as dead [54]. Both permanently attached and metamorphosed individuals were counted as settled, the remaining living cyprids were counted as swimmers. The EC50 and LC50 values of each compound were calculated using Sigma Plot (SYSTAT Software Inc.). All bioassays were conducted with six replicates per treatment.
Toxicity tests on Balanus amphitrite nauplii
Toxicity tests on nauplii of B. amphitrite were conducted as described [55]. Only positively phototactic stage II nauplii were used. Compounds were tested at concentrations of 0.5, 1, 2.5, 5, 10, 25 and 50 μg/mL with 6 replicates of each concentration and the seawater control. Tests were conducted by adding 10-15 nauplii to wells of a 24-well plate containing 2 mL of solution. The number of swimming nauplii was recorded after a 24 h exposure to the compounds. Non-swimming larvae were regarded as dead. LC50 values of each compound were calculated using Sigma Plot. All bioassays were conducted with six replicates per treatment.
Acknowledgments
The Greek research team for this project was co-funded by the European Social Fund and National Resources – (EPEAEK II) PYTHAGORAS II, the General Secretariat for the Research and Technology and the University of Athens. The British team was supported by awards from the US Office of Naval Research (N00014-02-1-0311) and from the Natural Environment Research Council (NER/B/S/ 2003/00273). Our special thanks to Ms S. Henry (School of Marine Science and Technology, Newcastle University, UK) for assistance with cyprid cultures and to Prof. D. Rittschof (Duke University Marine Laboratory, Beaufort, North Carolina, USA) for providing B. amphitrite adults. The Serbian team was supported by a bilateral research program of the Yugoslavian Federal Ministry for Development, Science and Environment.
Footnotes
Sample Availability: Samples of the compounds 1-8 are available from the authors.
References
- 1.Armstrong E., Boyd K., Burgess J. Prevention of marine biofouling using natural compounds from marine organisms. Biotech. Ann. Rev. 2000;6:221–241. doi: 10.1016/s1387-2656(00)06024-5. [DOI] [PubMed] [Google Scholar]
- 2.Rittschof D. Natural product antifoulants: one perspective on the challenges related to coatings developments. Biofouling. 2000;15:119–127. doi: 10.1080/08927010009386303. [DOI] [PubMed] [Google Scholar]
- 3.Bellas J. Comparative toxicity of alternative antifouling biocides on embryos and larvae of marine invertebrates. Sci. Total Environ. 2006;367:573–585. doi: 10.1016/j.scitotenv.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Braithwaite R.A., Fletcher R.L. The toxicity of Irgarol 1051 and Sea-Nine 211 to the non-target macroalga Fucus serratus with the aid of an image capture and analysis system. J. Exp. Mar. Biol. Ecol. 2005;322:111–121. doi: 10.1016/j.jembe.2005.01.015. [DOI] [Google Scholar]
- 5.Fernandez-Alba A.R., Hernando M.D., Piedra L., Chisti Y. Toxicity evaluation of single and mixed antifouling biocides measured with acute toxicity bioassays. Anal. Chim. Acta. 2002;456:303–312. [Google Scholar]
- 6.Kobayashi N., Okamura H. Effects of new antifouling compounds on the development of sea urchin. Mar. Pollut. Bull. 2002;44:748–751. doi: 10.1016/S0025-326X(02)00052-8. [DOI] [PubMed] [Google Scholar]
- 7.Kwok K.W.H., Leung K.M.H. Toxicity of antifouling biocides to the intertidal harpacticoid copepod Trigriopus japonicus (Crustacea, Copepoda): effects of temperature and salinity. Mar. Pollut. Bull. 2005;51:830–837. doi: 10.1016/j.marpolbul.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 8.i>Myers J.H., Gunthorpe L., Allinson G., Duda S. Effects of antifouling biocides to the germination and growth of the marine macroalga, Hormosira banksii (Turner) Desicaine. Mar. Pollut. Bull. 2006;52:1048–1055. doi: 10.1016/j.marpolbul.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Coehlo M.R., Bebianno M.J., Langston W.J. Routes of TBT uptake in the clam Rudiatpes decussates. II. Food as a vector of TBT uptake. Mar. Environ. Res. 2002;54:193–207. doi: 10.1016/s0141-1136(02)00106-x. [DOI] [PubMed] [Google Scholar]
- 10.Peterson S., Batley G., Scammell M. Tetracycline in antifouling paints. Mar. Pollut. Bull. 1993;26:96–100. doi: 10.1016/0025-326X(93)90098-5. [DOI] [Google Scholar]
- 11.Di Landa G., Ansanelli G., Ciccoli R., Cremisini C. Occurrence of antifouling paint booster biocides in selected harbors and marinas inside the Gulf of Napoli: a preliminary survey. Mar. Pollut. Bull. 2006;52:1541–1546. doi: 10.1016/j.marpolbul.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Dahlström M., Martensson L., Jonsson P., Amebrant T., Elwing H. Surface active adrenoreceptor compounds prevent the settlement of cyprid larvae of Balanus improvisus. Biofouling. 2000;16:191–198. [Google Scholar]
- 13.Clare A.S. Towards non-toxic antifouling. J. Mar. Biotechnol. 1998;6:3–6. [Google Scholar]
- 14.Hellio C., Tsoukatou M., Maréchal J.P., Aldred N., Beaupoil C., Clare A.S., Vagias C., Roussis V. Inhibitory effects of Mediterranean sponge extracts and metabolites on larval settlement of the barnacle, Balanus amphitrite. Mar. Biotechnol. 2005;7:297–305. doi: 10.1007/s10126-004-3150-x. [DOI] [PubMed] [Google Scholar]
- 15.Tsoukatou M., Hellio C., Vagias C., Harvala C., Roussis V. Chemical defense and antifouling activity of three Mediterranean sponges of the genus Ircinia. Z. Naturforsch. C. 2002;57:161–171. doi: 10.1515/znc-2002-1-227. [DOI] [PubMed] [Google Scholar]
- 16.Tang R.J., Cooney J.J. Effects of marine paints on microbial biofilm development on three materials. J. Ind. Microbiol. Biotechnol. 1998;20:275–280. [Google Scholar]
- 17.Sladić D., Gašić M.J. Reactivity and biological activity of the marine sesquiterpene hydroquinone avarol and related compounds from sponges of the order Dictyoceratida. Molecules. 2006;11:1–33. doi: 10.3390/11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer A.M.S., Hamann M.T. Marine pharmacology in 2000: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Mar. Biotechnol. 2004;6:37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blunt J.W., Copp B.R., Munro M.G.H., Northcote T.P., Princep M.R. Marine natural products. Nat. Prod. Rep. 2004;21:1–49. doi: 10.1039/b305250h. [DOI] [PubMed] [Google Scholar]
- 20.Mihopoulos N., Vagias C., Chinou I., Roussakis C., Scoullos M., Harvala C., Roussis V. Antibacterial and cytotoxic natural and synthesized hydroquinones from the sponge Ircinia spinosula. Z. Naturforsch. C. 1999;54:417–423. doi: 10.1515/znc-1999-5-618. [DOI] [PubMed] [Google Scholar]
- 21.Sera Y., Adachi K., Fujii K., Shizuri Y. A new antifouling hexapeptide from a Palauan sponge, Haliclona sp. J. Nat. Prod. 2003;66:719–721. doi: 10.1021/np020271i. [DOI] [PubMed] [Google Scholar]
- 22.i>Hirota H., Okino T., Yoshimura E., Fusetani N. Five new antifouling sesquiterpenes from two marine sponges of the genus Axinyssa and the nudibranch Phyllidia pustulosa. Tetrahedron. 1998;54:13791–13980. [Google Scholar]
- 23.Okino T., Yoshimura E., Hirota H., Fusetani N. Antifouling kalihinenes from the marine sponge Acanthella cavernosa. Tetrahedron Lett. 1995;36:8637–8640. doi: 10.1016/0040-4039(95)01861-B. [DOI] [Google Scholar]
- 24.Willemsen P. The screening of sponge extract for antifouling activity using a bioassay with laboratory reared cyprid larvae of the barnacle Balanus amphitrite. Int. Biodeter. Biodegr. 1994;10:361–373. doi: 10.1016/0964-8305(94)90094-9. [DOI] [Google Scholar]
- 25.Minale L., Riccio R., Sodano G. Avarol, a novel sesquiterpenoid hydroquinone with a rearranged drimane skeleton from the sponge Dysidea avara. Tetrahedron Lett. 1974;38:3401–3404. doi: 10.1016/S0040-4039(01)91918-5. [DOI] [Google Scholar]
- 26.Müller W.E.G., Zahn R.K., Gašić M.J., Dogović N., Maidhof A., Becker C., Diehl-Seifert B., Eich E. Avarol, a cytostatically active compound from the marine sponge Dysidea avara. Comp. Biochem. Phys. C. 1985;80:47–52. doi: 10.1016/0742-8413(85)90130-6. [DOI] [PubMed] [Google Scholar]
- 27.Müller W.E.G., Maidhof A., Zahn R.K., Schröder H.C., Gašić M.J., Heidemann D., Bernd A., Kurelec B., Eich E., Seibert G. Potent antileukemic activity of the novel cytostatic agent avarone and its analogues in vitro and in vivo. Cancer Res. 1985;45:4822–4826. [PubMed] [Google Scholar]
- 28.Müller W.E.G., Sladić D., Zahn R.K., Bässler K-H., Dogović N., Gerner H., Gašić M.J., Schröder H.C. Avarol-induced DNA strand breakage in vitro and in Friend erythroleukemia cells. Cancer Res. 1987;47:6565–6571. [PubMed] [Google Scholar]
- 29.De Giulio A., De Rosa S., Strazzullo G., Diliberto L., Obino P., Marongiu M.E., Pani A., La Colla P. Synthesis and evaluation of cytostatic and antiviral activities of 3' and 4'-avarone derivatives. Antivir. Chem. Chemoth. 1991;2:223–227. [Google Scholar]
- 30.Sarin P.S., Sun D., Thornton A., Müller W.E.G. Inhibition of replication of the etiological agent of acquired immune deficiency syndrome (human T-lymphotropic retrovirus/lymphadenopathy-associated virus) by avarol and avarone. J. Nat. Cancer Inst. 1987;78:663–666. [PubMed] [Google Scholar]
- 31.Loya S., Hizi A. The inhibition of human immunodeficiency virus type 1 reverse transcriptase by avarol and avarone derivatives. FEBS Lett. 1990;269:131–134. doi: 10.1016/0014-5793(90)81137-D. [DOI] [PubMed] [Google Scholar]
- 32.Ferrá M.L., Sanz M.J., Bustos G., Payá M., Alcaraz M.J., De Rosa S. Avarol and avarone, two new anti-inflammatory agents of marine origin. Eur. J. Pharmacol. 1994;253:75–82. doi: 10.1016/0014-2999(94)90759-5. [DOI] [PubMed] [Google Scholar]
- 33.Amigó M., Terencio M.C., Mitova M., Iodice C., Payá M., De Rosa S. Potential antipsoriatic avarol derivatives as antioxidants and inhibitors of PGE2 generation and proliferation in the HaCaT cell line. J. Nat. Prod. 2004;67:1459–1463. doi: 10.1021/np049873n. [DOI] [PubMed] [Google Scholar]
- 34.Seibert G., Raether W., Dogović N., Gašić M.J., Zahn R.K., Müller W.E.G. Antibacterial and antifungal activity of avarone and avarol. ZBL Bakt. Hyg. A. 1985;260:379–386. doi: 10.1016/s0176-6724(85)80026-2. [DOI] [PubMed] [Google Scholar]
- 35.Cozzolino B., De Giulio A., De Rosa S., Strazzullo G., Gašić M.J., Sladić D., Zlatović M. Biological activities of avarol derivatives, 1. amino derivatives. J. Nat. Prod. 1990;53:699–702. doi: 10.1021/np50069a027. [DOI] [Google Scholar]
- 36.Uriz M.J., Turon X., Galera J., Tur J.M. New light on the cell location of avarol within the sponge Dysidea avara (Dendroceratida) Cell Tissue Res. 1996;285:519–527. doi: 10.1007/s004410050668. [DOI] [Google Scholar]
- 37.Bhadury P., Wright P.C. Exploitation of marine algae: biogenic compounds for potential antifouling applications. Planta. 2004;219:561–578. doi: 10.1007/s00425-004-1307-5. [DOI] [PubMed] [Google Scholar]
- 38.Costerton J.W., Lewandowski Z., Caldwell D., Korber D., Lappin-Scott H.M. Microbial biofilms. Ann. Rev. Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 39.Abarzua S., Jakubowski S. Biotechnological investigation for the prevention of biofouling. I. Biological and biochemical principles for the prevention of biofouling. Mar. Ecol. Prog. Ser. II. 1995;123:301–312. [Google Scholar]
- 40.Clare A.S. Marine natural product antifoulants: status and potential. Biofouling. 1996;9:211–229. doi: 10.1080/08927019609378304. [DOI] [Google Scholar]
- 41.Steinberg P.D., de Nys R. Chemical mediation of colonization of seaweed surfaces. J. Phycol. 2002;38:621–629. doi: 10.1046/j.1529-8817.2002.02042.x. [DOI] [Google Scholar]
- 42.Hay M.E. Marine chemical ecology: what is known and what is next. J. Exp. Mar. Biol. Ecol. 1996;200:103–134. doi: 10.1016/S0022-0981(96)02659-7. [DOI] [Google Scholar]
- 43.Sarma A.S., Chattopadhyay P. Synthetic studies of trans-clerodane diterponoids and congeners: stereocontrolled total synthesis of (±)-avarol. J. Org. Chem. 1982;47:1727–1731. doi: 10.1021/jo00348a025. [DOI] [Google Scholar]
- 44.Rittschof D., Ali C.-H., Kok L.-M., Teo S.L.-M. Pharmaceuticals as antifoulants: concept and principles. Biofouling. 2003;19:207–212. doi: 10.1080/0892701021000083769. [DOI] [PubMed] [Google Scholar]
- 45.Božić T., Sladić D., Zlatović M., Novaković I., Trifunović S., Gašić M.J. Regioselectivity of conjugate additions to monoalkyl-1,4-benzoquinones. J. Serb. Chem. Soc. 2002;67:547–551. [Google Scholar]
- 46.Maréchal J-P., Culioli G., Hellio C., Thomas-Guyon H., Callow M.E., Clare A.S., Ortalo-Magne A. Seasonal variations in antifouling activity of crude extracts of the brown alga Bifurcaria bifurcata (Cystoseiraceae) against cyprids of Balanus amphitrite and the marine bacteria Cobetia marina and Pseudoalteromonas haloplanktis. J. Exp. Mar. Biol. Ecol. 2004;313:47–62. [Google Scholar]
- 47.Amsterdam D. In: Antibiotics in laboratory medicine. 4th ed. Loman V., editor. Williams and Wilkins; Baltimore, MD: 1996. pp. 52–111. [Google Scholar]
- 48.i>Hellio C., Bremer G., Pons A.M., Le Gal Y., Bourgougnon N. Inhibition of the development of microorganims (bacteria and fungi) by extracts of marine alga from Brittany, France. Appl. J. Microbiol. Biotechnol. 2000;54:543–549. doi: 10.1007/s002530000413. [DOI] [PubMed] [Google Scholar]
- 49.Hellio C., Maréchal J.P., Véron B., Bremer G., Clare A.S., Le Gal Y. Seasonal variation of antifouling activities of marine algae from the Brittany coast (France) Mar. Biotechnol. 2004;6:67–82. doi: 10.1007/s10126-003-0020-x. [DOI] [PubMed] [Google Scholar]
- 50.Billinghurst Z., Clare A.S., Fileman T., McEvoy J., Readman J., Depledge M.H. Inhibition of barnacle settlement by the environmental oestrogen 4-nonylphenol and the natural oestrogen 17β oestradiol. Mar. Pollut. Bull. 1998;36:833–839. [Google Scholar]
- 51.Hellio C., Simon-Colin C., Clare A.S., Deslandes E. Isethionic acid and floridoside, isolated from the red alga, Grateloupia turuturu, inhibit settlement of Balanus amphitrite cyprid larvae. Biofouling. 2004;20:139–145. doi: 10.1080/08927010412331279605. [DOI] [PubMed] [Google Scholar]
- 52.Maréchal J-P., Hellio C., Sebire M., Clare A.S. Settlement behaviour of marine invertebrate larvae measured by Ethovision 3.0. Biofouling. 2004;20:211–217. doi: 10.1080/08927010400011674. [DOI] [PubMed] [Google Scholar]
- 53.Guillard R., Ryther J. Studies of marine planktonic diatoms, I: Cyclotella nana (Hustedt) and Detonula confervacea (Cleve) Can. J. Microbiol. 1972;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- 54.Lau S., Qian P.Y. Inhibitory effect of phenolic compounds and marine bacteria on larval settlement of the barnacle Balanus amphitrite Darwin. Biofouling. 2000;16:47–58. doi: 10.1080/08927010009378429. [DOI] [Google Scholar]
- 55.Wu R.S.S., Lam P.K.S., Zhou B.S. A settlement inhibition assay with cyprid larvae of the barnacle Balanus amphitrite. Chemosphere. 1997;35:1867–1874. doi: 10.1016/S0045-6535(97)00238-5. [DOI] [Google Scholar]