Abstract
Microbial transformation of imbricatolic acid (1) by Aspergillus niger afforded 1α-hydroxyimbricatolic acid (2), while transformation with Rhizopus nigricans yielded 15-hydroxy-8,17-epoxylabdan-19-oic acid (3). When the diterpene 1 was added to a Cunninghamella echinulata culture, the main products were the microbial metabolites mycophenolic acid (4) and its 3-hydroxy derivative 5. All the structures were elucidated by spectroscopic methods. The cytotoxicity of these compounds towards human lung fibroblasts and AGS cells was assessed. While 4 and 5 showed low cytotoxicity, with IC50 values > 1000 µM against AGS cells and fibroblasts, 1α-hydroxyimbricatolic acid (2) presented moderate toxicity towards these targets, with IC50 values of 307 and 631 µM, respectively. The structure of 2 is presented for the first time.
Keywords: Aspergillus niger, Rhizopus nigricans, imbricatolic acid, biotransformation, diterpene, Cunninghamella echinulata, mycophenolic acid
Introduction
The diterpene imbricatolic acid (1) is one of the main constituents of the resin from the large tree Araucaria araucana (Mol.) Koch. The resin, as well as imbricatolic acid, have been recently shown to display gastroprotective effects in induced gastric lesion models in animals [1,2]. The gastroprotective effect of several semisynthetic derivatives of imbricatolic acid was assessed, but so far none of them contained hydroxy functions in the A ring.
Recent studies have reported the successful use of microorganisms for bioconversion of diterpenes. They include the biotransformation of stemodine by Rhizopus oryzae [3], the transformation of isosteviol by Aspergillus niger, Glomerella cingulata and Mortierella elongata [4], transformation of baccatin and 1β-hydroxybaccatin I with Aspergillus niger [5], microbial transformation of a pimarane derivative with Gibberella fujikuroi [6], of dehydroabietanol and teideadiol by Mucor plumbeus [7], stemodin and several Stemodia diterpenes with Aspergillus niger [8], Mucor plumbeus and Whetzelinia sclerotiorum [9].
The aim of the present work was to obtain new hydroxylated derivatives of imbricatolic acid by means of microbial transformation using Aspergillus niger, Cunninghamella echinulata and Rhizopus nigricans.
Results and Discussion
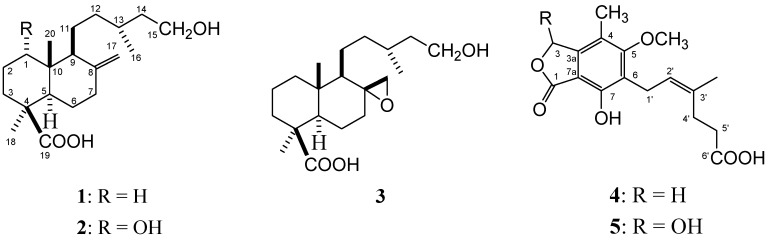
Microbial transformation of imbricatolic acid (1) by Aspergillus niger afforded a main compound, identified as 1α-hydroxyimbricatolic acid (2), while Rhizopus nigricans transformed imbricatolic acid to the epoxy derivative 3. From the Cunninghamella echinulata culture, the main compounds isolated were microbial metabolites, identified as mycophenolic acid (4) and its 3-hydroxy derivative 5 (Figure 1). The structures of the known compounds were determined by 1D- and 2D-NMR techniques and confirmed by comparing the physical and spectroscopic/spectrometric data with those from the literature (NMR and MS).
Figure 1.
Structures of compounds 1-5.
The molecular formula of 2 proved to be C20H34O4 from HREIMS, DEPT and 13C-NMR data. The 1H-NMR spectrum of compound 2 was close to that of 1, differing mainly by the presence of a broad singlet at δH 3.85 indicating a OH group, instead of a methylene group in the A ring. In the 13C-NMR spectrum of 2, eight methylene sp3 C atoms and an oxygenated methine (δC 71.20 d) were observed, instead of the nine methylene sp3 C atoms seen for imbricatolic acid. The position of the hydroxy function followed from the signal multiplicity and the HMBC correlations. Clear correlations between the H signal at δ 3.85 and C-5 (δC 48.55) and C-3 (δC 30.70) helped position the OH group at C-1. The H-20 methyl group at δH 0.59 also presented a clear HMBC correlation with the C at δ 71.20, supporting the proposed structure. The stereochemistry of the OH function was deduced from the coupling constants which are about 7.7 Hz for the β-hydroxy function while the α-isomer signal appeared as a broad singlet with very small coupling constants (< 1 Hz) [7,10]. The NMR spectral data of compound 2 are summarized in the Table 1.
Table 1.
1H- and 13C-NMR data of compound 2 (400 and 100 MHz, respectively, CDCl3).
| Position | δH (J in Hz) | δC, mult. |
|---|---|---|
| H | C | |
| 1 | 3.85, br s | 71.20, CH |
| 2 | 1.60, 2.21, m | 27.50, CH2 |
| 3 | 1.94, 1.56, m | 30.70, CH2 |
| 4 | - | 44.00, qC |
| 5 | 1.82, m | 48.55, CH |
| 6 | 1.87-2.00, m | 25.81, CH2 |
| 7 | 1.89, 2.36, m | 38.39, CH2 |
| 8 | - | 148.62, qC |
| 9 | 2.21, m | 48.34, CH |
| 10 | - | 44.00, qC |
| 11 | 1.50, 1.31, m | 19.58, CH2 |
| 12 | 1.15, 1.37, m | 35.18, CH2 |
| 13 | 1.62, m | 29.14, CH |
| 14 | 1.70, 1.29, m | 38.81, CH2 |
| 15 | 3.66, 3.72, m | 61.13, CH2 |
| 16 | 0.88, d (6.6) | 20.55, CH3 |
| 17 | 4.53 s, 4.86 s | 106.96, CH2 |
| 18 | 1.25, s | 28.71, CH3 |
| 19 | - | 183.41, qC |
| 20 | 0.59, s | 13.24, CH3 |
Among the several recently reported diterpene biotransformations by A. niger [9,11,12], only in the bioconversion of isoesteviol, in which the 1α,7α-dihydroxy derivative is obtained, was the 1α position hydroxylated [13]. Related compounds with an additional OH function include 3,15-dihydroxy-8(17)-labden-19-oic acid from Juniperus thurifera [14,15], the 6,15-dihydroxyderivative from Viburnum suspensum [16] and 7α-hydroxy-8(17)labdene-15,18-dioic acid from Haplopappus pulchellus [17]. A 7-β-hydroxy labdane derivative was described from Baccharis pedunculata [18] while other products were obtained from Eupatorium salvia [19]. Labdane derivatives with a 6-hydroxy function were isolated from Viburnum suspensum [16] while 3-hydroxy labdanes were obtained from Haplopappus chrysanthemifolius [20]. Beauveria bassiana ATCC 7159 was able to hydroxylate the stemodane and stemarane diterpenes stemodin and stemodinone, isolated from the Jamaican plant Stemodia maritima. The microorganisms yielded C-18 hydroxylated products of stemodin and stemodinone while stemarin was oxidized at C-1 to the corresponding β-configured alcohol as well as to the C-19 carboxylic acid [21]. Cunninghamella echinulata var. elegans hydroxylated stemodin to its 7α-hydroxy, 7β-hydroxy and 3β-hydroxy derivatives. Using this microorganism, stemarin was hydroxylated at the 6α position with oxidation of the C-19 methyl group to a carboxylic acid. Phanerochaete chrysosporium was able to hydroxylate stemodin to its 7 β-hydroxy as well as to the 3β-hydroxy and 11β-hydroxyderivatives and to hydroxylate stemodinone at C-19 [22]. These reports clearly illustrate the potential of microbial transformations to give selective oxidation products of diterpenoids.
Mycophenolic acid (4) has been reported as an immunosuppressive compound [23] and to present promising activity in the treatment of immune-mediated renal diseases [24]. The immunosuppressive and antitumor activities of mycophenolic acid have been recently reviewed [25]. Other biological activities reported for this compound include antifungal, antibacterial and antiviral effects [26]. To the best of our knowledge, this is the first report of mycophenolic acid production by Cunninghamella species. Until now the compound was reported only from cultures of Byssochlamys nivea [27] and mainly from several Penicillium species, such as P. brevi-compactum [28], P. roqueforti [29], P. roqueforti var. carneum [30], P. bialowiezense, P. chrysogenum [31], P. raciborkii [27], P. stoloniferum and other Penicillium spp. [32]. On the other hand, 3-hydroxymycophenolic acid (5) was obtained as a microbial transformation product of mycophenolic acid [33,34]. The total synthesis of mycophenolic acid has been reported [26].
Compound 2 presented moderate toxicity towards AGS cells and fibroblasts, with IC50 values of 307 and 631 µM, respectively. Compound 1 presented cytotoxicity IC50 values of 134 and 280 µM towards AGS cells and fibroblasts, respectively [2]. The cytotoxicity of the derivative 3 (IC50 values) was previously reported as 288 and 305 µM towards AGS cells and fibroblasts, respectively [2]. Mycophenolic acid (4) and its derivative 5 showed low cytotoxicity with IC50 values > 1000 µM against AGS cells and fibroblasts.
Experimental
General
Melting points were determined on a Koffler hot stage apparatus (Electrothermal 9100) and are uncorrected. Optical rotations were obtained on a Jasco DIP 370 polarimeter, and IR spectra were recorded on a Nicolet Nexus FT-IR instrument. The NMR spectra were recorded in CDCl3 on a Bruker Avance 400 NMR spectrometer at 400 MHz for 1H and 100 MHz for 13C, respectively. MS spectra were measured in a Varian unit at 70 eV. Silica gel 60 (Merck, 63-200 µm particle size) was used for column chromatography; precoated Silica gel plates (Merck, Kieselgel 60 F254, 0.25 mm) were used for TLC analysis. TLC spots were visualized by spraying the chromatograms with p-anisaldehyde-ethanol-acetic acid-H2SO4 (2:170:20:10 v/v) and heating at 110 °C for 3 min. Imbricatolic acid (15-hydroxylabd-8(17)-en-19-oic acid, 1) was isolated from Araucaria araucana resin as previously reported and recrystallized as colorless crystals, mp 102-105 °C [1,2].
Microorganisms
The microorganisms used were either from the American Type Culture Collection (ATCC): Aspergillus niger ATCC 16404, Cunninghamella echinulata ATCC 8688a or the Micoteca de la Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina: Rhizopus nigricans UBA 6.
Screening scale experiments
Liquid Czapek medium (30 mL) held in 125 mL Erlenmeyer flasks was innoculated with a spore suspension in a first fermentation stage. Stage I cultures were incubated on a rotatory shaker at 250 rpm and 28 ºC ± 2 °C for 48 h. The second fermentation stage was initiated by transferring the inoculum from the Stage I culture in a final volume of 10%. After 48 h incubation, substrate 1 (10 mg) dissolved in dimethylformamide was added in each flask. Samples (5 mL of incubation media) were withdrawn every 12, 24 and 48 h, partitioned with EtOAc (2 mL) and analyzed by TLC. Three experiments with two repetitions each were carried out for every fungal agent. Blank assays without substrate and without fungi were carried out.
Preparative scale experiments and product isolation
Aspergillus niger ATCC 16404 culture.
First fermentation stage was performed as described for the screening experiments. In Stage II the substrate at 200 mg L-1 was added to each of ten 5 L-Erlenmeyer flasks containing 48 h old culture (1 L). After 15 days, the culture was separated into culture medium and mycelium by filtration. Extraction of the culture filtrates with EtOAc (3 x 500 mL each) after acidification with HCl to pH 5, afforded 800 mg of EtOAc-solubles. Chromatography of the crude extract on silica gel (100 g, column length 42 cm, internal diameter 1.8 cm) with a CHCl3-acetone gradient afforded 56 fractions, pooled into nine groups according to the TLC patterns. From the fraction pools 1-2, some 85 mg imbricatolic acid (1) was recovered, while the pooled fraction 6 (70 mg), yielded 30 mg of 1-α,15-dihydroxylabd-8(17)-en-19-oic acid (1-α-hydroxyimbricatolic acid (2) as colorless crystals; mp 179-181 °C; + 56.77° (c = 0.303, MeOH); IR (KBr) νmax 3441, 2942, 2928, 1687, 1461, 1197, 1060, 1029, 895 cm-1; for NMR data, see Table 1; EIMS m/z 338 [M]+ (17), 320 (46), 275 (74), 234 (48), 219 (52), 201 (29), 189 (38), 182 (41), 173 (100), 169 (63), 159 (39), 137 (73), 133 (84), 123 (86), 197 (87), 95 (95), 81 (94), 55 (96); HREIMS m/z 338.485 (calcd. for C20H34O4, 338.485).
Cunninghamella echinulata ATCC 8688a culture.
First fermentation stage was performed as described for the screening experiments. In Stage II the substrate (200 mg L-1) was added to each of 12 5 L-Erlenmeyer flasks containing 48 h culture (1 L). After 14 days, the culture was separated into culture medium and mycelium by filtration. Extraction of the culture filtrates with EtOAc (3 x 500 mL each), after acidification with HCl to pH 5, afforded 4.9 g of EtOAc-solubles. Chromatography of the crude extract on 400 g silica gel with a petroleum ether (PE)-ethyl acetate (EtOAc) gradient afforded some 65 fractions of ca. 70 mL each. Fractions 1-9 contained no compounds of interest and were discarded. Fractions 10-18 yielded some 80 mg of imbricatolic acid (1), while fractions 26-28 afforded 500 mg of mycophenolic acid (4) [26] and the pooled fractions 29-46 contained 10.5 mg of 3-hydroxymycophenolic acid (5) [33,34]. The spectroscopic of the isolated compounds were in agreement with the structures and with published data.
Rhizopus nigricans UBA6 culture.
First fermentation stage was performed as was described for the screening experiments. In Stage II the substrate (200 mg L-1) was added to each ten 5 L-Erlenmeyer flasks containing 48 h culture (1 L). After 5 days, the culture was separated into culture medium and mycelium by filtration. Extraction of the culture filtrates with EtOAc (3 x 500 mL) after acidification with HCl to pH 5, afforded 680 mg of EtOAc-solubles. This EtOAc extract was chromatographed on 40 g silica gel with a PE, PE-EtOAc, EtOAc and EtOAc-MeOH gradient. Fractions 1 (PE, 100 mL), 2-3 (PE-EtOAc 9:1, 200 mL) and 4 (PE-EtOAc 8:2, 100 mL) did not contain any compounds of interest and were discarded. Fractions 5 and 6 (PE-EtOAc 7:3 and 1:1, 100 mL each) contained 120 mg of imbricatolic acid (1). Fractions 7-8 eluted with EtOAc (200 mL) and 9-10 (EtOAc-MeOH 9:1, 200 mL) afforded a mixture of imbricatolic acid (1) and a second, more polar compound which gave a red-violet spot with anisaldehyde-sulphuric acid reagent. Preparative TLC of the combined fractions 7-10 (silica gel, PE-EtOAc 1:1) afforded 60 mg of imbricatolic acid (1) and 30 mg of 15-hydroxy-8,17-epoxylabdan-19-oic acid (3) [2].
MRC-5 cell culture
The cytotoxic effect of the assayed compounds, expressed as cell viability, was assessed on a permanent fibroblast cell line derived from human lung (MRC-5) (ATCC CCL-171). MRC-5 fibroblasts were grown as monolayers in minimum essential Eagle medium (MEM), with Earle's salts, L-glutamine (2 mM) and sodium bicarbonate (2.2 gL-1), supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), penicillin (100 IUmL-1) and streptomycin (100 µgmL-1) in a humidified incubator with 5% CO2 in air at 37 °C. Cell passage was maintained between 10 and 16. The medium was changed every 2 days.
AGS cell culture
The cytotoxic effect of the assayed compounds, expressed as cell viability, was assessed on a permanent human gastric epithelial cell line (AGS) (ATCC CRL-1739). The AGS cells were grown as monolayers in Ham F-12 medium containing L-glutamine (1 mM) and sodium bicarbonate (1.5 gL-1), supplemented with 10% (v/v) heat-inactivated FBS, penicillin (100 IUmL-1) and streptomycin (100 µgmL-1) in a humidified incubator with 5% CO2 in air at 37 °C. The cell passage was maintained between 42 and 48. The medium was changed every 2 days.
Cytotoxicity assay
Confluent cultures of MRC-5 as well as AGS cells were treated with medium containing the diterpene derivatives at concentrations ranging from 0 up to 1000 µM. The products were first dissolved in DMSO and then in the corresponding culture medium supplemented with 2% FBS. The final content of DMSO in the test medium and controls was 1% (v/v). Cells were exposed for 24 h to the test medium with or without the compound (control). Each concentration was tested in quadruplicate together with the control and repeated three times in separate experiments. At the end of the incubation, the neutral red uptake (NR) assay was carried out [35]. To calculate the IC50 values (concentration that produces a 50% inhibitory effect on the evaluated parameter) the results were transformed to percentage of controls and the IC50 values were graphically obtained from the dose-response curves.
Acknowledgements
Financial support by FONDECYT, Grant Nº 1030583 and the Programa de Productos Bioactivos, University of Talca is gratefully acknowledged. Carlos Aranda thanks the Programa en Biotecnología Silvoagrícola for a postdoctoral grant.
Footnotes
Sample Availability: Samples of compounds 1 and 3 are available from the authors.
References
- 1.Schmeda-Hirschmann G., Astudillo L., Rodríguez J.A., Theoduloz C., Yáñez T. Gastro-protective effect of the Mapuche crude drug Araucaria araucana resin and its main constituents. J. Ethnopharmacol. 2005;101:271–276. doi: 10.1016/j.jep.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 2.Schmeda-Hirschmann G., Astudillo L., Sepúlveda B., Rodríguez J.A., Theoduloz C., Yáñez T., Palenzuela J.A. Gastroprotective effect and cytotoxicity of natural and semisynthetic labdane diterpenes from Araucaria araucana. Z. Naturforsch. C. 2005;60:511–522. doi: 10.1515/znc-2005-7-801. [DOI] [PubMed] [Google Scholar]
- 3.Martin G.D., Reynolds W.F., Reese P.B. Stemodane skeletal rearrangement: chemistry and microbial transformation. Phytochemistry. 2005;66:901–909. doi: 10.1016/j.phytochem.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Akihisa T., Hamasaki Y., Tokuda H., Ukiya M., Kimura Y., Nishino H. Microbial transformation of isosteviol and inhibitory effects on Epstein-Barr virus activation of the transformation products. J. Nat. Prod. 2004;67:407–410. doi: 10.1021/np030393q. [DOI] [PubMed] [Google Scholar]
- 5.Shen Y.C., Lo K.L., Lin C.L., Chakraborty R. Microbial transformation of baccatin VI and 1beta-hydroxy baccatin I by Aspergillus niger. Bioorg. Med. Chem. Lett. 2003;13:4493–4496. doi: 10.1016/j.bmcl.2003.08.069. [DOI] [PubMed] [Google Scholar]
- 6.Fraga B.M., González P., Hernández M.G., Chamy M.C., Garbarino J.A. Microbial transformation of 18-hydroxy-9,13-epi-ent-pimara-7,15-diene by Gibberella fujikuroi. J. Nat. Prod. 2003;66:392–397. doi: 10.1021/np020457h. [DOI] [PubMed] [Google Scholar]
- 7.Fraga B.M., Hernández M.G., Artega J.M., Suárez S. The microbiological transformation of the diterpenes dehydroabietanol and teideadiol by Mucor plumbeus. Phytochemistry. 2003;63:663–668. doi: 10.1016/S0031-9422(03)00291-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen A.R.M., Reese P.B. Biotransformation of terpenes from Stemodia maritima by Aspergillus niger ATCC 9142. Phytochemistry. 2002;59:57–62. doi: 10.1016/S0031-9422(01)00355-7. [DOI] [PubMed] [Google Scholar]
- 9.Chen A.R.M., Ruddock P.L.D., Lamm A.S., Reynolds W.F., Reese P.B. Stemodane and stemarane diterpenoid hydroxylation by Mucor plumbeus and Whetzelinia sclerotiorum. Phytochemistry. 2005;66:1898–1902. doi: 10.1016/j.phytochem.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Gouiric S.C., Feresin G.E., Tapia A.A., Rossomando P.C., Schmeda-Hirschmann G., Bustos D.A. 1β,7β-Dihydroxydehydroabietic acid, a new biotransformation product of dehydroabietic acid by Aspergillus niger. World J. Microbiol. Biotechnol. 2004;20:281–284. [Google Scholar]
- 11.Schmeda-Hirschmann G., Astudillo L., Palenzuela J.A. Biotransformation of solidagenone by Alternaria alternata, Aspergillus niger and Curvularia lunata cultures. World J. Microbiol. Biotechnol. 2004;20:93–97. [Google Scholar]
- 12.Orden A.A., Cifuente D.A., Borkowski E.J., Tonn C.E., Kurina M. Stereo- and regioselective hydroxylation of grindelic acid derivatives by Aspergillus niger. Nat. Prod. Res. 2005;19:625–631. doi: 10.1080/14786410512331330693. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira B.H., dos Santos M.C., Leal P.C. Biotransformation of the diperpenoid, isosteviol, by Aspergillus niger, Penicillium chrysogenum and Rhizopus arrhizus. Phytochemistry. 1999;51:737–741. doi: 10.1016/s0031-9422(99)00058-8. [DOI] [PubMed] [Google Scholar]
- 14.San Feliciano A., Miguel del Corral J.M., Lopez J.L., de Pascual-Teresa B. Labdane acids from polar extracts of Juniperus thurifera. Phytochemistry. 1992;31:1719–1722. [Google Scholar]
- 15.San Feliciano A., Miguel del Corral J.M., Lopez J.L., de Pascual-Teresa B. Further diterpene acids from Juniperus thurifera. Phytochemistry. 1993;33:1165–1167. [Google Scholar]
- 16.Iwagawa T., Yaguchi S., Hase T., Okubo T., Kim M. Diterpene glucosides from Viburnum suspensum. Phytochemistry. 1993;32:1515–1518. doi: 10.1016/0031-9422(93)85169-R. [DOI] [Google Scholar]
- 17.Zdero C., Bohlmann F., Niemeyer H. Friedolabdanes and other constituents from Chilean Haplopappus species. Phytochemistry. 1991;30:3669–3677. doi: 10.1016/0031-9422(91)80089-J. [DOI] [Google Scholar]
- 18.Jakupovic J., Schuster A., Wasshausen D.C. Acetylenes and labdanes from Baccharis pedunculata. Phytochemistry. 1991;30:2785–2787. [Google Scholar]
- 19.González G.A., Bermejo B.J., Diaz J.G., Rodríguez Perez E.M., Yanes A.C., Rauter P., Pozo J. Diterpenes and other constituents of Eupatorium salvia. Phytochemistry. 1990;29:321–323. [Google Scholar]
- 20.Faini F., Labbe F., Torres R., Delle Monache F., Delle Monache G. Diterpenes from Haplopappus chrysanthemifolius. Phytochemistry. 1999;52:1547–1550. doi: 10.1016/S0031-9422(99)00395-7. [DOI] [Google Scholar]
- 21.Buchanan G.O., Reese P.B. Biotransformation of diterpenes and diterpene derivatives by Beauveria bassiana ATCC 7159. Phytochemistry. 2001;56:141–151. doi: 10.1016/S0031-9422(00)00403-9. [DOI] [PubMed] [Google Scholar]
- 22.Lamm A.S., Reynolds W.F., Reese P.B. Bioconversion of Stemodia maritima diterpenes and derivatives by Cunninghamella echinulata var. elegans and Phanerochaete chrysosporium. Phytochemistry. 2006;67:1088–1093. doi: 10.1016/j.phytochem.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Nelson P.H., Eugui E., Wang C.C., Allison A.C. Synthesis and immunosuppressive activity of some side-chain variants of mycophenolic acid. J. Med. Chem. 1990;33:833–838. doi: 10.1021/jm00164a057. [DOI] [PubMed] [Google Scholar]
- 24.Dal Canton A., Amore A., Barbano G., Coppo R., Emma F., Grandaliano G., Klersy C., Perfumo F., Rizzoni G., Schena F.P., Sepe V. One-year angiotensin-converting enzyme inhibition plus mycophenolate mofetil immunosuppression in the course of early IgA nephropathy: a multicenter, randomised, controlled study. J. Nephrol. 2005;18:136–140. [PubMed] [Google Scholar]
- 25.Casadio F., Croci S., D’Errico Grigioni A., Corti B., Grigioni W.F., Landuzzi L., Lollini P.L. Renal transplantation toward the definition of immunosuppressive regimens with antitumor activity. Transplant. P. 2005;37:2144–2147. doi: 10.1016/j.transproceed.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 26.Covarrubias-Zúñiga A., González-Lucas A., Domínguez M.M. Total synthesis of mycophenolic acid. Tetrahedron. 2003;59:1989–1994. [Google Scholar]
- 27.Puel O., Tadrist S., Galtier P., Oswald I.P., Delaforge M. Byssochlamys nivea as a source of mycophenolic acid. Appl. Environm. Microbiol. 2005;71:550–553. doi: 10.1128/AEM.71.1.550-553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doerfler D.L., Bartman C.D., Campbell I.M. Mycophenolic acid production by Penicillium brevicompactum in two media. Can. J. Microbiol. 1979;25:940–943. doi: 10.1139/m79-142. [DOI] [PubMed] [Google Scholar]
- 29.Schneweis I., Meyer K., Hormansdorfer S., Bauer J. Mycophenolic acid in silage. Appl. Environm. Microbiol. 2000;66:3639–3641. doi: 10.1128/AEM.66.8.3639-3641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boysen M., Skouboe P., Frisvad J., Rossen L. Reclassification of the Penicillium roqueforti group into three species on the basis of molecular genetic and biochemical profiles. Microbiology. 1996;142:541–549. doi: 10.1099/13500872-142-3-541. [DOI] [PubMed] [Google Scholar]
- 31.Frisvad J.C., Smedsgaard J., Larsen T.O., Samson R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004;49:201–241. [Google Scholar]
- 32.Chapman and Hall/CRC; Boca Raton, FL, USA: 2006. Dictionary of Natural Products on CD-ROM. [Google Scholar]
- 33.Jekkel A., Barta I., Kónya A., Süt J., Boros S., Horváth G., Ambrus G. Microbiological transformation of mycophenolic acid. J. Mol. Catal. B: Enzym. 2001;11:423–426. [Google Scholar]
- 34.Jekkel A., Barta I., Boros S., Süt J., Horváth G., Szabó Z., Ambrus G. Microbial transformation of mycophenolic acid Part II. J. Mol. Catal. B: Enzym. 2002;19–20:209–214. [Google Scholar]
- 35.Rodríguez J.A., Haun M. Cytotoxicity of trans-dehydrocrotonin from Croton cajucara (Euphorbiaceae) on V79 cells and rat hepatocytes. Planta Med. 1999;65:522–526. doi: 10.1055/s-1999-14008. [DOI] [PubMed] [Google Scholar]