Abstract
Objectives:
We sought to determine the rate of chemotherapy-related cardiotoxicity, and estimate adherence to cardiac monitoring recommendations, among chemotherapy-treated breast cancer patients.
Background:
Heart Failure (HF) is a known complication associated with cancer therapies. Little is known regarding the rate of chemotherapy-related cardiotoxicity, and the adherence to cardiac monitoring recommendations among chemotherapy-treated breast cancer patients.
Methods:
Patients >18 years old diagnosed with non-metastatic invasive breast cancer between 2009–2014, treated with chemotherapy within 6 months of their diagnosis were identified in the Truven Health MarketScan database. HF, comorbidities, and treatment details were identified using diagnosis and billing codes. Analyses included descriptive statistics, Cox Proportional Hazard regression, and logistic regression.
Results:
16,456 patients were included; the median age was 56 years. Cardiotoxicity was identified in 4.2% of patients. Trastuzumab (HR=2.01, 95%CI 1.72–2.36), anthracyclines (HR=1.53, 95%CI 1.30–1.80), Deyo comorbidity score (HR=1.38, 95%CI 1.15–1.66; HR=2.47, 95%CI 1.94–3.15 for scores of 1 and ≥2 respectively), hypertension (HR=1.28, 95CI% 1.091.51), and valve disease (HR=1.93, 95%CI 1.48–2.51) were associated with an increased risk of cardiotoxicity. Patients ≤35 years old (HR=0.37, 95%CI 0.19–0.72) and 36–49 years old (HR=0.49, 95%CI 0.38–0.62) were less likely to have cardiotoxicity compared to patients ages 65+. Among 4,325 trastuzumab-treated patients, guideline-adherent cardiac monitoring was identified in 46.2% of patients. Anthracyclines (OR=1.58, 95%CI 1.35–1.87), taxanes (OR=1.63, 95% CI 1.27–2.08), and radiation (OR=1.22, 95%CI 1.08–1.39) were associated with guideline adherent monitoring.
Conclusions:
HF is an uncommon complication of breast cancer therapies. The risk was higher among trastuzumab or anthracycline-treated patients and lower in younger patients. Cardiac monitoring among trastuzumab-treated patients should be a priority among high-risk patients and in the presence of comorbidities or other chemotherapies such as anthracyclines.
Keywords: Breast Cancer, Cardio-oncology, Cardiac Monitoring, Cardiotoxicity, Trastuzumab
INTRODUCTION
After secondary malignancies, cardiovascular disease is the leading cause of morbidity and mortality among breast cancer survivors (1). These high rates are in part due to the cardiac toxicities of cancer therapies. Trastuzumab-based chemotherapy is the cornerstone of the systemic therapy for patients with HER2-positive tumors (2). Trastuzumab is a monoclonal antibody with excellent tolerance, its use, however, is associated with cardiotoxicity (2). Data from clinical trials suggest that the trastuzumab-cardiotoxicity rates range from 4.1% to 10% (36). Trastuzumab causes damage to the cardiac myocytes that can lead to heart failure (HF) through a type II cardiotoxicity, characterized by its reversibility (2). Despite being reversible with patients recovering cardiac function, to date, it is not possible to predict who will develop this complication; therefore, it is important to understand the incidence in the general population and identify at-risk patients.
The National Comprehensive Cancer Network (NCCN) recommends cardiac monitoring before initiating trastuzumab treatment, and every 3 months while on the treatment (i.e. at 3, 6, 9, and 12 months) (7). Cardiac monitoring is usually performed with an echocardiogram or with radionuclide ventriculography (multiple-gated acquisition [MUGA] scans) (8). There is little information on the patterns of cardiac monitoring in breast cancer patients. In a previous work we found that among 2,203 breast cancer patients older than 65 years old, 64% received suboptimal cardiac monitoring during trastuzumab therapy (8). Thavendiranathan et al. found that while the absolute risk of cancer treatment-related cardiotoxicity was lower in younger patients, the relative risk of cancer treatment-related cardiotoxicity was similar between older and younger patients, indicating the need for equal consideration of surveillance for cardiac dysfunction in this young population that has been typically considered at low risk for such events (9). To the best of our knowledge, there are no data from insurance claims on both the rates and factors associated with cardiotoxicity and guideline-adherent cardiac monitoring in young American patients. In this large cohort of breast cancer patients, we estimated the rate and the determinants of cardiotoxicity associated with the use of chemotherapy and trastuzumab- based chemotherapy. In addition, we calculated the rate of cardiac monitoring among trastuzumab-treated patients and described the pattern of cardiac monitoring according to age.
METHODS
Data Source and Data Extraction
Female patients over 18 years of age diagnosed with non-metastatic invasive breast cancer between 2009–2014 were identified in the Truven Health MarketScan database. This database includes de-identified patient-level health data from medical claims and prescription drug claims for insured employees, spouses, and their dependents around the United States. Data are from large employers, managed care organizations, hospitals, electronic medical record (EMR) providers, Medicare and Medicaid, and are often used for health services research.
Patients were included if they had been treated with chemotherapy within the first 6 months after their diagnosis. Male breast cancer patients and those patients with a previous history of HF or previous cancer were excluded from the study. Patients were followed from the date of their breast cancer diagnosis until they either died or lost insurance coverage. The last follow-up date was December 31, 2015.
Definitions
Cardiotoxicity was defined as an incident case of HF following a breast cancer diagnosis. HF after breast cancer diagnosis was identified using International Classification of Diseases version 9 (ICD-09) diagnosis codes 425, 428, and 785.51 in inpatient, facility, and outpatient claims. HF, therefore, denotes symptomatic and asymptomatic events, as data on symptoms were not available on the MarketScan database. Patients were noted as having HF if there was at least one claim in the inpatient file or at least two claims that were more than 30 days apart in the outpatient files.
Among patients treated with trastuzumab-based chemotherapy, we evaluated the rates of cardiac monitoring. Guideline-adherent cardiac monitoring was defined as a baseline cardiac evaluation within 4 months before the first dose of trastuzumab, and a subsequent follow-up cardiac evaluation at least every 4 months during trastuzumab therapy (8). Our group used this 4 month cut-off in previous work and it was chosen to accommodate for differences in scheduling, resources, or levels of accessibility to medical care (8). Baseline cardiac monitoring was defined as a test before any trastuzumab dose; follow-up cardiac monitoring was defined as monitoring every 4 months after initiating treatment. Methods for cardiac monitoring such as echocardiograms and MUGA scans were identified in the facility, outpatient and inpatient claims using Current Procedural Terminology (CPT) codes (Echocardiograms [93303–4, 99306–8, 99320–1, 93325], MUGA scans [78414, 78433, 78451–4, 78472, 78478, 78480]). We also evaluated identified cardiac-Magnetic Resonance Imaging (cMRI)[ (75557, 75559, 75561, 75563, 75565)].
The Deyo Comorbidity score was calculated from claims within 6 months before diagnosis (10). Pre-existing conditions were identified using the following ICD-9 codes: hypertension (401–409, exclude 402.11, 402.91), coronary artery disease (CAD) (410–414, exclude 414.1, 36.01, 36.1), valve disease (394–397, 424, exclude 424.9, 35), and diabetes (250). Using the Healthcare Procedure Coding System (HCPCS), chemotherapy drugs were identified using the following codes: trastuzumab (J9355), anthracyclines (J9000, J9001, J9010, J9178, J9180), and taxanes (J9170, J9171, J9264, J9265).
Statistical Methods
Descriptive statistics were used to determine the rate of cardiotoxicity and the rate of guideline-adherent cardiac monitoring. X2 test or Wilcoxon’s test were used to compare the demographic and clinical features of patients who did and did not experience these outcomes of interest. A time-dependent Cox regression model was used to evaluate the risk of cardiotoxicity in our entire cohort (N=16,456). Trastuzumab was the only time-dependent variable, and was defined as any use following a breast cancer diagnosis. Other variables in the model included age group (≤35, 36–49, 50–64, and 65+), year of breast cancer diagnosis (2009, 2010, 2011, 2012, 2013, and 2014), trastuzumab, anthracyclines, taxanes, radiation, Deyo comorbidity score, hypertension, valve disease, insurance (PPO, HMO, and Other), and geographical region (Northeast, North Central, South, West, and Unknown). Results are expressed in hazard ratios (HR) and 95% CI. A sensitivity analysis using only inpatient claims -as a proxy for severity-was performed to calculate the rate of cardiotoxicity. We used a previously described method to incorporate a time-dependent variable in a Kaplan-Meier curve (11). In simple terms, this method updates the cohort at all event times, and splits survival time according to covariate status. Comparisons were made using the log-rank test.
A logistic regression model was used to determine the odds of receiving guideline- adherent cardiac monitoring among the 4,325 trastuzumab-treated patients. Variables in the model included age, year of diagnosis, anthracyclines, taxanes, radiation, Deyo comorbidity score, hypertension, valve disease, insurance, and region. Results are expressed as odds ratios (ORs) and 95% CI. We also compared the rates of guideline-adherent cardiac monitoring according to age group at baseline and at follow-up among the patients who had been treated with trastuzumab.
Statistical analyses were performed using SAS version 9.4. The research was reviewed by the institutional review board of The University of Texas MD Anderson Cancer Center and was exempt under the codes of regulations.
RESULTS
A total of 16,456 patients were included in this study. The median age was 56 years. Among the included patients, 4,325 received trastuzumab-based chemotherapy.
Cardiotoxicity
In our cohort, 692 (4.2%) patients developed HF after chemotherapy. The median time to event was 8 months. Patient characteristics according to cardiotoxicity are listed in Table-1. Among patients treated with trastuzumab, the rate was 8.3% as compared to 2.7% for those who were not treated with trastuzumab (P<0.001). In comparison, the rates for anthracycline users versus non-anthracycline users were 4.6% versus 4.0% (P=0.048). In the sensitivity analysis using only inpatient claims, we found that the rates for cardiotoxicity were the same for trastuzumab users and nonusers at 1.7% (P=<0.001) (Supplemental Table-1).
TABLE-1.
Patient Characteristics According to Cardiotoxicity Among Patients Ages 18–80+ With Breast Cancer (N=16456).
| Cardiotoxicity | |||
|---|---|---|---|
| Patient Characteristic | Total; N | Yes; N (%) | P |
| All Patients | 16456 | 692 (4.2) | <0.001 |
| Age, years | <0.001 | ||
| <35 | 426 | 9 (2.1) | |
| 36–49 | 4116 | 121 (2.9) | |
| 50–64 | 8914 | 314 (3.5) | |
| 65+ | 3000 | 248 (8.3) | |
| Year of Diagnosis | 0.166 | ||
| 2009 | 3324 | 162 (4.9) | |
| 2010 | 3186 | 134 (4.2) | |
| 2011 | 3256 | 136 (4.2) | |
| 2012 | 2546 | 98 (3.8) | |
| 2013 | 2432 | 105 (4.3) | |
| 2014 | 1712 | 57 (3.3) | |
| Any Trastuzumab Use After Diagnosis | <0.001 | ||
| No | 12131 | 322 (2.7) | |
| Yes | 4325 | 360 (8.3) | |
| Anthracyclines | 0.048 | ||
| No | 10870 | 433 (4.0) | |
| Yes | 5586 | 259 (4.6) | |
| Taxanes | <0.001 | ||
| No | 1621 | 96 (5.9) | |
| Yes | 14835 | 596 (4.0) | |
| Radiation | 0.968 | ||
| No | 5481 | 230 (4.2) | |
| Yes | 10975 | 462 (4.2) | |
| Deyo Comorbidity Score | <0.001 | ||
| 0 | 12680 | 436 (3.4) | |
| 1 | 3023 | 168 (5.6) | |
| 2+ | 753 | 88 (11.7) | |
| Hypertension | <0.001 | ||
| No | 10522 | 350 (3.3) | |
| Yes | 5934 | 342 (5.8) | |
| Diabetes | <0.001 | ||
| No | 14475 | 546 (3.8) | |
| Yes | 1981 | 146 (7.4) | |
| Coronary Artery Disease | <0.001 | ||
| No | 15816 | 615 (3.9) | |
| Yes | 640 | 77 (12.0) | |
| Valve Disease | <0.001 | ||
| No | 15824 | 628 (4.0) | |
| Yes | 632 | 64 (10.1) | |
| Insurance | 0.001 | ||
| PPO | 9608 | 361 (3.8) | |
| HMO | 2103 | 92 (4.4) | |
| Other | 4745 | 239 (5.0) | |
| Region | 0.003 | ||
| North Central | 3963 | 193 (4.9) | |
| Northeast | 2779 | 140 (5.0) | |
| South | 6507 | 234 (3.6) | |
| Unknown | 289 | 10 (3.5) | |
| West | 2918 | 115 (3.9) | |
P<0.05.
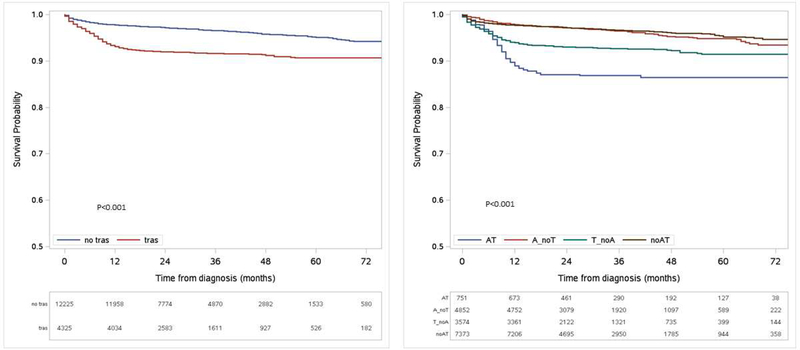
Figure-1 contains the Kaplan-Meier curve for time to HF. The Kaplan-Meier plots use trastuzumab as a time-dependent variable. Patients treated with anthracyclines and trastuzumab were at the highest risk, followed by patients that received trastuzumab-based chemotherapy. The lowest risk was seen among patients treated with anthracyclines and those that received chemotherapy with regimens not containing trastuzumab or anthracyclines. Cardiotoxicity was identified in 2.1% of patients ages ≤35 (N=426), 2.9% of patients ages 36–49 (N=4116), 3.5% of patients ages 50–64 (N=8914), and 8.3% of patients ages 65+ (N=3000). Table-2 contains the complete univariable and multivariable Cox regression models. After adjusting for potential confounders, trastuzumab-treated patients (HR=2.01, 95%CI 1.72–2.36) and those treated with anthracycline-containing regimens (HR=1.53, 95%CI 1.30–1.80) were more likely to develop HF. Using the 65+ age group as a reference, all other age groups had a lower risk of HF, with patients ages ≤35 having the lowest risk (HR=0.37, 95% CI 0.19–0.75). Other variables that were associated with an increased risk of HF included comorbidities (HR=1.38, 95%CI 1.15–1.66; HR=2.47, 95%CI 1.94–3.15 for Deyo scores of 1 and ≥2 respectively), hypertension (HR=1.28, 95 CI% 1.09–1.51), and valve disease (HR=1.93, 95%CI 1.48–2.51). Supplemental Table-2 contains the same models for determining the risk of cardiotoxicity, using only inpatient claims.
Figure-1. Kaplan-Meier Survival Plots for HF-free survival for patients with breast cancer by incorporating trastuzumab use as a time-dependent variable.
(A.) Stratified by trastuzumab use. (B.) Stratified by trastuzumab and anthracycline use. AT, anthracycline and trastuzumab; A_noT, anthracycline and no trastuzumab; T_noA, trastuzumab and no anthracycline; and noAT, no anthracycline or trastuzumab
TABLE-2.
Cox Regression Model Using Trastuzumab As a Time-Dependent Variable to Evaluate the Risk of Cardiotoxicity Among Breast Cancer Patients diagnosed between 2009–2014 (N=16456).
| Patient Characteristic | Crude HR (95% CI) | Adjusted HR (95% CI) | ||
|---|---|---|---|---|
| Age, years | ||||
| 65+ | Ref | Ref | ||
| ≤35 | 0.09 | (0.01 – 0.66) | 0.37 | (0.19 – 0.72) |
| 36–49 | 0.38 | (0.26 – 0.55) | 0.49 | (0.38 – 0.62) |
| 50–64 | 0.49 | (0.38 – 0.63) | 0.52 | (0.44 – 0.63) |
| Year of Diagnosis | ||||
| 2009 | Ref | Ref | ||
| 2010 | 1.02 | (0.75 – 1.39) | 0.91 | (0.72 – 1.15) |
| 2011 | 1.04 | (0.75 – 1.44) | 1.03 | (0.82 – 1.30) |
| 2012 | 0.99 | (0.69 – 1.43) | 0.98 | (0.76 – 1.27) |
| 2013 | 0.93 | (0.61 – 1.40) | 1.16 | (0.90 – 1.50) |
| 2014 | 1.13 | (0.71 – 1.80) | 0.98 | (0.72 – 1.33) |
| Trastuzumab | ||||
| No | Ref | Ref | ||
| Yes | 1.83 | (1.57 – 2.13) | 2.01 | (1.72 – 2.36) |
| Anthracyclines | ||||
| No | Ref | Ref | ||
| Yes | 1.74 | (1.39 – 2.16) | 1.53 | (1.30 – 1.80) |
| Taxanes | ||||
| No | Ref | Ref | ||
| Yes | 0.70 | (0.53 – 0.94) | 0.81 | (0.65 – 1.01) |
| Radiation | ||||
| No | Ref | Ref | ||
| Yes | 0.79 | (0.63 – 0.99) | 0.95 | (0.81 – 1.11) |
| Deyo Comorbidity Score | ||||
| 0 | Ref | Ref | ||
| 1 | 1.50 | (1.11 – 2.02) | 1.38 | (1.15 – 1.66) |
| 2+ | 3.04 | (2.08 – 4.45) | 2.47 | (1.94 – 3.15) |
| Hypertension | ||||
| No | Ref | Ref | ||
| Yes | 1.63 | (1.28 – 2.08) | 1.28 | (1.09 – 1.51) |
| Valve Disease | ||||
| No | Ref | Ref | ||
| Yes | 2.05 | (1.45 – 2.90) | 1.93 | (1.48 – 2.51) |
| Insurance | ||||
| PPO | Ref | Ref | ||
| HMO | 1.09 | (0.78 – 1.53) | 1.12 | (0.89 – 1.41) |
| Other | 1.23 | (0.97 – 1.56) | 1.09 | (0.92 – 1.29) |
| Region | ||||
| Northeast | Ref | Ref | ||
| North Central | 1.25 | (0.88 – 1.76) | 0.90 | (0.72 – 1.12) |
| South | 1.13 | (0.81 – 1.57) | 0.72 | (0.58 – 0.89) |
| Unknown | 1.65 | (0.74 – 3.68) | 0.74 | (0.39 – 1.42) |
| West | 0.99 | (0.66 – 1.50) | 0.82 | (0.64 – 1.05) |
Cardiac Monitoring
Of the 4,325 patients treated with trastuzumab-based chemotherapy, 73.5% (n=3181) underwent cardiac monitoring at baseline. While there are no guidelines to conduct cardiac monitoring with cMRI, we identified that 15 out of 4325 (0.3%) trastuzumab-treated patients had this test performed during their treatment. Guideline-adherent cardiac monitoring was identified in 46.2% or 1997 patients (N=4325). Table-3 shows patient characteristics according to recommended cardiac monitoring. Anthracyclines, taxanes, radiation, and region were associated with guideline-adherent cardiac monitoring. In the adjusted model (Table-4), patients diagnosed more recently (OR=1.29, 95%CI 1.05–1.60; OR=1.40, 95%CI 1.11–1.76 for 2013 v 2009 and 2014 v 2009), those treated with anthracyclines (OR=1.58, 95%CI 1.35–1.87), taxanes (OR=1.63, 95%CI 1.27–2.08), radiation (OR=1.22, 95% CI 1.08–1.39), and had insurance other than HMO or PPO (OR=1.16, 95%CI 1.01–1.34 for other v PPO) had higher odds of receiving guideline-adherent cardiac monitoring. Patients who lived in the West region were less likely to receive guideline adherent cardiac monitoring (OR=0.78, 95%CI 0.63–0.96), as compared to those from the Northeast.
TABLE-3.
Trastuzumab Users Only - Patient Characteristics According to Recommended Cardiac Monitoring Among Trastuzumab-Treated Breast Cancer Patients (N=4325).
| Recommended Cardiac Monitoring | |||
|---|---|---|---|
| Patient Characteristic | Total; N | Yes; N (%) | P |
| All Patients | 4325 | 1997 (46.2) | <0.001 |
| Heart Failure | <0.001 | ||
| No | 3965 | 1789 (45.1) | |
| Yes | 360 | 208 (57.8) | |
| Age, years | 0.457 | ||
| <35 | 112 | 45 (40.2) | |
| 36–49 | 1019 | 485 (47.6) | |
| 50–64 | 2381 | 1097 (46.1) | |
| 65+ | 813 | 370 (45.5) | |
| Year of Diagnosis | 0.166 | ||
| 2009 | 733 | 339 (46.2) | |
| 2010 | 816 | 350 (42.9) | |
| 2011 | 801 | 341 (42.6) | |
| 2012 | 652 | 319 (48.9) | |
| 2013 | 710 | 375 (52.8) | |
| 2014 | 512 | 273 (53.3) | |
| Anthracyclines | <0.001 | ||
| No | 3574 | 1579 (44.2) | |
| Yes | 751 | 418 (55.7) | |
| Taxanes | <0.001 | ||
| No | 327 | 114 (34.9) | |
| Yes | 3998 | 1883 (47.1) | |
| Radiation | 0.002 | ||
| No | 1631 | 703 (43.1) | |
| Yes | 2694 | 1294 (48.0) | |
| Deyo Comorbidity Score | 0.959 | ||
| 0 | 3383 | 1559 (46.1) | |
| 1 | 738 | 342 (46.3) | |
| 2+ | 204 | 96 (47.1) | |
| Hypertension | 0.471 | ||
| No | 2804 | 1306 (46.6) | |
| Yes | 1521 | 691 (45.4) | |
| Diabetes | 0.320 | ||
| No | 3849 | 1767 (45.9) | |
| Yes | 476 | 230 (48.3) | |
| Coronary Artery Disease | 0.595 | ||
| No | 4159 | 1917 (46.1) | |
| Yes | 166 | 80 (48.2) | |
| Valve Disease | 0.737 | ||
| No | 4147 | 1917 (46.2) | |
| Yes | 178 | 80 (44.9) | |
| Insurance | 0.113 | ||
| PPO | 2538 | 1141 (45.0) | |
| HMO | 484 | 224 (46.3) | |
| Other | 1303 | 632 (48.5) | |
| Region | 0.005 | ||
| North Central | 1049 | 508 (48.4) | |
| Northeast | 740 | 363 (49.1) | |
| South | 1676 | 769 (45.9) | |
| Unknown | 73 | 37 (50.7) | |
| West | 787 | 320 (40.7) | |
P<0.05.
TABLE-4.
Trastuzumab Users Only - Logistic Regression Model Evaluating the Odds of Recommended Cardiac Monitoring Among Breast Cancer Patients Treated with Trastuzumab (N=4325).
| Patient Characteristic | Crude OR (95% CI) | Adjusted OR (95% CI) | ||
|---|---|---|---|---|
| Age, years | ||||
| 65+ | Ref | Ref | ||
| <35 | 0.80 | (0.54 – 1.20) | 0.73 | (0.48 – 1.11) |
| 36–49 | 1.09 | (0.90 – 1.31) | 1.02 | (0.83 – 1.26) |
| 50–64 | 1.02 | (0.87 – 1.20) | 0.98 | (0.83 – 1.16) |
| Year of Diagnosis | ||||
| 2009 | Ref | Ref | ||
| 2010 | 0.87 | (0.72 – 1.06) | 0.86 | (0.71 – 1.06) |
| 2011 | 0.88 | (0.72 – 1.07) | 0.88 | (0.72 – 1.08) |
| 2012 | 1.12 | (0.91 – 1.39) | 1.14 | (0.92 – 1.42) |
| 2013 | 1.33 | (1.09 – 1.64) | 1.29 | (1.05 – 1.60) |
| 2014 | 1.37 | (1.09 – 1.71) | 1.40 | (1.11 – 1.76) |
| Anthracyclines | ||||
| No | Ref | Ref | ||
| Yes | 1.59 | (1.35 – 1.86) | 1.58 | (1.35 – 1.87) |
| Taxanes | ||||
| No | Ref | Ref | ||
| Yes | 1.66 | (1.31 – 2.11) | 1.63 | (1.27 – 2.08) |
| Radiation | ||||
| No | Ref | Ref | ||
| Yes | 1.22 | (1.08 – 1.38) | 1.22 | (1.08 – 1.39) |
| Deyo Comorbidity Score | ||||
| 0 | Ref | Ref | ||
| 1 | 1.01 | (0.86 – 1.19) | 1.03 | (0.87 – 1.21) |
| 2+ | 1.04 | (0.78 – 1.38) | 1.06 | (0.79 – 1.42) |
| Hypertension | ||||
| No | Ref | Ref | ||
| Yes | 0.95 | (0.84 – 1.08) | 0.92 | (0.80 – 1.05) |
| Valve Disease | ||||
| No | Ref | Ref | ||
| Yes | 0.95 | (0.70 – 1.28) | 0.96 | (0.70 – 1.30) |
| Insurance | ||||
| PPO | Ref | Ref | ||
| HMO | 1.05 | (0.87 – 1.28) | 1.09 | (0.89 – 1.34) |
| Other | 1.15 | (1.01 – 1.32) | 1.16 | (1.01 – 1.34) |
| Region | ||||
| Northeast | Ref | Ref | ||
| North Central | 0.98 | (0.81 – 1.18) | 1.01 | (0.83 – 1.23) |
| South | 0.88 | (0.74 – 1.05) | 0.93 | (0.78 – 1.11) |
| Unknown | 1.07 | (0.66 – 1.73) | 1.22 | (0.74 – 2.01) |
| West | 0.71 | (0.58 – 0.87) | 0.78 | (0.63 – 0.96) |
HF was more frequently identified among patients undergoing recommended cardiac monitoring (10.4% v 6.5%; P<0.001), suggesting that as more patients are screened, more patients are likely to be found as having HF. When evaluating only inpatient claims, we observed that the rates of HF were 2.0% among those who adhere to cardiac monitoring guidelines and 1.5% for those that did not (P=0.210).
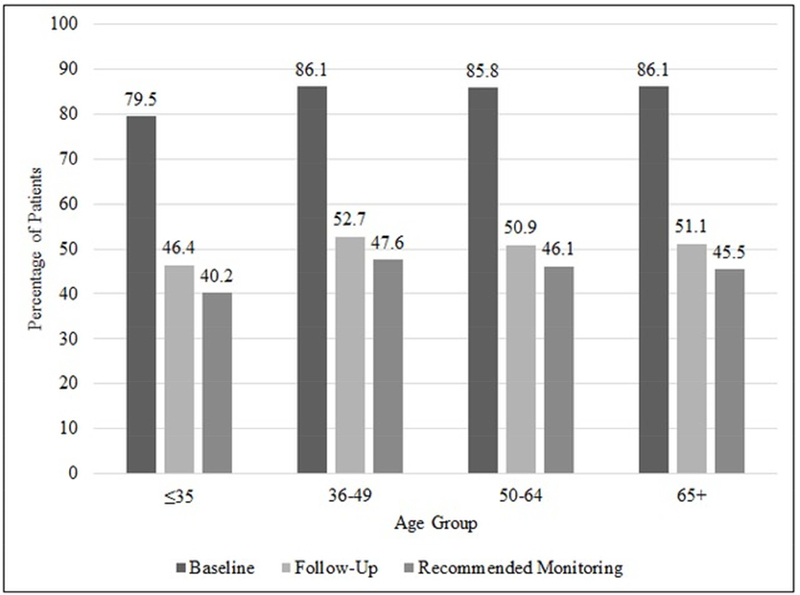
In Figure-2, we examined whether the rate of cardiac monitoring differed according to age and compared the rate of recommended cardiac monitoring. Overall, the rates were similar at baseline and at follow-up for all age groups. 79.5% of patients ages ≤35 received cardiac monitoring at baseline as compared to 86.1% of patients ages 65+. At follow-up, among patients ages ≤35, 40.2% received the recommended cardiac monitoring as compared to patients ages 65+, of which 45.5% had received the recommended cardiac monitoring. Patients aged ≤35 had the lowest rate of recommended cardiac monitoring, and the highest was seen in patients ages 36–49.
Figure-2. Rate of Cardiac Monitoring and Recommended Cardiac Monitoring by Age Group.
The percentage of cardiac monitoring at baseline (before trastuzumab treatment), at follow-up, and the overall rate of recommended cardiac monitoring by age group in trastuzumab-treated breast cancer patients (n=4325).
DISCUSSION
In this large cohort of breast cancer patients, we observed that 8.3% of the trastuzumab- treated patients developed cardiotoxicity compared to 2.7% among those who had not been treated with trastuzumab. Among patients who received trastuzumab, guideline-adherent cardiac monitoring was identified in 46.2% of patients.
To the best of our knowledge, this study is the first of its kind to estimate cardiotoxicity rates and cardiac monitoring in American women with breast cancer using the MarketScan database, including both younger and older women. The literature has primarily focused on older women, who have higher rates of HF than those reported in clinical trials (12). Our study particularly focuses on young women, who tend to have fewer comorbidities, and likely are relatively like the patients included in the pivotal trastuzumab clinical trials (13–15). Thavendiranathan et al. noted that as compared to older breast cancer patients, younger breast cancer patients have a longer life expectancy and may receive more aggressive chemotherapy (9). Thus, this is a critical group for analysis with regards to cardiotoxicity and cardiac monitoring.
Our calculated rate of cardiotoxicity was within the range of those reported in clinical trials. As expected, with increasing age, there was a consistent increase in the risk of cardiotoxicity. Deyo comorbidity score, hypertension, and valve disease were all associated with an increased risk of HF. This observation is in line with our previous findings. We reported that comorbidities were associated with increased risk of cardiotoxicity among older breast cancer patients (16). In addition, we quantified the risk of cardiotoxicity associated with other cancer therapies. Anthracycline and trastuzumab use was associated with a higher risk of HF. This finding is consistent with the known fact that anthracycline and trastuzumab use is associated with an increased risk of HF compared to no chemotherapy (17). Interestingly, taxanes were protective in our study, while the mechanisms for this observation is not clear and clinical trial data have not shown this observation, it has been reported that combination treatments with taxanes may be less cardiotoxic, and that modern adjuvant regimens of taxanes apparently do not increase anthracycline cardiotoxicity (18). We observed that radiation therapy had no association with risk of HF. While some suggest that left chest wall radiation therapy increases the risk of a cardiac event, we were not able to test this association since we cannot reliably identify laterality in this dataset (19).
A limitation of claims-based research is that we cannot identify the exact cause of the HF. We do not have data on the left ventricular ejection fraction (LVEF). It is possible that our estimates include events associated with causes other than cancer therapy; however, if this occurred, the misclassification would have been non-differential affecting potentially all the patients in the cohort and not biasing the direction of the estimate. We performed a sensitivity analysis evaluating exclusively inpatient claims as a marker for severity. The rates of HF requiring hospitalization were the same for both trastuzumab users and non-trastuzumab users. In addition, the estimates were much lower than what we observed in the original analysis that had both inpatient and outpatient claims, suggesting that most of the trastuzumab-related HF is diagnosed and treated in the outpatient setting.
We previously described in a cohort of Medicare patients (>65) that 36% received recommended cardiac monitoring (8). In our current work, including a younger patient population, we found that 46.2% of all trastuzumab-treated breast cancer patients received recommended cardiac monitoring. Furthermore, as expected, we found that younger breast cancer patients were less likely to receive cardiac monitoring during trastuzumab treatment as compared to older patients. We found that those who receive the recommended cardiac monitoring are more likely to be identified as having HF, meaning that there may be more asymptomatic cases of HF that would go undetected without cardiac monitoring. We must note, however, that our sensitivity analysis using inpatient claims allowed us to determine that the HF identified using cardiac monitoring was not severe enough to require hospitalization and was likely asymptomatic. The clinical implications of the diagnosis of asymptomatic HF are hard to determine and are beyond the scope of this study.
Across all age groups, baseline rates of cardiac monitoring were higher than at follow-up. Similarly, in a study conducted in Australia, 37.7% of patients received cardiac assessment pretreatment, and then only 26.4% received it both before and during therapy (20). The high rates at baseline, as opposed to follow-up, are likely due to the tendency for cardiotoxicity to appear in the first 3 months of treatment (13) (21). Other factors may be a lack of awareness of cardiac monitoring, as well as a perceived lack of clinical relevance or need among physicians.
Our results also capture an interesting time trend, as a more recent diagnosis was associated with increased odds of receiving the guideline-adherent cardiac monitoring. In a 2007 study by Subar et al, among 585 American breast cancer patients under age 65, only 15.9% received the full cardiac assessment (22). Our rate is higher, which may be reflective of an increased awareness of cardiotoxicity and an improved dissemination of the cardiac monitoring guidelines.
Comorbidity score, hypertension, and valve disease, and were not associated with increased odds of recommended cardiac monitoring. This is in line with our previous findings, where the administration of anthracyclines was associated with recommended cardiac monitoring, but comorbidities were not (8). Consistently, Lu et al. observed in a cohort of 3,418 patients with HER2+ metastatic breast cancer that trastuzumab, taxanes, anthracyclines, and older age predicted cardiac assessment (20). Subar et al. also reported that prior anthracycline treatment and duration of trastuzumab therapy were associated with increased odds of receiving cardiac monitoring (22).
Our study is unique. In this large cohort we examined time and geographic trends, insurance information, and a variety of comorbidities and cancer therapies. However, our study is subject to the limitations of claims-based research. Our cohort includes only patients with private insurance, which could limit generalizability. Because older women are more likely to be insured by Medicare, they may be underrepresented in this study. We were also unable to examine how these outcomes may have differed by cancer stage, race and ethnicity, and by rural or urban settings. Over-testing for cardiotoxicity and the impact of lifestyle factors, such as smoking or obesity may have also been areas of interest, but we were unable to evaluate them (15) (23) (24). The low rates of cardiac monitoring in women could have many possible explanations. They could reflect a low perceived need on the part of physicians, instead of unawareness of the guidelines. In our previous findings, physician-level characteristics had more influence on adherence to cardiac monitoring recommendations as compared to patient-level characteristics (8). The database used for this project did not allow us to examine this association. We must remember that while cardiac monitoring is recommended in different guidelines, such recommendations are not based on category 1 data, and the timing recommended and the intervals of testing are rather arbitrary (25). In examining the rate of both cardiac monitoring and cardiotoxicity we could begin to address the controversial issue of whether cardiac monitoring is warranted in young breast cancer patients (25–26).
Our study demonstrates that cancer treatment history and comorbidities are important risk factors for cardiotoxicity. Biomarkers may be promising and cost-effective in the prediction of cardiotoxicity as compared to cardiac monitoring (2) (27). The number of cancer survivors is expected to increase over time and we will continue to see patients develop treatment-related cardiotoxicity. Thus, more research, evidence-based guidelines, and tools for prediction of cancer treatment-related cardiotoxicity are needed.
PERSPECTIVES
Competency in Systems-Based Practice
This study can have implications in the use of cardiac monitoring in breast cancer patients. Our results suggest a low adherence to the guidelines for cardiac monitoring, as well as a time trend such that adherence is increasing over time. It is important to examine the reasons for the lack of adherence to cardiac monitoring. Our findings can begin to address the need for quality improvement in the cardiovascular care for cancer patients, as well as the evaluation of the cost- effectiveness for cardiac monitoring.
Competency in Patient Care and Procedural Skills
This study provides important information on the rates of cardiac toxicities associated with cancer therapies outside of a clinical trial. In a large patient population, we identified cardiotoxicity rates according to regimen and age, thus providing practicing clinicians important information that should be considered during treatment planning and counseling. Our data also adds to the understanding of the risk factors associated with HF in this patient population and thus, can help identify patients at higher risk who may benefit from cardiac monitoring.
Translational Outlook 1:
Since cancer therapies are associated with rare but clinically relevant cardiac toxicities, we should continue to look for a more cost-effective way of detecting cardiotoxicity, understanding that the primary objective is not only to identify toxicities but to improve outcomes.
Translational Outlook 2
Guideline-adherent monitoring may be potentially a marker of quality cancer care, that may lead to improved cardiovascular health in cancer patients. Additional research is needed to understand the reasons for the low rates of adherence to cardiac monitoring guidelines.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported in part by the National Cancer Institute’s Cancer Center support grant awarded to MD Anderson Cancer Center (2P30 CA016672), by the Cancer Prevention and Research Institute of Texas (CPRIT) grant (RP160674). Mariana Chavez-MacGregor, MD, MSc, and Sharon H. Giordano MD, MPH, are supported by the Susan G. Komen Breast Cancer Foundation (SAC150061 and SAC110053).
This project was supported by CPRIT RP160674, Komen SAC 150061, and NIH P30 CA016672.
ABBREVATIONS LIST
- CAD
Coronary Artery Disease
- cMRI
Cardiac Magnetic Resonance Imaging
- EMR
Electronic Medical Record
- HF
Heart Failure
- ICD
International Classification of Diseases
- MUGA
Multiple-gated acquisition
- NCCN
National Comprehensive Cancer Network
Footnotes
Dislcosures: Authors have no relationship with industry and no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Haque R, Prout M, Geiger AM, Kamineni A, Thwin SS, Avila C, et al. Comorbidities and cardiovascular disease risk in older breast cancer survivors. Am J Manag Care. 2014;20:86–92. [PMC free article] [PubMed] [Google Scholar]
- [2].Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, et al. Trastuzumabinduced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–6. [DOI] [PubMed] [Google Scholar]
- [3].Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE Jr., Ewer MS, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2positive breast cancer. J Clin Oncol. 2012;30:3792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huszno J, Les D, Sarzyczny-Slota D, Nowara E. Cardiac side effects of trastuzumab in breast cancer patients - single centere experiences. Contemp Oncol (Pozn). 2013;17:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xue J, Jiang Z, Qi F, Lv S, Zhang S, Wang T, et al. Risk of trastuzumab-related cardiotoxicity in early breast cancer patients: a prospective observational study. J Breast Cancer. 2014;17:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, Version 3.2014.
- [8].Chavez-MacGregor M, Niu J, Zhang N, Elting LS, Smith BD, Banchs J, et al. Cardiac Monitoring During Adjuvant Trastuzumab-Based Chemotherapy Among Older Patients With Breast Cancer. J Clin Oncol. 2015;33:2176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thavendiranathan P, Abdel-Qadir H, Fischer HD, Camacho X, Amir E, Austin PC, et al. Breast Cancer Therapy-Related Cardiac Dysfunction in Adult Women Treated in Routine Clinical Practice: A Population-Based Cohort Study. J Clin Oncol. 2016;34:2239–46. [DOI] [PubMed] [Google Scholar]
- [10].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- [11].Snapinn SM, Jiang QI, Iglewicz B. Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. The American Statistician. 2005;59(4): 301–7. [Google Scholar]
- [12].Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60:2504–12. [DOI] [PubMed] [Google Scholar]
- [13].Seferina SC, de Boer M, Derksen MW, van den Berkmortel F, van Kampen RJ, van de Wouw AJ, et al. Cardiotoxicity and Cardiac Monitoring During Adjuvant Trastuzumab in Daily Dutch Practice: A Study of the Southeast Netherlands Breast Cancer Consortium. Oncologist. 2016;21:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chin-Yee NJ, Yan AT, Kumachev A, Ko D, Earle C, Tomlinson G, et al. Association of hospital and physician case volumes with cardiac monitoring and cardiotoxicity during adjuvant trastuzumab treatment for breast cancer: a retrospective cohort study. CMAJ Open. 2016;4:E66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chavez-MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31:4222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Morris PG, Hudis CA . Trastuzumab-related cardiotoxicity following anthracycline-based adjuvant chemotherapy: how worried should we be? J Clin Oncol. 2010;28:3407–10. [DOI] [PubMed] [Google Scholar]
- [18].Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008;14:14–24. [DOI] [PubMed] [Google Scholar]
- [19].Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular Disease Mortality Among Breast Cancer Survivors. Epidemiology. 2016;27:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lu CY, Srasuebkul P, Drew AK, Chen K, Ward RL, Pearson SA. Trastuzumab therapy in Australia: which patients with HER2+ metastatic breast cancer are assessed for cardiac function? Breast 2013;22:482–7. [DOI] [PubMed] [Google Scholar]
- [21].Tarantini L, Cioffi G, Gori S, Tuccia F, Boccardi L, Bovelli D, et al. Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J Card Fail. 2012;18:113–9. [DOI] [PubMed] [Google Scholar]
- [22].Subar M, Lin W, Chen W, Pittman DG. Lack of uniformity in cardiac assessment during trastuzumab therapy. Breast J. 2011;17:383–90. [DOI] [PubMed] [Google Scholar]
- [23].Jones AL, Barlow M, Barrett-Lee PJ, Canney PA, Gilmour IM, Robb SD, et al. Management of cardiac health in trastuzumab-treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer. 2009;100:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guenancia C, Lefebvre A, Cardinale D, Yu AF, Ladoire S, Ghiringhelli F, et al. Obesity As a Risk Factor for Anthracyclines and Trastuzumab Cardiotoxicity in Breast Cancer: A Systematic Review and Meta-Analysis. J Clin Oncol. 2016;34:3157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dang CT, Yu AF, Jones LW, Liu J, Steingart RM, Argolo DF, et al. Cardiac Surveillance Guidelines for Trastuzumab-Containing Therapy in Early-Stage Breast Cancer: Getting to the Heart of the Matter. J Clin Oncol. 2016;34:1030–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dang C, Guo H, Najita J, Yardley D, Marcom K, Albain K, et al. Cardiac Outcomes of Patients Receiving Adjuvant Weekly Paclitaxel and Trastuzumab for Node-Negative, ERBB2Positive Breast Cancer. JAMA Oncol. 2016;2:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.