Abstract
Major histocompatibility complexes (MHC) have been used for more than two decades in clinical and pre-clinical approaches of tumor immunotherapy. They have been proven efficient for detecting anti-tumor-specific T cells when utilized as soluble multimers, immobilized on cells or artificial structures such as artificial antigenpresenting cells (aAPC) and have been shown to generate effective anti-tumor responses. In this review we summarize the use of soluble MHC class I complexes in tumor vaccination studies, highlighting the different strategies and their contradicting results. In summary, we believe that soluble MHC class I molecules represent an exciting tool with great potential to impact the understanding and development of immunotherapeutic approaches on many levels from monitoring to treatment.
Keywords: MHC-class I, T cells, vaccination, tumor, immunotherapy
Introduction
MHC class I and class II molecules have been evolutionarily optimized for the presentation of a variety of peptide antigens (Ag’s), which are then recognized by T cell receptors (TCR) present on the surface of T cells. To date over thirty unique TCR-MHC class I and over twenty unique TCR-MHC class II complex structures have been described in detail that result in effective CD8+ and CD4+ T cell stimulation, respectively, when loaded with corresponding Ag’s [1].
While both MHC class I and II have been utilized for immunotherapy approaches, this review will focus on the use of MHC class I molecules. MHC class I molecules have been used in many multimeric formulations, from dimeric to dodecameric (reviewed in [2] and [3]), for detection and analysis of antigen-specific CD8+ T cell responses. Specifically, after immobilization on artificial structures such as paramagnetic beads, liposomes or biodegradable backbones of different sizes and shapes, MHC class I molecules have been used to efficiently stimulate or modulate antigen-specific CD8+ T cell responses in vitro and in vivo (reviewed in [4–7]). More recently MHC class I molecules have also been utilized for redirection of antigenspecific CD8+ T cells to tumor cells, which they would otherwise not recognize [8– 13]. Furthermore, MHC class I molecules have been utilized to deplete antigenspecific CD8+ T cells. In these cases, they were conjugated to alpha emitting epitopes or toxins, or they were immobilized onto a surface of a paramagnetic or biodegradable bead together with a death inducing second signal such as an anti-Fas monoclonal antibody (mAb) (reviewed in [14] and [15–18]). Also noteworthy is that Yoshida et al. show in an elegant way how one can use the significant technological improvements in mass spectrometry based immunopeptidomics and in silico methodologies for the identification of tumor-antigens by utilizing transferred secreted human MHC class I molecules presenting peptides derived from intracellular proteins, which are then further processed and used for vaccination [19].
Finally, soluble MHC class I molecules have been used in various vaccination protocols resulting in a wide range of contradicting outcomes, reaching from tolerance induction to induction of anti-tumor responses [20–28]. Here, we will discuss possible reasons and explanations for either favorable or unfavorable immunotherapeutic soluble peptide-MHC-class I complex (pMC) based vaccination strategies. This work wants to contribute to the claim of Hu et al. that the full toolbox of immunotherapeutic approaches to cancer therapy has to be revisited to unleash the immune system’s full potential [29].
Possible mechanism of action for soluble pMC
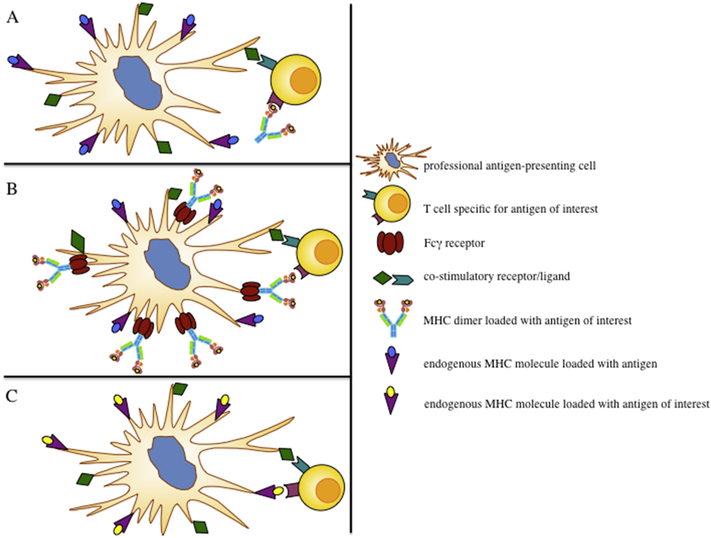
If a T cell engages a pMC (signal 1), it depends on the presence and type of the costimulatory signal (signal 2) if it results in activation, anergy or depletion. While immobilized pMC’s have been described to activate naïve CD8+ T cells in vitro by a CD28/B7 and LFA-1/ICAM-1 independent mechanism [21] following the “strengthof-signal” hypothesis [30,31], activation is also influenced by an adjuvant induced pro-inflammatory environment in combination with co-stimulatory signals provided by professional antigen-presenting cells (APC) or activated T cells (Fig. 1A).
Figure 1:
Schematic of possible mechanisms of action for soluble peptide MHC class I complexes (pMC). (A) Direct interaction of soluble pMC with T cells. (B) Interaction of T cells with Fcγ receptor bound pMC’s on professional antigenpresenting cells (APC). (C) Recognition of pMC-antigens as a result of antigen crosspresentation. In general the nature and extent of the induced T cell response will be dependent on a pro-inflammatory environment and co-stimulation provided by APC.
A second mechanism is the direct stimulation of a TCR by Fcγ-receptor (Fcγ-R□ bound pMC on the surface of an APC. It has been demonstrated that antibody Fc functionalized pMC’s can bind via Fcγ-RI and Fcγ-RIII onto professional antigenpresenting cells which enables them to activate antigen-specific T cell responses. This can be further enhanced through maturation of the APC with immune-stimulatory agents, such as lipo-poly-saccharide (LPS) [24], Poly (I:C) [32], and anti-CD40 mAb [23,33,34]. These agents cause up-regulation of Fcγ-R and expression of costimulatory molecules. Thus mature APC can present more pMC and provide better co-stimulation, which results in enhanced T cell stimulation, activation and proliferation (Fig. 1B).
Finally, pMC can be internalized by APC such as immature DC, by a mechanism called phagocytosis and/or pinocytosis. This initial uptake of pMC’s initiates a process described as cross-presentation. After internalization the pMC can be exported into the DC cytosol and processed by the proteasome. Subsequently, the dissociated peptide of a pMC will then be loaded onto host MHC class I molecules (reviewed in [35,36]). Alternatively, the pMC will be degraded into various peptides in the phagosome and directly loaded onto MHC class I molecules resulting in the presentation of multiple antigenic peptides, all different from the original loaded peptide of the pMC (reviewed in [37]). Though nature and extent of the immune response will depend on the DC phenotype (Fig. 1C).
While all three mechanisms may contribute to the generation of a pMC induced immune response, to date it is not clear as to which extent each mechanism contributes to the formation of this response.
However, it has been demonstrated that cross-presentation cannot be the only mechanism, as vaccination of TCR transgenic 2C mice that are of H2b background with soluble QL9-peptide loaded Ld-Ig molecules initiated a 2C T cell response. While 2C cells recognize QL9-peptide in the context of an Ld-MHC molecule in an allo-response, the QL9-peptide cannot be presented on the host Kb-MHC molecules. Therefore, the 2C response must be induced by one of the other two mechanisms [23]. Furthermore, it could be demonstrated that anti-CD40 mAb induced maturation of APC prior to pMC immunization was crucial for an effective immune response. Administration of anti-CD40 mAb together with or after pMC administration did not result in formation of a robust immune response, which further supports that the pMC is presented on the cell surface and not internalized [23].
Context of Stimulation
In general, to ensure a proper CD8+ T cells response upon re-stimulation a costimulatory signal during the initial stimulation is required. Furthermore, while primary CD8+ T cell responses can be independent of CD4+ T cell, dependent on antigen levels and presence of danger signals, the generation of a long-lived functional memory CD8+ T cell response requires sufficient CD4+ T cell help (reviewed in [38]).
Recently we demonstrated that pre-treatment with anti-CD40 mAb prior to pMC immunization initiated and significantly increased formation of a long-lived memory CD8+ T cell response in vivo [23]. This is in line with findings demonstrating that CD40/CD40L signaling between CD4+ T cells and APC renders the later one into a stimulatory cell for efficient CD8+ T cell priming [39]. Therefore, application of antiCD40 mAb leads to APC maturation and effective co-stimulation along with pMC resulting in a robust memory CD8+ T cell response. Interestingly, Goldstein et al. demonstrated efficient activation of CD44- naïve CD8+ T cell by stimulation with immobilized pMC’s independent of CD28/B7 and LFA-1/ICAM-1 signaling. This in vitro data was in line with the assumption that generation of a primary T cell response does not require an obligatory co-stimulation but whether these T cells developed a memory phenotype was not investigated [21,22]. Another study from Carey and colleagues, co-applying LPS and pMC demonstrated effective CD8+ T cell priming leading to prolonged survival after in vivo HSV-1 challenge [24]. Similarly, Sakita et al. and Maile et al. could demonstrate the formation of an in vivo anti-pMC T cell response but neither study provided any data regarding the T cell phenotype [25,26]. Furthermore, both studies utilized pMC isolated from E.coli inclusion bodies that might bear the risk of LPS contamination or non-specific immune stimulation by a different glycosylation pattern of the pMC [40].
In summary, we feel that more complete, comparative studies are necessary to answer the question as to what extent pMC immunization requires co-stimulation and in what form. However, there is already clear evidence that the addition of proper costimulation enhances pMC induced T cell response [20,24] and the formation of a long lived memory T cell population [23]. Thus, co-stimulation represents a crucial factor for pMC induced cytotoxic T cell responses.
Dosage, timing and route of administration
The timing of pMC administration is another crucial factor for the generation of either a protective anti-tumor or a suppressive T cell response to prolong transplant survival. In a rat model, Fried et al. demonstrated prolonged cardiac graft survival when animals were immunized with pMC daily for 14 days [27] and O’Herrin et al. described inhibition of allo-reactivity in mice through four immunizations with pMC [28]. In a comparative approach Maile et al. demonstrated that a single dose treatment with HY peptide loaded Db tetramers resulted in graft rejection whereas multiple injections, each with the same amount of the single dose treatment HY peptide, slowed down the rejection [26]. In the absence of co-stimulation, these experiments indicate that multiple pMC immunizations can result in the formation of a suppressive T cell response rather than the formation of a cytotoxic T cell response by single dose administration of pMC.
In contrast, the study of Savage et al. using the same animal model but a different HY peptide, showed 5 fold expansion of peptide specific T cells after triple immunization with HY loaded Db tetramers on day 0, 1 and 3 without additional co-stimulation [20]. One possible explanation for these findings is the previous immunization of female B6 mice with male B6 splenocytes several weeks before HY loaded Db tetramer immunization. This suggested the induction of a baseline HY-specific T cell response could provide co-stimulation during HY loaded Db tetramer immunization, a mechanism similar to the one that has been described by Paine et al. in the human system [41]. The study of Savage et al. did not provide direct co-stimulation along with multiple pMC immunizations, however, it is the only one priming an in vivo HYspecific T cell response prior to pMC immunization. All other studies utilizing multiple pMC applications neither provided co-stimulation nor in vivo priming [26– 28], suggesting that in the absence of co-stimulation the fate of immunization outcome (activation or anergy) is determined by pMC immunization timing. However, pMC immunization along with co-stimulations seems to result in the formation of an effective antigen-specific T cell response independent of timing.
Furthermore, it has been demonstrated that the formation of a cytotoxic T cell response could be further increased by a booster injection. To this purpose Schuurhuis et al. and our group demonstrated that a second booster injection or a tumor challenge should be applied between day 14 and 30 after initial immunization, providing enough time to induce a robust T cell response and the formation of a protective memory response [23,42].
Many studies have been performed utilizing pMC’s in a range from 0.5 – 1000 μg/mouse either applied in a single dose or in multiple steps. In general there is no correlation between amount of pMC applied and efficacy of the pMC-specific T cell response induced. Only two studies provided titration data suggesting that one time administration of high amounts of pMC resulted in a more robust pMC-specific T cell response formation in vivo [23,25]. Thus, dosage of pMC’s can vary and influence the strength of a pMC-specific T cell response but it seems not to determine the outcome of the immunization in terms of T cell activation or anergy induction. In addition, several of the discussed studies have analyzed the impact of the route of pMC administration i.p.[20,24,26–28] or s.c. [23,25] and concluded that the demonstrated effects of pMC immunization are independent of the route of administration.
Conclusion
Soluble pMC immunization has proven to induce a strong anti-tumor and anti-viral response when applied under pro-inflammatory conditions or with proper costimulation and with the correct timing. At the same time pMC immunization has the potential to be applied for tolerance induction to a specific Ag, facilitating the possibility of transplant protection when utilized without co-stimulus and in multistep immunization protocols. Furthermore, in light of recent progress in identification of tumor neo-Ag’s [19],[43–45], pMC based immunization could turn into an new approach of highly personalized cancer immunotherapy. Thus, we believe that the data gathered on soluble pMC immunization justifies further investigation for possible application in various immunological disorders. We hope this review highlights the importance and provokes further effort in this intriguing field of research.
Acknowledgements
This work was in part supported by the Patenschaftsprogramm 2016 of the Goethe University Frankfurt am Main (AS), German Cancer Foundation (grant no. 70112372) (CS); the National Institutes of Health (P01-AI072677 and R01CA108835), TEDCO/Maryland Innovation Initiative and NexImmune, Inc. MD Biotech Center (JPS).
List of abbreviations:
- MHC
major histocompatibility complex
- mAb
monoclonal antibody
- APC
antigen presenting cell
- aAPC
artificial antigen presenting cell
- DC
dendritic cell
- Ag’s
antigens
- pMC
peptide-MHC-class I complex
- TCR
T cell
Footnotes
Conflict of interest
A.S. and C.S. have nothing to disclose. Under a licensing agreement between NexImmune and the Johns Hopkins University, J.P.S. and M.O. are entitled to shares of royalty received by the University on sales of aAPC products described in this article. They also own NexImmune stock, which is subject to certain restrictions under University policy. Dr. Schneck is a member of the company’s Board of Directors and Scientific Advisory Board. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J, T Cell Antigen Receptor Recognition of Antigen-Presenting Molecules., Annu. Rev. Immunol (2014). doi: 10.1146/annurev-immunol-032414-112334. [DOI] [PubMed] [Google Scholar]
- [2].Casalegno-Garduño R, Schmitt A, Yao J, Wang X, Xu X, Freund M, Schmitt M, Multimer technologies for detection and adoptive transfer of antigen-specific T cells., Cancer Immunol. Immunother 59 (2010) 195–202. doi: 10.1007/s00262-009-0778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Huang J, Zeng X, Sigal N, Lund PJ, Su LF, Huang H, Chien Y, Davis MM, Detection, phenotyping, and quantification of antigen-specific T cells using a peptide-MHC dodecamer., Proc. Natl. Acad. Sci. U. S. A 113 (2016) E1890–7. doi: 10.1073/pnas.1602488113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Oelke M, Schneck JP, Overview of a HLA-Ig based “Lego-like system” for T cell monitoring, modulation and expansion., Immunol. Res 47 (2010) 248–56. doi: 10.1007/s12026-009-8156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sunshine JC, Green JJ, Nanoengineering approaches to the design of artificial antigen-presenting cells., Nanomedicine (Lond). 8 (2013) 1173–89. doi: 10.2217/nnm.13.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Perica K, Kosmides AK, Schneck JP, Linking form to function: Biophysical aspects of artificial antigen presenting cell design., Biochim. Biophys. Acta. (2014). doi: 10.1016/j.bbamcr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eggermont LJ, Paulis LE, Tel J, Figdor CG, Towards efficient cancer immunotherapy: advances in developing artificial antigen-presenting cells., Trends Biotechnol. 32 (2014) 456–65. doi: 10.1016/j.tibtech.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mous R, Savage P, Remmerswaal EBM, van Lier RAW, Eldering E, van Oers MHJ, Redirection of CMV-specific CTL towards B-CLL via CD20targeted HLA/CMV complexes., Leukemia. 20 (2006) 1096–102. doi: 10.1038/sj.leu.2404185. [DOI] [PubMed] [Google Scholar]
- [9].Savage P, Dyson J, Milrain M, Mathews D, King B, Chan HTC, Barber L, Epenetos A, Ogg G, McMichael A, Glennie MJ, French RR, Immunotherapy with antibody-targeted HLA class I complexes: results of in vivo tumour cell killing and therapeutic vaccination., Tumour Biol. 28 (2007) 205–11. doi: 10.1159/000107416. [DOI] [PubMed] [Google Scholar]
- [10].King BC, Hamblin AD, Savage PM, Douglas LR, Hansen TH, French RR, Johnson PWM, Glennie MJ, Antibody-peptide-MHC fusion conjugates target non-cognate T cells to kill tumour cells., Cancer Immunol. Immunother 62 (2013) 1093–105. doi: 10.1007/s00262-013-1408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schmittnaegel M, Levitsky V, Hoffmann E, Georges G, Mundigl O, Klein C, Knoetgen H, Committing Cytomegalovirus-Specific CD8 T Cells to Eliminate Tumor Cells by Bifunctional Major Histocompatibility Class I Antibody Fusion Molecules., Cancer Immunol. Res (2015). doi: 10.1158/2326-6066.CIR-15-0037. [DOI] [PubMed] [Google Scholar]
- [12].Schütz C, Varela JC, Perica K, Haupt C, Oelke M, Schneck JP, Antigenspecific T cell Redirectors: a nanoparticle based approach for redirecting T cells., Oncotarget 7 (2016) 68503–12. doi: 10.18632/oncotarget.11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Robert B, Guillaume P, Luescher I, Doucey MA, Cerottini JC, Romero P, Mach JP, Redirecting anti-viral CTL against cancer cells by surface targeting of monomeric MHC class I-viral peptide conjugated to antibody fragments., Cancer Immun 1 (2001) 2 http://www.ncbi.nlm.nih.gov/pubmed/12747763 (accessed August 31, 2017). [PubMed] [Google Scholar]
- [14].Schütz C, Oelke M, Schneck JP, Mackensen A, Fleck M, Killer artificial antigen-presenting cells: the synthetic embodiment of a “guided missile”., Immunotherapy. 2 (2010) 539–50. doi: 10.2217/imt.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shen C, He Y, Cheng K, Zhang D, Miao S, Zhang A, Meng F, Miao F, Zhang J, Killer artificial antigen-presenting cells deplete alloantigen-specific T cells in a murine model of alloskin transplantation., Immunol. Lett 138 (2011) 144–55. doi: 10.1016/j.imlet.2011.04.002. [DOI] [PubMed] [Google Scholar]
- [16].Schütz C, Fleck M, Mackensen A, Zoso A, Halbritter D, Schneck JP, Oelke M, Killer artificial antigen-presenting cells: a novel strategy to delete specific T cells., Blood. 111 (2008) 3546–52. doi: 10.1182/blood-2007-09113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schütz C, Fleck M, Schneck JP, Oelke M, Killer Artificial Antigen Presenting Cells (KaAPC) for Efficient In Vitro Depletion of Human Antigen-specific T Cells, J. Vis. Exp (2014). doi: 10.3791/51859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang W, Fang K, Li M-C, Chang D, Shahzad KA, Xu T, Zhang L, Gu N, Shen CL, A biodegradable killer microparticle to selectively deplete antigenspecific T cells in vitro and in vivo., Oncotarget. (2016). doi: 10.18632/oncotarget.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yoshida M, Ishioka Y, Ozawa T, Okuyama H, Iguchi M, Ota T, Ito T, Nagira M, Morita A, Tanaka H, Naito H, Kidoya H, Takakura N, Soluble HLAassociated peptide from PSF1 has a cancer vaccine potency, Sci. Rep 7 (2017) 1–10. doi: 10.1038/s41598-017-11605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Savage P, Millrain M, Dimakou S, Stebbing J, Dyson J, Expansion of CD8+ cytotoxic T cells in vitro and in vivo using MHC class I tetramers., Tumour Biol. 28 (2007) 70–6. doi: 10.1159/000099152. [DOI] [PubMed] [Google Scholar]
- [21].Goldstein JS, Chen T, Brunswick M, Mostowsky H, Kozlowski S, Purified MHC class I and peptide complexes activate naive CD8+ T cells independently of the CD28/B7 and LFA-1/ICAM-1 costimulatory interactions., J. Immunol 160 (1998) 3180–7. http://www.ncbi.nlm.nih.gov/pubmed/9531273 (accessed January 14, 2015). [PubMed] [Google Scholar]
- [22].Goldstein J, Mostowsky H, Tung J, Hon H, Brunswick M, Kozlowski S, Naive alloreactive CD8 T cells are activated by purified major histocompatibility complex class I and antigenic peptide., Eur. J. Immunol 27 (1997) 871–8. doi: 10.1002/eji.1830270411. [DOI] [PubMed] [Google Scholar]
- [23].Schütz C, Zoso A, Peng S, Bennett JD, Schneck JP, Oelke M, MHC-Ig induces memory T cell formation in vivo and inhibits tumour growth, Immunity, Inflamm. Dis 2 (2014) 181–192. doi: 10.1002/iid3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Carey B, DeLay M, Strasser JE, Chalk C, Dudley-McClain K, Milligan GN, Brunner HI, Thornton S, Hirsch R, A soluble divalent class I MHC/IgG1 fusion protein activates CD8+ T cells in vivo., Clin. Immunol 116 (2005) 65–76. doi: 10.1016/j.clim.2005.02.013. [DOI] [PubMed] [Google Scholar]
- [25].Sakita I, Hörig H, Sun R, Wang F, Nathenson SG, In vivo CTL immunity can be elicited by in vitro reconstituted MHC/peptide complex., J. Immunol. Methods 192 (1996) 105–15. http://www.ncbi.nlm.nih.gov/pubmed/8699005 (accessed January 19, 2015). [DOI] [PubMed] [Google Scholar]
- [26].Maile R, Wang B, Schooler W, Meyer A, Collins EJ, Frelinger JA, Antigenspecific modulation of an immune response by in vivo administration of soluble MHC class I tetramers., J. Immunol 167 (2001) 3708–14. http://www.ncbi.nlm.nih.gov/pubmed/11564786 (accessed January 7, 2015). [DOI] [PubMed] [Google Scholar]
- [27].Fried A, Berg M, Sharma B, Bonde S, Zavazava N, Recombinant dimeric MHC antigens protect cardiac allografts from rejection and visualize alloreactive T cells., J. Leukoc. Biol 78 (2005) 595–604. doi: 10.1189/jlb.0205078. [DOI] [PubMed] [Google Scholar]
- [28].O’Herrin SM, Slansky JE, Tang Q, Markiewicz MA, Gajewski TF, Pardoll DM, Schneck JP, Bluestone JA, Antigen-specific blockade of T cells in vivo using dimeric MHC peptide., J. Immunol 167 (2001) 2555–60. http://www.ncbi.nlm.nih.gov/pubmed/11509595 (accessed January 14, 2015). [DOI] [PubMed] [Google Scholar]
- [29].Hu Z, Ott PA, Wu CJ, Towards personalized, tumour-specific, therapeutic vaccines for cancer, Nat. Rev. Immunol. 18 (2018) 168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bluestone JA, New perspectives of CD28-B7-mediated T cell costimulation., Immunity. 2 (1995) 555–9. http://www.ncbi.nlm.nih.gov/pubmed/7540940 (accessed June 12, 2017). [DOI] [PubMed] [Google Scholar]
- [31].Lenschow DJ, Walunas TL, Bluestone JA, CD28/B7 system of T cell costimulation., Annu. Rev. Immunol 14 (1996) 233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- [32].Wischke C, Zimmermann J, Wessinger B, Schendler A, Borchert H-H, Peters JH, Nesselhut T, Lorenzen DR, Poly(I:C) coated PLGA microparticles induce dendritic cell maturation., Int. J. Pharm 365 (2009) 61–8. doi: 10.1016/j.ijpharm.2008.08.039. [DOI] [PubMed] [Google Scholar]
- [33].Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, Offringa R, Toes RE, CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy., Nat. Med 5 (1999) 774–9. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- [34].von Bergwelt-Baildon M, Shimabukuro-Vornhagen A, Popov A, KleinGonzalez N, Fiore F, Debey S, Draube A, Maecker B, Menezes I, Nadler LM, Schultze JL, CD40-activated B cells express full lymph node homing triad and induce T-cell chemotaxis: potential as cellular adjuvants., Blood. 107 (2006) 2786–9. doi: 10.1182/blood-2004-01-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Apcher S, Prado Martins R, Fåhraeus R, The source of MHC class I presented peptides and its implications, Curr. Opin. Immunol. 40 (2016) 117–122. doi: 10.1016/j.coi.2016.04.002. [DOI] [PubMed] [Google Scholar]
- [36].Ge Q, Stone JD, Thompson MT, Cochran JR, Rushe M, Eisen HN, Chen J, Stern LJ, Soluble peptide-MHC monomers cause activation of CD8+ T cells through transfer of the peptide to T cell MHC molecules., Proc. Natl. Acad. Sci. U. S. A 99 (2002) 13729–34. doi: 10.1073/pnas.212515299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Joffre OP, Segura E, Savina A, Amigorena S, Cross-presentation by dendritic cells., Nat. Rev. Immunol 12 (2012) 557–69. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- [38].Bevan MJ, Helping the CD8(+) T-cell response., Nat. Rev. Immunol. 4 (2004) 595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- [39].Clarke SR, The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity., J. Leukoc. Biol 67 (2000) 607–14. http://www.ncbi.nlm.nih.gov/pubmed/10810999 (accessed January 14, 2015). [DOI] [PubMed] [Google Scholar]
- [40].Tan FYY, Tang CM, Exley RM, Sugar coating: bacterial protein glycosylation and host-microbe interactions., Trends Biochem. Sci (2015). doi: 10.1016/j.tibs.2015.03.016. [DOI] [PubMed] [Google Scholar]
- [41].Paine A, Kirchner H, Immenschuh S, Oelke M, Blasczyk R, Eiz-Vesper B, IL-2 upregulates CD86 expression on human CD4(+) and CD8(+) T cells., J. Immunol. 188 (2012) 1620–9. doi: 10.4049/jimmunol.1100181. [DOI] [PubMed] [Google Scholar]
- [42].Schuurhuis DH, van Montfoort N, Ioan-Facsinay A, Jiawan R, Camps M, Nouta J, Melief CJM, Verbeek JS, Ossendorp F, Immune complex-loaded dendritic cells are superior to soluble immune complexes as antitumor vaccine., J. Immunol 176 (2006) 4573–80. http://www.ncbi.nlm.nih.gov/pubmed/16585547 (accessed January 19, 2015). [DOI] [PubMed] [Google Scholar]
- [43].Duan F, Duitama J, Al Seesi S, Ayres CM, Pawashe AP, Blanchard T, Mcmahon D, Sidney J, Sette A, Baker BM, Mandoiu II, Srivastava PK, Comprehensive RN, Cancer H, Dame N, Genomic and bio-informatic profiling of mutational neo-epitopes reveals new rules to predict anti-cancer immunogenicity, J. Exp. Med 211 (2014) 2231–2248. doi: 10.1084/jem.20141308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fritsch EF, Rajasagi M, Ott PA, Brusic V, Hacohen N, Wu CJ, HLAbinding properties of tumor neoepitopes in humans., Cancer Immunol. Res 2 (2014) 522–9. doi: 10.1158/2326-6066.CIR-13-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bassani-Sternberg M, Bräunlein E, Klar R, Engleitner T, Sinitcyn P, Audehm S, Straub M, Weber J, Slotta-Huspenina J, Specht K, Martignoni ME, Werner A, Hein R, H Busch D, Peschel C, Rad R, Cox J, Mann M, Krackhardt AM, Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry., Nat. Commun 7 (2016) 13404. doi: 10.1038/ncomms13404. [DOI] [PMC free article] [PubMed] [Google Scholar]