Abstract
Introduction:
A clustering of relatives with dystonia has been reported in families with musician’s dystonia suggesting a genetic contribution to this disease. The aim of the present study was to determine whether interhemispheric inhibition (IHI) measured with transcranial magnetic stimulation is impaired in healthy family members rendering it a suitable endophenotypic marker for musician’s dystonia.
Methods:
Patients with musician’s hand dystonia (n ¼ 21), patients with sporadic writer’s cramp (n ¼ 15), their healthy family members (n ¼ 27), healthy musicians (n 12) and healthy non-musicians (n = 12) were included. An extended interview about the family history and musical activity was performed. IHI in both hemispheres was measured using transcranial magnetic stimulation.
Results:
A stepwise regression analysis revealed musical activity (p ¼ 0.001) and a family history of Endophenotype dystonia (p ¼ 0.008) but not dystonia per se, age, handedness or gender as relevant factors modulating IHI.
Conclusion:
These data support the notion of a genetic background of musician’s hand dystonia and suggests that reduced IHI is a possible endophenotypic marker of this disorder.
Keywords: TMS, Dystonia, Musicians dystonia, Endophenotype
1. Introduction
Focal hand dystonia is a movement disorder that impairs hand control during certain tasks such as writing or playing a musical instrument [1,2].
A clustering of focal hand dystonia has been reported in families of index patients with musician’s dystonia suggesting autosomal dominant inheritance with very low penetrance [3,4].
This is inline with reports on other types of dystonia including cervical dystonia. In these patients and their first-degree relatives impaired temporal discrimination of sensory stimuli appears to be an endophenotype inherited in an autosomal dominant fashion with low penetrance [5–7]. Similarly, abnormal vibration induced illusions might be an endophenotypic trait in patients with focal dystonia [8]. Interhemispheric inhibition (IHI) determined with paired-pulse transcranial magnetic stimulation (TMS) was reported to be reduced in the affected hemisphere in patients with focal hand dystonia in the study of Nelson et al. [9,10] In the studies of Beck et al. and Sattler et al. [11,12] it was reduced in those dystonia patients who also had mirror dystonia. IHI that is mediated by transcallosal fibres [13,14] may represent a marker of bimanual control and could therefore also be a valuable endophenotypic feature in dystonia patients.
Highly skilled hand functions require independence of hand movements. Reduced IHI associated with mirror movements could be a contributing factor to the development of disturbed hand function, particularly in highly demanding tasks like playing an instrument at a professional level [15].
In the present study, IHI was tested in musician’s hand dystonia and writer’s cramp patients, their healthy first-degree family members and two control groups (healthy musicians and healthy non-musicians).
Our aim was to further elucidate the impact of a familial background of dystonia as a marker of a genetic susceptibility, the presence of dystonia as a sign and musical activity as an index of hand skill function on IHI to determine whether IHI could serve as an endophenotypic marker for focal hand dystonia.
Our main hypotheses were i) that musical activity is associated with stronger IHI and ii) that a positive family history of dystonia reflecting genetic susceptibility and dystonia as a sign are associated with weaker IHI.
2. Methods
2.1. Subjects
Twenty-one professional musicians with musician’s hand dystonia (14 men, mean age 50 years (9 ± SD), range 35–80 years), 15 patients with sporadic writer’s cramp (7 men, mean age 52 years (15 ± SD), range 26–73 years), 27 healthy first-degree family members of the patients with musician’s hand dystonia or writer’s cramp (12 men, mean age 41 years (20 ± SD), range 19–77 years), 12 professional healthy musicians (7 men, mean age 48 years (8 ± SD), range 36–59 years) and 12 healthy non-musicians (7 men, mean age 50 years (5 ± SD), range 40–57 years) participated in the study. Patients and their relatives were recruited by experienced movement disorders specialists at the Hanover Institute of Music Physiology and Musician’s Medicine and the Movement Disorders Clinics in the Neurology Departments of the University hospitals of Lübeck and Hamburg, Germany between 2010 and 2012. A diagnosis of musician’s hand dystonia and writer’s cramp was made according to published standard criteria [16,17]. Healthy musicians and healthy non-musicians were recruited by the same examiners in Lübeck or Hamburg, Germany. Clinical details of participants are given in Table 1.
Table 1.
Demographics of the different groups.
| Familial Musician’s hand dystonia | Sporadic Musician’s hand dystonia | Sporadic Writer’s cramp | Healthy first-degree family members | Healthy musicians | Healthy nonMusicians | |
|---|---|---|---|---|---|---|
| Number | 12 | 9 | 15 | 27 | 12 | 12 |
| Gender (F/M) | 4/8 | 3/6 | 8/7 | 15/12 | 5/7 | 5/7 |
| Age, y, mean ± SD (R) | 49 ± 4 (40–55) | 52 ± 13 (35–80) | 52 ± 15 (26–73) | 41 ± 20 (19–77) | 48 ± 8 (36–59) | 50 ± 5 (40–57) |
| Handedness (right/left/ambidextrous) | 11/0/1 | 9/0/0 | 10/4/1 | 25/2/0 | 11/0/1 | 11/0/1 |
| Side of dystonia (right/left/bilateral) | 6/3/3 | 5/4/0 | 11/2/2 | n.a. | n.a. | n.a. |
| Onset age, y, mean ± SD (R) | 31 ± 6 (24–42) | 35 ± 14 (20–66) | 40 ± 13 (20–65) | n.a. | n.a. | n.a. |
| Duration of dystonia, y, mean ± SD (R) | 18 ± 6 (9–28) | 18 ± 6(11–29) | 12 ± 11 (1–34) | n.a. | n.a. | n.a. |
| Family history of dystonia (pos./neg.) | 12/0 | 0/12 | 0/15 | 27/0 | 2/10 | 0/12 |
| Musical activity (professional-/amateur-/non-musician) | 12/0/0 | 9/0/0 | 0/0/16 | 3/14/10 | 12/0/0 | 0/0/12 |
| Instrument group: | 5 | 4 | 4 | 6 | n.a. | |
| Woodwind | 2 | 1 | 3 | 1 | ||
| String | 5 | 4 | 1 | 1 | ||
| Brass | 4 | 4 | ||||
| Plucking | 4 | |||||
| Keyboard | 1 | |||||
| Drum | ||||||
| Age of first practice on the instrument, y, mean ± SD (R) | 10 ± 3(7–18) | 10 ± 2(7–14) | n.a. | 7 ± 2(6–12) | 9 ± 3(5–17) | n.a. |
Y = years, SD = standard deviation, R = range, n.a. = not applicable.
The vast majority of participants were right-handed (68 right handed, 6 left handed and 5 ambidextrous) according to the Edinburgh handedness inventory [18](Table 1). Gender distribution, age and handedness did not differ significantly between groups. Healthy musicians were matched for instrument groups to the musician’s hand dystonia patients. All musician’s hand dystonia and writer’s cramp patients had isolated dystonia with simple task-specific cramps and the majority (43 of 46) only had unilateral symptoms on their right hand. None of the patients had mirror dystonia. They had not received botulinum toxin injections for at least three months prior to the study. Twelve musician’s hand dystonia patients had a positive family history of dystonia according to the examination procedure published previously [4]. Thus, 12 cases with familial musician’s hand dystonia, 9 with sporadic musician’s hand dystonia and 15 with sporadic writer’s cramp were included. The musical activity of all participants was classified into the categories professionals, amateur and no musician. All participants provided written informed consent. The study was approved by the Hamburg Ethics Board.
2.2. EMG recording
Subjects were seated in a comfortable armchair. A TMS coil and subject head holder (Brainsight TMS frame; Roque Research Inc. Montreal; Canada) were adjusted to a frame surrounding the subjects’ chair. The head holder fixed to the frame allowed for a comfortable sitting position with the subjects’ heads resting on the holder and neck muscles relaxing. The coil holder ensured an accurate positioning of the TMS coils onto the subjects’ heads. The arms of the subjects were supported by a pillow so that arm muscles were completely relaxed. Subjects were instructed to relax but to keep their eyes open throughout the experiment.
EMG was recorded with disc surface electrodes placed over first dorsal interosseous muscles bilaterally, using a belly-tendon montage. In addition to the target first dorsal interosseous muscle where motor evoked potential (MEP) were measured, contralateral first dorsal interosseous muscle was also recorded to capture baseline EMG activity during measurements. EMG signals were continuously monitored acoustically with loudspeakers and visually by means of an oscilloscope. The ground electrode was placed at the wrist. EMG signals were amplified and filtered (20 Hz–1 kHz) with a D360 amplifier (Digitimer Limited, Welwyn Garden City, UK). The signals were sampled at 5000 Hz, digitised using a laboratory interface (Micro1401, Cambridge Electronics Design (CED), Cambridge, UK) and stored on a personal computer for display and later off-line data analysis.
2.3. TMS technique
Measurements were performed with two Magstim 200 magnetic stimulators, each connected with a figure-of-eight shaped coil with an outer winding diameter of approximately 70 mm (“baby coil”; Magstim Company, Whitland, Dyfed, UK) with handles perpendicular to the coil windings (“Branding-Iron-Style”) both for conditioning pulse and test pulse to measure IHI.
The coil was placed tangentially to the scalp at a 45°angle away from the midline, approximately perpendicular to the line of the central sulcus inducing a posterior to anterior directed current in the brain. We determined the optimal position for activation of the first dorsal interosseous muscles by moving the coil in 0.5 cm steps around the presumed primary motor hand area of both hemispheres. The sites where stimuli of slightly suprathreshold intensity consistently produced the largest MEPs with the steepest negative slope in the corresponding first dorsal interosseous muscle (referred to as “motor hot spot”) were marked with a wax pen. TMS coils were fixed to the frame using coil holders and placed at the marked stimulation sites.
Resting motor threshold was defined as the minimum stimulus intensity that produced an MEP of more than 50 μV in 5 out of 10 consecutive trials. It was expressed as a percentage of maximum stimulator output and was determined bilaterally over the primary motor hand areas. The intensity of the test pulse was set at an intensity that, when it was given alone, would evoke an EMG response of approximately 1 mV peak-to-peak size in the left first dorsal interosseous muscle.
IHI was probed using a conditioning-test paradigm. Conditioning pulses were applied to left and test pulse given to right primary motor hand area and vice versa. The intensity was always set at 120% of the resting motor threshold of the conditioned primary motor hand area.
IHI was tested at interstimulus intervals (ISIs) of 6, 7, 8, 9 and 10 ms. These six conditions (test pulse was given alone for 20 times and five conditioning pulse at different ISIs 10 times each) were applied randomly in a block of 70 trials.
2.4. Data analyses
Measurements were made on individual trials. Mean peak-to-peak MEP amplitudes were determined. IHI was calculated for each ISI as a percent value in relation to the mean test pulse amplitude from right to left and from left to right hemisphere. Thus, high values represent weak IHI and vice versa.
2.5. Statistical analyses
Repeated measures ANOVA were used to test for main effects and interactions of the factors ISI (6,7,8,9,10 ms) and group (familial musician’s hand dystonia, sporadic musician’s hand dystonia, healthy first-degree family members, healthy musicians, healthy non-musicians, writer’s cramp) and the factor side (right or left hemisphere). For group comparisons of resting motor threshold and test MEP size one factor ANOVA (group) was used. Greenhouse-Geisser correction was used to correct for non-sphericity. Post hoc paired sample t-tests with Bonferroni correction were used.
For comparisons between two datasets like resting motor thresholds between hemispheres we used paired samples t-tests.
After excluding significant interactions of ISIs and side with the factor group we performed our main analysis of the impact of different clinical and demographic factors on mean IHI.
We performed a hierarchical multiple regression analysis with a first step analysis of the factors sex, age and presence of dystonia, factors previously described as influencing IHI [10,11,19]. In a second step, the factors musical activity (professional; amateur, non musician) and family history of dystonia were considered.
3. Results
3.1. Resting motor thresholds and unconditioned MEP amplitudes
Resting motor threshold was significantly lower in the left hemisphere (42.5 SD 8.7 versus 45.7% SD 8.0 maximum stimulator output) (F (5,86)=30.6; p < 0.001) but there was no group difference F (5,86)=0.77; p=0.58) or interaction of side × group (F(5,86)=0.97; p=0.44). MEP amplitudes did not differ betweenhemispheres (left 0.64 mV SD 0.55; right=0.56 mV SD 0.39) (F(5,86)=2.9; p=0.87), groups (F (5,86)=1.1; p=0.38) and there was no interaction of these factors (F (5,86)=1.3;p=0.29).
3.2. Interhemispheric inhibition
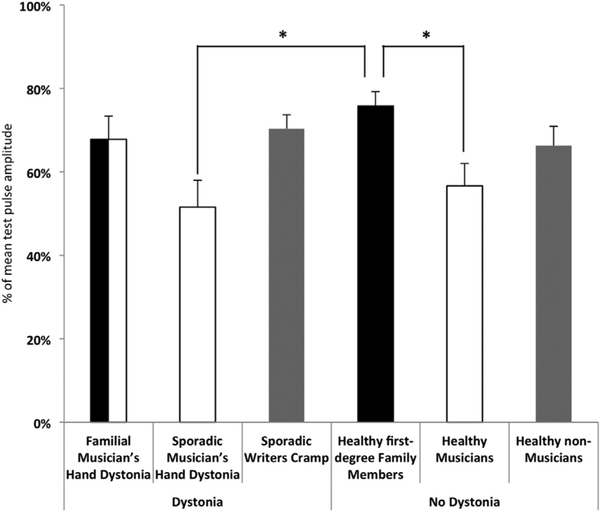
IHI differed between groups (F (5,86) = 4,0, p 0.03). There was no difference between sides (F (5,86) = 0.8, p=0.36) and also no interaction of group × side (F (5,86)=0.99, p = 0.43). The factor ISI showed a significant effect (F (4,344) = 16.3, p < 0.001) but there was no interaction of ISI with the factor group (F (4; 344)=1.1; p = 0.34). Also, there was no three way interaction of factors group, side and ISI (F (20; 344) = 0.67; p = 0.86). Post hoc tests of thegroup differences revealed a significant difference of IHI between healthy musicians and healthy first-degree family members (p = 0.07) and between sporadic musicians’ hand dystonia and healthy first-degree family members (p=0.17) (Fig. 1).
Fig. 1.
Group differences of interhemispheric inhibition (IHI). Mean IHI values for the different groups are given. The presence of the factor dystonia is symbolized by the sorting of columns with groups with dystonia on the right and those without on the left side of the figure. The factor family history of dystonia is symbolized by the colour black and the factor professional musician by the colour white. The grey columns indicate groups without any of these factors. Note that the group familial musician’s hand dystonia include both factors. The asterixis indicate a significant difference in the post hoc test (Bonferroni correction) between groups (p < 0.05).
Because there was no interaction of the factor group with any other factors, (side or ISI) we used mean values of IHI for further analysis.
3.3. Influence of age, dystonia, family history of dystonia and musical activity on interhemispheric inhibition
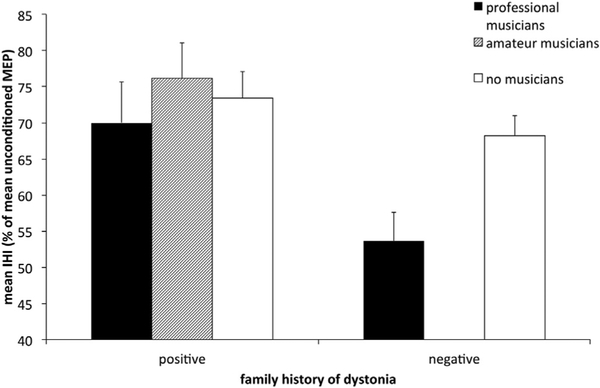
A multiple stepwise regression analysis of the factors age, sex, dystonia, handedness, “musical activity” and “family history of dystonia” revealed that the factors “musical activity” and “family history of dystonia” significantly influenced the degree of IHI (Fig. 2). The model (r2 = 0.16; F (86) = 8.2; p=0.001) revealed that 16% of the variability on IHI is explained by the included factors “family history of dystonia” (B = −12.6; β = −0.34; p = 0.001) and “musical activity” (B = 5.5; β = −0.27; p = 0.008).
Fig. 2.
Strength of interhemispheric inhibition (IHI) in relation to a family history of dystonia and musical activity. Mean values of IHI are displayed as a function of the status of musical activity and the presence of a family history of dystonia. Mean IHI values and standard error of mean (error bars) are given.
The p-values for the excluded factors in the model were the following: dystonia p = 0.95; gender p = 0.89; handedness p = 0.77; age p = 0.7.
4. Discussion
The main findings in this study are that IHI was more pronounced in people with a high degree of musical activity and reduced in people with a family history of dystonia. Importantly and unexpectedly, dystonia as a clinical sign was not a determinant of IHI.
4.1. Interhemispheric inhibition and musical activity
For a professional musician a high degree of independent, finely tuned bilateral and at times synchronized manual performance are prerequisites. There is good evidence that the ability of independent manual activity is predominantly mediated by transcallosal projections [15,19,20].
In early childhood, children typically have mirror movements of the hands during motor tasks which is associated with weak IHI at that age [19]. Using diffusion tensor imaging, less fractional anisotropy in the area III of the corpus callosum and less IHI was found in preschool children compared to adults [21]. This finding is in line with a study showing a gradual maturation of the connectivity of the motor part of the callosal fibre system during childhood and adolescence [22].
Similarly, it was shown in adults that the degree of mirror movements is inversely correlated with the degree of IHI [15]. On a structural level, the fractional anisotropy of callosal fibres connecting the hand motor areas is correlated positively with the degree of IHI [23]. In addition, deterioration of hand motor performance in elderly subjects correlates with some measures of interhemispheric inhibition [20].
Given that manual performance apparently depends, at least in part, on IHI and the integrity of callosal fibres, a positive correlation between musical activity and the degree of IHI, as found in the present study, is probably not surprising. However, in the study by Ridding et al. [24] IHI was reduced, rather than increased in healthy musicians both at rest and with the target muscle being active. The main difference between the study of Ridding and the present study relates to the fact that Ridding and colleagues had included a smaller group of musicians (n = 6) and did not specifically examine the influence of family history of dystonia or the degree of musical activity on IHI. Reduced IHI in their group of musicians might have been the result of factors other than musical activity.
Supporting the role of interhemispheric connections for musical performance, a recent diffusion tensor imaging MRI study addressing the diffusibility of callosal connections in the auditory systems showed a higher transcallosal connectivity of the planum temporale of both hemispheres in professional musicians [25].
4.2. Interhemispheric inhibition and dystonia
Our initial hypothesis was that dystonia as a sign would be associated with weak IHI. However, we could not confirm this. Dystonia as a clinical sign did not explain the variability of IHI in our cohort. At first sight, this finding may appear counterintuitive. However, previous studies addressing this issue are also equivocal. For instance, whereas Nelson et al. [10] found IHI to be reduced in the affected hemisphere in writer’s cramp patients at ISIs of 10e12 ms with the target muscles at rest, IHI reductions in focal hand dystonia patients were related to mirror movements but not dystonia per se in two other studies [11,12]. The latter is in line with findings in healthy subjects showing that reduced IHI is correlated with the degree of mirror movements [15] and underscores the hypothesis that the capability of decoupling both hands is associated with greater IHI. Taken together, there is no striking evidence that dystonia per se is directly related to interhemispheric interaction between primary motor hand areas.
4.3. Family history of dystonia
We could confirm our hypothesis that a positive family history of dystonia is associated with reduced IHI. Apparently, weak IHI reflects an important genetic trait for a reduced capability to decouple bi-manual performance leading to a higher risk of developing dystonia. IHI may thus represent a useful endophenotypic marker for the risk of developing dystonia.
In dystonia, many alterations in cortical connectivity and plasticity have been described (for review see Ref. [26]) both in genetically determined monogenic and genetically undetermined forms of dystonia [27–29]. Typically, inheritance in monogenetic forms of dystonia is autosomal dominant [30]. The presence and distribution of endophenotypes including an increased temporal discrimination threshold and abnormal vibration induced illusions in dystonia patients and their relatives also suggests autosomal dominant inheritance in most other forms of isolated dystonia [3–5,8,31,32].
Using the same classification scheme as in a previously published study [4], reduced IHI in the present study was related to the factor family history of dystonia. The factors musical activity (see above) and family history combined accounted for 16% of the variability of IHI in our study. Given that the former correlated positively but the latter negatively with the strength of IHI, there was no net effect in the group of musician’s hand dystonia patients, i.e. IHI findings in this group did not differ from those in any of the healthy controls groups (Fig. 1). Similarly, because none of the factors was relevant in the group of writer’s cramp patient, this group also did not differ from healthy non-musicians or healthy family members (Fig. 1). Strong IHI was present only in the groups of healthy musicians and those with sporadic musician’s hand dystonia compared to the group of healthy first degree family members. This is most likely explained by the fact that in the group of healthy musicians and those with sporadic musician’s hand dystonia a high degree of musical activity was combined with a lack of a familial history of dystonia, both factors associated with stronger IHI, whether or not dystonia was present. In the group of first degree family members the factor familial history of dystonia is present, resulting in reduced IHI.
Although the overall impact of the factor family history of dystonia on IHI was small it may nonetheless reflect an important genetic trait of reduced bi-manual decoupling as a risk factor for developing dystonia.
5. Conclusions
In these groups of professional musicians with and without musician’s hand dystonia, sporadic writer’s cramp patients and their relatives, the strength of IHI was influenced positively by musical activity and negatively by the presence of a positive family history of dystonia but not by the presence of dystonia per se.
Acknowledgements
The authors thank the patients, family members and healthy controls for their participation in the study. This work was supported by research grants from the Dystonia Coalition (NS065701 from the NIH Office of Rare Diseases Research in the National Center for Advancing Translational Sciences) and the University of Lübeck. CK is a recipient of a Schilling Award from the Hermann and Lilly Schilling Foundation.
Footnotes
Financial disclosure
The authors report no conflicts of interest.
Dr. Bäumer received honaria form Merz Pharmaceuticals, Ipsen Pharma and Allergan. He served on the scientific advisory board for Merz Pharmaceuticals.
Prof. Schmidt: reports no disclosures.
Dr. Heldmann: reports no disclosures.
Dr. Landwehr: reports no disclosures.
Dr Simmer: reports no disclosures.
Dr. Tönniges:reports no disclosures.
Prof. Münte: reports no disclosures.
Prof Lohmann: receives research support from the German Research Foundation and the Dystonia Coalition.
Prof. Altenmüller serves in the Editorial board of following Journals: Journal of Interdisciplinary Music Studies, Medical Problems of Performing Artists, Musicae Scientiae, Music and Medicine, BMC Movement Disorders.
He receives a research-grant from the German Hertie Foundation (HF 1205).
He receives royalties from the publication of the book “Music, Motor Control and the Brain” which appeared at Oxford University press 2006 and from the book “Emotions in Vocal Communication in Animal and Men” which appeared 2013 in Oxford University Press.
He received honoraria for teaching courses on the application of Botulinum-toxin A by following enterprises: Allergan (Botox), Ipsen Pharma (Dysport), Desitin, Merz (Xeomin).
Prof Klein: serves as medical advisor to Centogene and received an honorarium for speaking at Biogen Idec.
Prof Münchau is member of the scientific advisory board of the German Tourette Society; he received grants by Pharm Allergan, Ipsen, Merz Pharmaceuticals, Actelion and honoraria for lectures from Pharm Allergan, Ipsen, Merz Pharmaceuticals, Actelion, GlaxoSmithKline and Desitin. He has been supported by the Possehl-Stiftung, Lübeck, Dystonia Coalition (USA), Tourette Syndrome Association (Germany), European Huntington Disease Network, N.E.MO. Charity supporting the research of paediatric movement disorders, the Ärztekammer Schleswige–Holstein and the Fortbildungsakademie der Deutschen Gesellschaft für Neurologie. He has received the following academic research support: European Union: Multicentre Tics in Children Studies (EMTICS) a spart of the FP 7 program (HEALTH.2011.2.2.1–3: Addictive and/or compulsive behaviour in children and adolescents: translating pre-clinical results into therapies), PI, 2011–2015; Deutsche Forschungsgemeinschaft (DFG): project „Intentional inhibition and self-control in Gilles de la Tourette syndrome” (MU 1692/3–1), PI, 2010–2014; DFG: project “Connectivity and plasticity in cortical motor networks in Parkin gene associated parkinsonism and dopa responsive dystonia” (SFB 936), PI, 2011–2015, DFG: project „Psychophysische Methoden im Kontext pharmakologischer und verhaltens-therapeutischer Ansätze zur Behandlung des Gilles de la Tourette Syndroms” (MU 1692/4–1), PI, 2015e2017. He receives royalties from the publication of the book Gene Therapy.
References
- [1].Fahn S, Bressman SB, Marsden CD, Classification of dystonia, Adv. Neurol 78 (1998) 1–10. [PubMed] [Google Scholar]
- [2].Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, Hallett M, Jankovic J, Jinnah HA, Klein C, Lang AE, Mink JW, Teller JK, Phenomenology and classification of dystonia: a consensus update, Mov. Disord 28 (2013) 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schmidt A, Jabusch HC, Altenmuller E, Hagenah J, Bruggemann N, Hedrich K, Saunders-Pullman R, Bressman SB, Kramer PL, Klein C, Dominantly transmitted focal dystonia in families of patients with musician’s cramp, Neurology 67 (2006) 691–693. [DOI] [PubMed] [Google Scholar]
- [4].Schmidt A, Jabusch HC, Altenmuller E, Hagenah J, Bruggemann N, Lohmann K, Enders L, Kramer PL, Saunders-Pullman R, Bressman SB, Munchau A, Klein C, Etiology of musician’s dystonia: familial or environmental? Neurology 72 (2009) 1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Walsh R, O’Dwyer JP, Sheikh IH, O’Riordan S, Lynch T, Hutchinson M, Sporadic adult onset dystonia: sensory abnormalities as an endophenotype in unaffected relatives, J. Neurol. Neurosurg. Psychiatry 78 (2007) 980–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tinazzi M, Fiaschi A, Frasson E, Fiorio M, Cortese F, Aglioti SM, Deficits of temporal discrimination in dystonia are independent from the spatial distance between the loci of tactile stimulation, Mov. Disord 17 (2002) 333–338. [DOI] [PubMed] [Google Scholar]
- [7].Hutchinson M, Kimmich O, Molloy A, Whelan R, Molloy F, Lynch T, Healy DG, Walsh C, Edwards MJ, Ozelius L, Reilly RB, O’Riordan S, The endophenotype and the phenotype: temporal discrimination and adult-onset dystonia, Mov. Disord 28 (2013) 1766–1774. [DOI] [PubMed] [Google Scholar]
- [8].Frima N, Nasir J, Grunewald RA, Abnormal vibration-induced illusion of movement in idiopathic focal dystonia: an endophenotypic marker? Mov. Disord 23 (2008) 373–377. [DOI] [PubMed] [Google Scholar]
- [9].Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD, Interhemispheric inhibition of the human motor cortex, J. Physiol 453 (1992) 525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nelson AJ, Hoque T, Gunraj C, Ni Z, Chen R, Impaired interhemispheric inhibition in writer’s cramp, Neurology 75 (2010) 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Beck S, Shamim EA, Richardson SP, Schubert M, Hallett M, Inter-hemispheric inhibition is impaired in mirror dystonia, Eur. J. Neurosci 29 (2009) 1634–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sattler V, Dickler M, Michaud M, Meunier S, Simonetta-Moreau M, Does abnormal interhemispheric inhibition play a role in mirror dystonia? Mov. Disord 29 (2014) 787–796. [DOI] [PubMed] [Google Scholar]
- [13].Gould HJ 3rd, Cusick CG, Pons TP, Kaas JH, The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys, J. Comp. Neurol 247 (1986) 297–325. [DOI] [PubMed] [Google Scholar]
- [14].Matsunami K, Hamada I, Effects of stimulation of corpus callosum on pre-central neuron activity in the awake monkey, J. Neurophysiol 52 (1984) 676–691. [DOI] [PubMed] [Google Scholar]
- [15].Hubers A, Orekhov Y, Ziemann U, Interhemispheric motor inhibition: its role in controlling electromyographic mirror activity, Eur. J. Neurosci 28 (2008) 364–371. [DOI] [PubMed] [Google Scholar]
- [16].Altenmuller E, Focal dystonia: advances in brain imaging and understanding of fine motor control in musicians, Hand Clin. 19 (2003) 523–538. [DOI] [PubMed] [Google Scholar]
- [17].Bressman SB, Raymond D, Wendt K, Saunders-Pullman R, De Leon D, Fahn S, Ozelius L, Risch N, Diagnostic criteria for dystonia in DYT1 families, Neurology 59 (2002) 1780–1782. [DOI] [PubMed] [Google Scholar]
- [18].Oldfield RC, The assessment and analysis of handedness: the Edinburgh inventory, Neuropsychologia 9 (1971) 97–113. [DOI] [PubMed] [Google Scholar]
- [19].Heinen F, Glocker FX, Fietzek U, Meyer BU, Lucking CH, Korinthenberg R, Absence of transcallosal inhibition following focal magnetic stimulation in preschool children, Ann Neurol 43 (1998) 608–612. [DOI] [PubMed] [Google Scholar]
- [20].Coppi E, Houdayer E, Chieffo R, Spagnolo F, Inuggi A, Straffi L, Comi G, Leocani L, Age-related changes in motor cortical representation and inter-hemispheric interactions: a transcranial magnetic stimulation study, Front. Aging Neurosci 6 (2014) 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Koerte I, Heinen F, Fuchs T, Laubender RP, Pomschar A, Stahl R, Berweck S, Winkler P, Hufschmidt A, Reiser MF, Ertl-Wagner B, Anisotropy of callosal motor fibers in combination with transcranial magnetic stimulation in the course of motor development, Invest Radiol. 44 (2009) 279–284. [DOI] [PubMed] [Google Scholar]
- [22].Kwon HG, Son SM, Jang SH, Development of the transcallosal motor fiber from the corticospinal tract in the human brain: diffusion tensor imaging study, Front. Hum. Neurosci 8 (2014) 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U, Human motor corpus callosum: topography, somatotopy, and link between microstructure and function, J. Neurosci 27 (2007) 12132–12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ridding MC, Brouwer B, Nordstrom MA, Reduced interhemispheric inhibition in musicians, Exp. Brain Res 133 (2000) 249–253. [DOI] [PubMed] [Google Scholar]
- [25].Elmer S, Hanggi J, Jancke L, Interhemispheric transcallosal connectivity between the left and right planum temporale predicts musicianship, performance in temporal speech processing, and functional specialization, Brain Struct. Funct 221 (2014) 331–344. [DOI] [PubMed] [Google Scholar]
- [26].Quartarone A, Hallett M, Emerging concepts in the physiological basis of dystonia, Mov. Disord 28 (2013) 958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Edwards MJ, Huang YZ, Wood NW, Rothwell JC, Bhatia KP, Different patterns of electrophysiological deficits in manifesting and non-manifesting carriers of the DYT1 gene mutation, Brain 126 (2003) 2074–2080. [DOI] [PubMed] [Google Scholar]
- [28].Rothwell JC, Edwards M, Huang YZ, Bhatia KP, Physiological studies in carriers of the DYT1 gene mutation, Rev. Neurol. Paris 159 (2003) 880–884. [PubMed] [Google Scholar]
- [29].Ikoma K, Samii A, Mercuri B, Wassermann EM, Hallett M, Abnormal cortical motor excitability in dystonia, Neurology 46 (1996) 1371–1376. [DOI] [PubMed] [Google Scholar]
- [30].Lohmann K, Klein C, Genetics of dystonia: what’s known? What’s new? What’s next? Mov. Disord 28 (2013) 899–905. [DOI] [PubMed] [Google Scholar]
- [31].Bradley D, Whelan R, Walsh R, Reilly RB, Hutchinson S, Molloy F, Hutchinson M, Temporal discrimination threshold: VBM evidence for an endophenotype in adult onset primary torsion dystonia, Brain 132 (2009) 2327–2335. [DOI] [PubMed] [Google Scholar]
- [32].Kimmich O, Molloy A, Whelan R, Williams L, Bradley D, Balsters J, Molloy F, Lynch T, Healy DG, Walsh C, O’Riordan S, Reilly RB, Hutchinson M, Temporal discrimination, a cervical dystonia endophenotype: penetrance and functional correlates, Mov. Disord 29 (2014) 804–811. [DOI] [PubMed] [Google Scholar]