Abstract
A 20-year-old patient, who had double outlet right ventricle, mitral atresia, pulmonary atresia, and bilateral superior vena cava and underwent successful lateral tunnel total cavo-pulmonary connection at 6 years old, presented with frequent watery diarrhea, general malaise, and tetany. He was known to have intractable protein-losing enteropathy (PLE) from 7 years of age that was resistant to various treatments. To keep hemodynamics stable, he required intravenous albumin infusion every day. Fontan fenestration partially improved his condition and allowed to stop albumin infusion, however still he showed muddy stool and cachexia with low serum albumin <20 g/L and immunoglobulin <3 g/L. Because of serious risk of infection, we placed him on regular subcutaneous immunoglobulin supplementation with rescue intravenous immunoglobulin that improved his PLE within a month and allowed him to be discharged. This case illustrates that immunoglobulin supplementation can be one of the choices of treatment for intractable PLE.
<Learning objective: Combined subcutaneous and intravenous immunoglobulin infusion therapy can be one of the choices of treatment for intractable protein-losing enteropathy that does not respond to multiple medications.>
Keywords: Protein-losing enteropathy, Fontan type operation, Immunoglobulin therapy, Subcutaneous immunoglobulin supplementation
Introduction
Protein-losing enteropathy (PLE) is one of the significant complications with ominous prognosis long after Fontan-type operation [1]. Various treatments including hemodynamic correction with pulmonary artery stent and inter-atrial fenestration, diet, medications such as steroid, heparin, diuretics, pulmonary vasodilator, and somatostatin analogue have been reported with limited success [2]. We report a case with intractable PLE successfully treated with combined intravenous and subcutaneous immunoglobulin supplementation (SCIg).
Case report
A 20-year-old patient, who had double outlet right ventricle, mitral atresia, pulmonary atresia, and bilateral superior vena cava and underwent successful lateral tunnel total cavo-pulmonary connection at 6 years of age, presented with frequent watery diarrhea of >10 times a day with output of >2000 g per day, general malaise, and tetany without proteinuria.
He had suffered from intractable PLE for 13 years that required 14 episodes of hospitalization. His treatments included high protein and low-fat diet, multiple courses of medical treatments such as intravenous or oral corticosteroid administration, heparin infusion, somatostatin analogue infusion, and high-dose oral spironolactone, and hemodynamic correction with stent implantation into pulmonary artery.
On admission, his body height was 160 cm and body weight was 46.6 kg with transcutaneous oxygen saturation of 95%. Laboratory data showed significantly low serum albumin concentration of 18.7 g/L and immunoglobulin G (IgG) of 0.86 g/L. No pathological bacteria or virus were found from his stool. Echocardiography showed mild common atrio-ventricular valve regurgitation. Scintigraphy confirmed exacerbated PLE. To keep hemodynamics stable, he required intravenous albumin infusion every day.
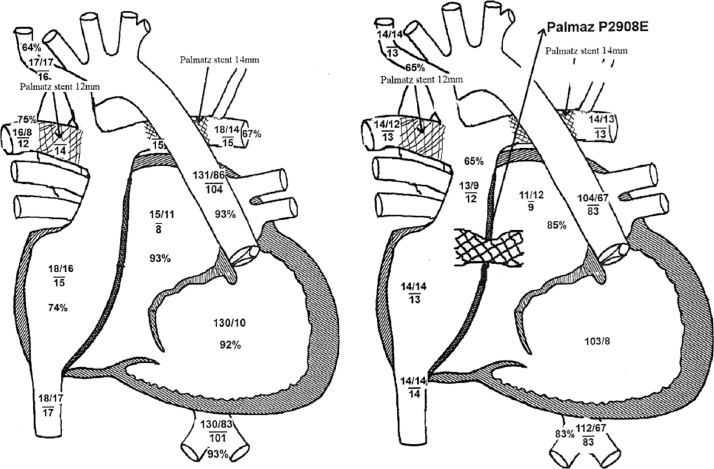
In this admission, we tried methylprednisolone pulse therapy, continuous epoprostenol infusion, and high-dose heparin infusion, however his stool and his laboratory data did not improve. Cardiac catheterization at this time revealed high central venous pressure (CVP) of 17 mmHg and atrial pressure of 8 mmHg giving pressure gradient of 9 mmHg that must have worsened his PLE, although he did not show significant pressure gradient in previous cardiac catheterizations. Because his pulmonary arteries were already acceptably dilated with pulmonary artery index of 201 mm2/M2, we made transcatheter interatrial fenestration, using PALMATZ P2908E™ stent (Cordis, Baar, Switzerland) (Fig. 1), that decreased his diarrhea to 2–3 times a day with improved colonoscopic findings. Three months later, cardiac catheterization revealed decreased CVP to 14 mmHg with pressure gradient of 3 mmHg and increased cardiac index from 3.55 to 4.22 L/min/m2.
Fig. 1.
Hemodynamic change after interatrial fenestration using stent. Central venous pressure and arterial oxygen saturation decreased but cardiac output increased.
Even with the hemodynamic improvement, still he suffered from malabsorption that his prothrombin time-international normalized ratio (PT-INR) never prolonged with maximal dose of warfarin (15 mg/day) and he showed signs of adrenal insufficiency such as oliguria and fatigue at each time we tried to switch intravenous predonisolone (10 mg/day) to oral dexamethazone (maximal 15 mg/day) or hydrocortisone (maximal 120 mg/day). Thus he required total parenteral alimentation and intravenous infusion of medications and he had to stay in hospital for over 1 year. As a result, he suffered from malnutrition and showed cachexia with body weight of 33.4 kg.
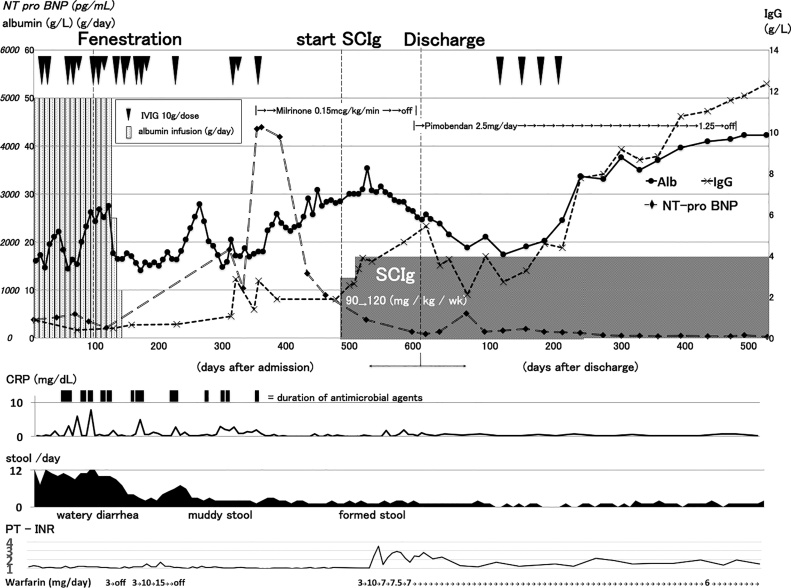
About 350 days after admission, he showed signs of heart failure such as gallop rhythm and elevation of N-terminal pro-brain natriuretic peptide (NT-pro BNP) without specific cause. We placed him on continuous infusion of phosphodiesterase 3 inhibitor, milrinone with 0.15 microgram per kg per min and his NT-pro BNP decreased to almost normal range. Although serum albumin also increased with milrinone infusion, serum immunoglobulin did not increase >5 g/L. We thought he was at great risk of serious infections and decided to place him on weekly subcutaneous immunoglobulin (SCIg), Hizentra™ (CSL Behring, King of Prussia, PA, USA); 20% solution of SCIg. We started SCIg for 90 mg/kg/week as initial dose and increased to 120 mg/kg/week as maintenance dose. Without any change in medical treatment, his serum IgG increased to >3 g/L within a month after starting SCIg, and his stool character improved from muddy to formed for once or twice a day with increasing serum albumin (Fig. 2).
Fig. 2.
Clinical course of the patient.
Alb, albumin; CRP, C-reactive protein; IgG, immunoglobulin G; SCIg, subcutaneous immunoglobulin infusion; PT-INR, prothrombin time-international normalized ratio; NT-pro BNP, N-terminal pro-brain natriuretic peptide.
As a representation of improved enteral absorption, his PT-INR kept 1.6–2.7 by 7 mg/day of oral warfarin therapy and he showed no signs of adrenal insufficiency with oral hydrocortisone (80 mg/day). After starting SCIg, he had no signs of infection and we could have switched all medication from intravenous to oral, including milrione to pimobendan, and let him be discharged 4 months later.
After discharge, we have added 4 courses of IVIg on SCIg to keep his serum IgG levels above 5.0 g/L. Since the last administration of IVIg, he did not require additional dose of IVIg for about 1year, and his condition had improved with time. At the latest visit 1 year after discharge, his laboratory data showed serum albumin of 42.3 g/L and IgG of 12.3 g/L. There have been no documented major side effects of SCIg. As a mild side effect, he suffered from pain of infusion site that could be dealt with using local analgesic ointment.
Discussion
This case illustrates that combination of intravenous and subcutaneous immunoglobulin supplementation can be one of the choices of treatment for intractable PLE after Fontan type operation. In this case, regular SCIg dramatically improved PLE, brought him into remission, and allowed him to be discharged, although multiple medications did not significantly improve his condition. This is the first English report of the effective control of PLE using SCIg after Fontan-type operation.
There is a little information available about the effectiveness of supplemental IgG in PLE, although many patients with PLE show significantly reduced gamma globulin levels, particularly IgG [3], because low molecular proteins are easily lost into stool. Only Zaupper et al. [4] reported effectiveness of high-dose intravenous immunoglobulin, 1–1.4 g/kg/month, in 4 patients with PLE after Fontan-type operation. The biggest difference between their patients and ours is disease severity, time length of PLE, and treatment history. Although no patients were placed on heparin or corticosteroid in that study, our patient has been suffering from PLE for 13 years that required multiple medications including heparin and corticosteroid.
The major advantages of SCIg over IVIg treatment for PLE must be patient’s convenience and medical cost. SCIg can be done by patient himself at home; however, IVIg needs venous line placement and short-term admission. Also we chose SCIg from medico-economic standpoint of view, because SCIg needed 90–120 mg/kg/week × 4 times of immunoglobulin (360–480 mg/kg) for a month costing approximately 3022–4029 yen/kg, although IVIg should have required 1–1.4 g/kg of immunoglobulin for a month costing 8683–12,155 yen/kg in Japan.
The mechanisms of this improvement of PLE achieved by regular immunoglobulin supplementation is not clear, because etiology and pathology of PLE after Fontan type operation is complicated and not fully understood. Probably immunological process including multiple minor infections and inflammation may be involved. Lenz et al. suggested that PLE after Fontan type operation is triggered by enteral infections [5]. In agreement with that report, our patient showed improvement of PLE at the same time when he stopped showing infection after SCIg.
On the other hand, anti-inflammatory function of IVIG might be involved in this improvement of PLE. Ostrow et al. suggested that, inflammatory cytokines such as tumor necrosis factor alpha contribute to the development of PLE after Fontan operation as adult congestive heart failure [6]. Obviously, IVIg has been known to inhibit inflammatory pathway induced by tumor necrosis factor alpha directly and indirectly [7], [8].
It is not known if SCIg could have worked alone or required combination with IVIg. However, in fact, 4 doses of IVIg dramatically increased albumin and IgG as shown in Fig. 2. Even with SCIg, he could not hold IgG >5 g/L, which is the target level to prevent frequent infection, and we gave him IVIg as loading. Once his IgG level exceeded 5 g/L, his IgG increased further only with SCIg but without additional IVIg. This phenomenon might be a key to understand one of the mechanisms of this success in this patient. In other words, we might have increased the dosage of SCIg further and have the same kind of effect. However, it requires more experience to answer to this question.
Alhough there is the possibility that milrinone improved both hemodynamics and his PLE indirectly, milrinone did not increase serum IgG. In addition, after discharge, only with 4 courses of IVIg without milrinone infusion, his serum albumin and immunoglobulin increased. This clinical course indicated effectiveness of immunoglobulin therapy for PLE.
Intravenous and subcutaneous immunoglobulin supplementation can be one of the choices of treatment for intractable PLE after Fontan operation.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Umar S.B., DiBaise J.K. Protein-losing enteropathy: case illustrations and clinical review. Am J Gastroenterol. 2010;105:43–49. doi: 10.1038/ajg.2009.561. [DOI] [PubMed] [Google Scholar]
- 2.Rychik J. Protein-losing enteropathy after Fontan operation. Congenit Heart Dis. 2007;2:288–300. doi: 10.1111/j.1747-0803.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- 3.Chakrabarti S., Keeton B.R., Salmon A.P., Vettukattil J.J. Acquired combined immunodeficiency associated with protein losing enteropathy complicating Fontan operation. Heart. 2003;89:1130–1131. doi: 10.1136/heart.89.10.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaupper L.B., Nielsen B.W., Herlin T. Protein-losing enteropathy after the total cavopulmonary connection: impact of intravenous immunoglobulin. Congenit Heart Dis. 2011;6:624–629. doi: 10.1111/j.1747-0803.2011.00568.x. [DOI] [PubMed] [Google Scholar]
- 5.Lenz D., Hambsch J., Schneider P., Häusler H.-J., Sauer U., Hess J. Protein-losing enteropathy in patients with Fontan circulation: is it triggered by infection? Crit Care. 2003;7:1–7. doi: 10.1186/cc2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrow A.M., Freeze H., Rychik J. Protein-losing enteropathy after Fontan operation: investigations into possible pathophysiologic mechanisms. Ann Thorac Surg. 2006;82:695–700. doi: 10.1016/j.athoracsur.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 7.Nakatani K., Takeshita S., Tsujimoto H., Sekine I. Intravenous immunoglobulin (IVIG) preparations induce apoptosis in TNF-alpha-stimulated endothelial cells via a mitochondria-dependent pathway. Clin Exp Immunol. 2002;127:445–454. doi: 10.1046/j.1365-2249.2002.01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozicky L.K., Zhao Z.Y., Menzies S.C., Fidanza M., Reid G.S., Wilhelmsen K. Intravenous immunoglobulin skews macrophages to an anti-inflammatory, IL-10-producing activation state. J Leukoc Biol. 2015;98:983–994. doi: 10.1189/jlb.3VMA0315-078R. [DOI] [PubMed] [Google Scholar]