Abstract
Pannus formation is a known complication of valve replacement surgery. However, few cases describe pannus formation in the mitral position, and they are mostly associated with prosthetic valves rather than only annuloplasty. We describe the case of a 62-year-old woman who first underwent reductive annuloplasty using a Carpentier-Edwards 28 mm ring prosthesis in 2009 that performed well on regular follow-up for 5 years. In 2014, the patient presented with significant weight gain, shortness of breath (New York Heart Association functional class III) and signs of severe mitral stenosis. Echocardiography demonstrated the formation of a pannus and increased pressure gradients. Removal of the ring prosthesis and pannus-like fibrotic tissue was performed. The native valve was left in place as it had supple leaflets and performed well once properly debrided. The patient remained well throughout a 24-month follow-up.
<Learning objective: This case report presents a rare phenomenon that will help familiarize readers with this complication type. Pannus formation of this type is rarely documented and encountered.>
Keywords: Pannus, Mitral annuloplasty, Stenosis, Obesity
Introduction
Pannus formation is a rare yet well-documented complication of aortic valve replacement surgery. Some case reports describe pannus formation in the mitral position following valve replacement [1], [2]. This phenomenon following mitral annuloplasty is rare. We present herein an interesting case of pannus formation following mitral valve (MV) annuloplasty.
Case report
A 62-year-old woman was admitted for elective MV annuloplasty. She was complaining primarily of shortness of breath, NewYork Heart Association (NYHA) functional class III. She also suffered from obesity [body mass index (BMI) 34], hypertension, dyslipidemia, type 2 diabetes, and sleep apnea.
She underwent MV repair surgery for significant chronic mitral regurgitation due to A2 leaflet prolapse. MV repair was performed. A chordal transfer from P2 to A2 was done and a Carpentier-Edwards (CE) 28 mm annuloplasty ring was implanted. Post-operative echocardiogram showed resolution of the regurgitation with normal pressure gradients and a MV area (MVA) of 2.1 cm2.
Five years later, the patient returned with shortness of breath. Her BMI had increased to 50 since her first surgery. Transthoracic echocardiography (TTE) showed increased left atrial volume index with a mean mitral pressure gradient of 9 mmHg and MVA of 1.27 cm2,
Stress echocardiography was also performed and showed a reduced functional capacity, achieving only 2.8 Mets and 79% of the maximal heart rate. There were no signs of cardiac ischemia but the mean mitral pressure gradient increased from 9 mmHg at rest to 22 mmHg during exercise. The pulmonary artery pressure was measured at 53 mmHg.
Cardiac catheterization showed mean mitral pressure of 13.5 mmHg for a MVA of 1.4 cm2, or 0.68 cm2/m2 when indexed for patient size. It was then decided that the best option was a redo surgery.
Transesophageal echocardiography (TEE) was performed intra-operatively and showed severe stenosis of the MV. On 3D reconstruction, there seemed to be a membrane located just above the mitral annulus (Fig. 1). The operation was performed through median sternotomy with aortic and bi-caval cannulations. Antegrade (Del Nido) cardioplegia was administered. The MV was accessed using a trans-atrial approach. Important mitral stenosis (MS) was confirmed. Pannus-like fibrotic tissues surrounding the ring and mitral annulus were removed. Finally, removal of the CE 28 mm ring prosthesis was completed. Overgrowth of this pannus-like fibrotic tissue was also removed at and under the mitral annulus, although the ring remained stiff. The leaflets were unaffected by the pannus and had preserved mobility and flexibility. After consideration and thorough confirmation of the competence of the MV, it was decided to leave the existing valve in place.
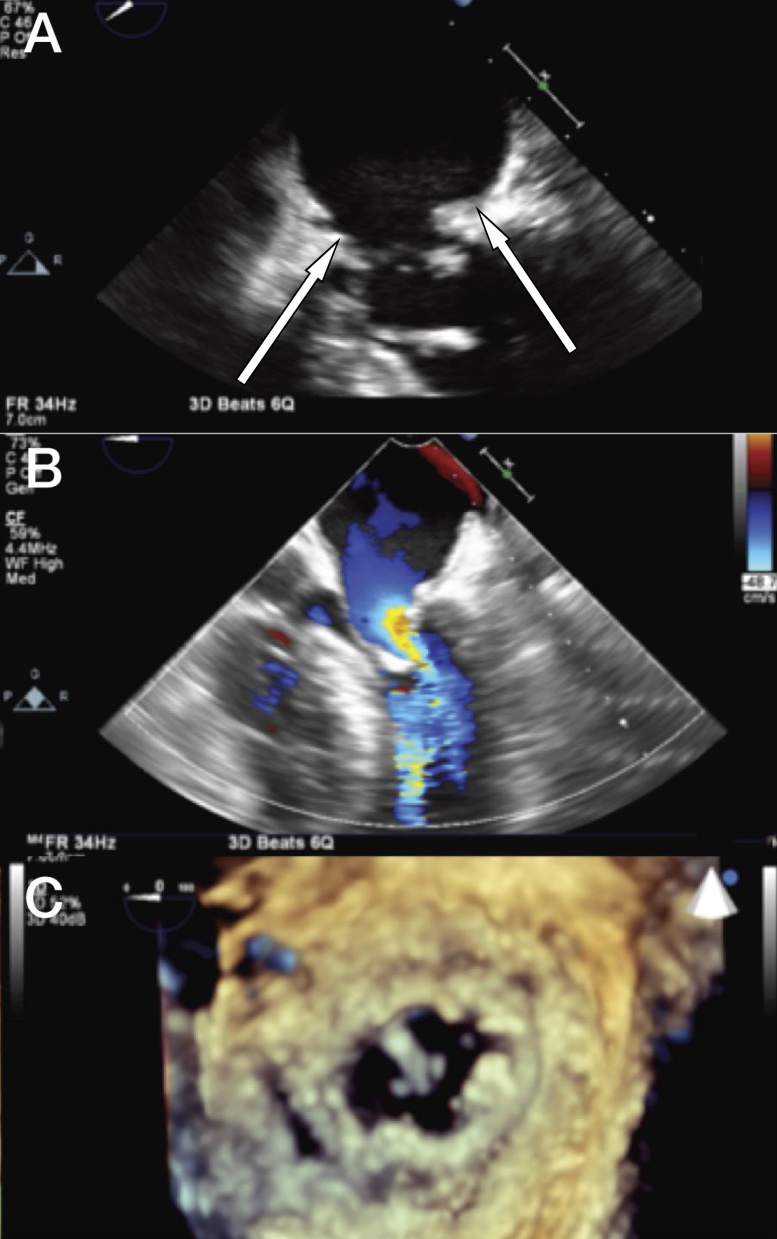
Fig. 1.
2D and 3D transesophageal views and surgical view of the mitral valve pre-operatively. (A) Mid-esophageal view showing abnormal tissue at the mitral valve level (arrows at pannus) and (B) color flow Doppler acceleration. Chordal transfer (P2 to A2) is also visualized. (C) Transesophageal 3D view from the atrial perspective of the mitral ring, mitral valve and abnormal tissue over the ring.
Post-extra corporal circulation TEE (Fig. 2) showed no mitral regurgitation and the mean mitral pressure gradient was measured at 3 mmHg. The patient recovered quickly and 5 days later control TTE showed a mean mitral pressure gradient of 3 mmHg and MVA of 2.2 cm2.
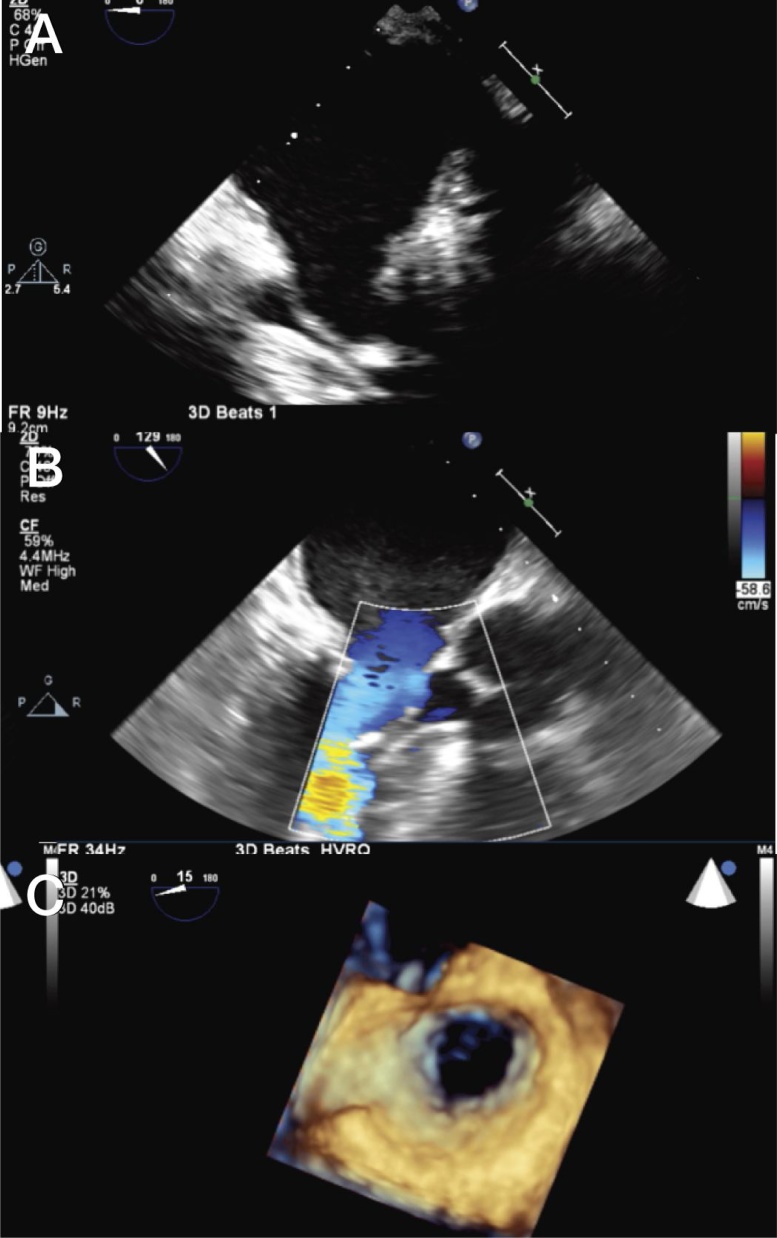
Fig. 2.
2D and 3D transesophageal views of the mitral valve post-operatively. (A) Mid-esophageal view of the mitral valve without any abnormal tissue and (B) long axis with normal color flow Doppler. (C) Atrial perspective showing the wide open mitral valve after removal of ring and pannus.
Discussion
This case illustrates a unique combination of pannus formation and MS induced by significant weight gain many years after MV repair with a CE 28 mm ring. The resulting MS was corrected by removal of the annuloplasty ring and fibrotic tissue, while leaving the intact leaflets in place.
Several factors have been associated with a higher risk for developing MS after MV repair, including the use of flexible Duran annuloplasty rings versus rigid CE rings, complete annuloplasty rings versus partial bands, small versus large anterior leaflet opening angle, and anterior leaflet tip opening length [3].
Suh et al. [4] compared a group of patients, mostly with degenerative MR, who received a Duran ring annuloplasty to another group that received a CE ring annuloplasty. They found that the patients with the flexible Duran ring had significantly smaller MVA and higher mean trans-mitral pressure gradient (TMPG), resulting in a higher incidence of functional MS. They stated that Duran ring recipients experienced a gradual annual increase in mean TMPG at a rate of 0.19 mmHg/year, which was not experienced by the patients with the rigid CE ring. Significant pannus formation around the MV annulus could be detected on cardiac computed tomography and was significantly higher in the Duran ring patients, whereas the leaflet thickness was not different between the two groups, confirming that the obstruction was at the level of the annulus, and possibly caused by the pannus formation. These findings may indicate that MS after MV repair is a progressive process that starts early after the repair [4]. Although we used a CE flexible ring rather than Duran ring, our patient developed pannus formation which is most likely due to effect of BMI which was significantly increased (34 vs 50). The consequence of this process leads to the observed stenosis.
Long-term surveillance of clinical outcomes and survival and evolution of the native MV will be interesting. The authors believed the removal of the simple annular ring would decrease the inflammatory response that caused the overgrowth of the tissue obstructing the MV, which would prevent the recurrence of this complication. Assessment of the remodeling of this valve will be interesting to follow.
Conflict of interest
None.
Funding
No funding was received for this work.
References
- 1.Ogino T., Sakata K., Yamagishi T., Hagiwara S., Ohshima K., Iino Y. Mitral valve malfunction of a St. Jude Medical prosthetic due to pannus formation and thrombosis. Kitakanto Med J. 2012;62:301–303. Available from: https://www.jstage.jst.go.jp/article/kmj/62/3/62_301/_pdf. [Accessed August 20, 2017] [Google Scholar]
- 2.Abudiab M., McKay J., Deavers M., Zoghbi W., Chang S. Pannus of mitral valve prosthesis causing left ventricular outflow tract obstruction. Methodist Debakey Cardiovasc J. 2016;12:183. doi: 10.14797/mdcj-12-3-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung C.H., Kim J.B., Choo S.J., Kim K.S., Song H., Song M.G. Long-term outcomes after mitral ring annuloplasty for degenerative mitral regurgitation: Duran ring versus Carpentier-Edwards ring. J Heart Valve Dis. 2007;16:536–545. [PubMed] [Google Scholar]
- 4.Suh Y.J., Chang B.C., Im D.J., Kim Y.J., Hong Y.J., Hong G.R. Assessment of mitral annuloplasty ring by cardiac computed tomography: correlation with echocardiographic parameters and comparison between two different ring types. J Thorac Cardiovasc Surg. 2015;150:1082–1090. doi: 10.1016/j.jtcvs.2015.07.019. [DOI] [PubMed] [Google Scholar]