Abstract
The combination of persistent pulmonary hypertension of the newborn (PPHN) and transposition of the great arteries (TGA) has serious impacts on treatment and prognosis, often with adverse outcomes. We report the case of a male full-term newborn with TGA with intact ventricular septum and severe PPHN who died 2 h after birth; further, we examined his vascular histology. On autopsy, lung histology showed mild fibrous hypertrophy in the intima and moderate medial hypertrophy of the minimal pulmonary artery. Hypoplasia of the pulmonary artery was not detected. Pulmonary congestion was detected and pneumatization was poor. Debris was present in the alveoli. Hemosiderin deposition was detected, suggesting prenatal hemostasis or hemorrhage. Severe PPHN may have occurred because of pulmonary arterial spasm accompanying pulmonary congestion which had been in the fetal stage. A wide range of lesions can be present in the pulmonary vascular bed in TGA. The pathologies of pulmonary vascular tissues with TGA and PPHN are not uniform.
<Learning objective: Neonates with transposition of the great arteries with intact ventricular septum (TGA/IVS) may have severe pulmonary hypertension. The progression of pulmonary vascular disease in TGA/IVS is unpredictable; therefore, the delivery of a fetus diagnosed with TGA/IVS should be performed at an institution in which catheterization and balloon atrial septostomy can be performed.>
Keywords: Transposition of the great arteries/intact ventricular septum, Persistent pulmonary hypertension of the newborn, Histological findings
Introduction
Transposition of the great arteries (TGA) is one of the most common cyanotic congenital heart diseases (CHDs) and accounts for 5–7% of all patients with CHD [1]. TGA is reportedly associated with persistent pulmonary hypertension of the newborn (PPHN) in 1–3% of cases [1]. The combination of PPHN and TGA has serious implications for treatment and prognosis [1], [2], often with fatal outcomes. Data concerning the incidence and prognosis of the combination of TGA and PPHN are limited. In infants aged <6 months with TGA, an intact ventricular septum (TGA/IVS), and a closed patent ductus arteriosus (PDA), pulmonary vascular changes of more than Heath-Edwards grade 2 are unusual [3]. Grade 2 lesions have been reported more often in infants aged <6 months [3]. PPHN results from failure of the pulmonary vasculature to relax at birth. We report a case of a newborn with TGA/IVS and severe PPHN who died at 2 h after birth and examine the patient's vascular histology.
Case report
A 37-year-old woman was referred to us at 30 weeks’ gestation due to inadequate visualization of three vessels at another institution. Detailed scanning demonstrated normal growth, no anomalies, and a normal four-chamber view; however, the great vessels were inadequately evaluated. The patient returned at 32 weeks’ gestation for targeted fetal echocardiography. Two-dimensional (2D) and color Doppler ultrasound revealed TGA (with D-loop). A patent foramen ovale (PFO) was detected but was not restrictive or aneurysmal (Fig. 1A). We performed 2D ultrasound on the day before delivery, but the shunt through the PFO was only right-to-left, and the flow through the PDA was pulsatile, with a flow rate of 0.9 m/s.
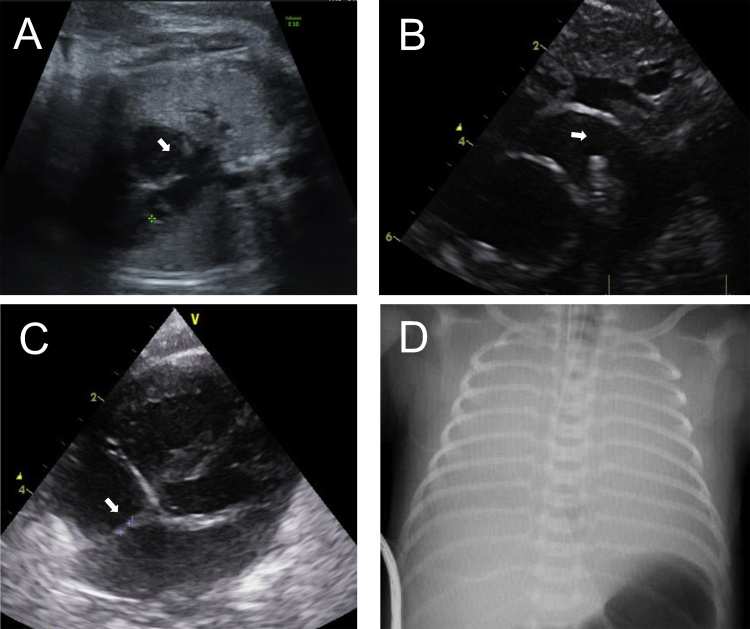
Fig. 1.
(A) Transabdominal ultrasound at 37 weeks’ gestation (the day before delivery). PFO (arrow) was not restrictive or aneurysmal. Color Doppler detected only right-to-left flow. (B, C) Postnatal echocardiography. (B) The PDA was not closed and not constricted. The diameter of the PDA was 3.3 mm. The flow was mainly right-to-left. (C) PFO was only a small orifice on imaging (arrow) and Doppler. (D) There was hardly any lung pneumatization on chest X-ray, suggesting respiratory problems. PDA, patent ductus arteriosus; PFO, patent foramen ovale.
The patient (a male) was born in our hospital at 37 weeks’ gestation through cesarean section (performed due to the mother's prior history of cesarean section) with a birth weight of 3081 g and Apgar scores of 4 and 5. The amniotic fluid was clean. There were no findings of premature placental abruption. He was intubated soon after birth because of increasing respiratory distress and cyanosis. Despite a high ventilatory setting, he remained severely hypoxic and acidotic.
On the neonate's arrival at the neonatal intensive care unit, severe cyanosis was observed with a preductal oxygen saturation (SpO2) of 50% and a postductal SpO2 of 60%. Owing to severe persistent hypoxemia, progressive signs of end-organ deterioration (lactate 20 mmol/L) and severe acidosis (pH 6.425; pCO2, 139 mmHg; BE, −27.5 mmol/l) persisted. Intravenous infusion of prostaglandin E1 was initiated to maintain ductal patency. Postnatal echocardiography confirmed prenatal findings, but a restrictive PFO and the flow at the ductal level were mainly right-to-left (Fig. 1B and C), suggesting elevated pulmonary vascular resistance. Chest X-ray hardly revealed any lung pneumatization, suggesting respiratory problems (Fig. 1D). After initial medical treatment, the patient remained severely hypoxemic despite mechanical ventilation, 100% inspired oxygen, moderate hyperventilation, inhaled nitric oxide therapy (30 ppm), inotropic support with dopamine, and volume expansion. The neonate's SpO2 worsened gradually, with preductal SpO2 as low as 30% and postductal SpO2 as low as 40% at 1.5 h of birth. Sinus bradycardia and ventricular dysfunction gradually progressed, and the neonate suffered cardiac arrest and died while we planned and prepared for balloon atrial septostomy (BAS).
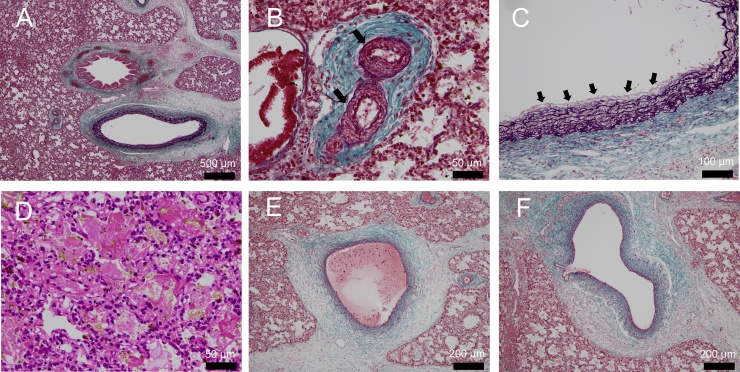
At autopsy, airway abnormality was not detected macroscopically. Lung histology showed mild fibrous hypertrophy in the intima and moderate medial hypertrophy of the pulmonary artery (Heath-Edwards grade 3) but hypoplasia of the pulmonary artery was not detected. In the pulmonary vein system, characteristic findings of pulmonary vein obstruction, such as intimal hyperplasia and media thickening, were not detected. Pulmonary congestion was detected and pneumatization was poor. Debris was present in the alveoli. Hemosiderin deposition was detected, suggesting prenatal hemostasis or hemorrhage. Anatomical abnormalities, such as alveolar capillary dysplasia, which is observed in PPHN, were not observed (Fig. 2).
Fig. 2.
Histological sections of postmortem lung specimens. (A, B, C, E, F) Elastica-Masson's trichrome stain showing connective tissue. (D) Hematoxylin–eosin staining. Lung histology showed moderate medial hypertrophy (B, arrow) and mild fibrous hypertrophy in the intima (C, arrow) of the pulmonary artery. In the pulmonary vein system, characteristic findings of pulmonary vein obstruction, such as intimal hyperplasia and media thickening, were not detected (E, F). Hemosiderin deposition was detected, suggesting prenatal hemostasis or hemorrhage (D).
Discussion
PPHN affects 0.4–6.8 per 10,000 live births and is a common cause of hypoxia in neonates, with a mortality rate of 10–50% [4], [5]. The combination of TGA/IVS and PPHN early in life is often fatal [1], [2], [4], [5], [6]. The mechanism of TGA/IVS complicated with PPHN is not well described and is complicated. There is a general consensus that effective pulmonary blood flow decreases after birth because of PPHN with associated right-to-left shunting at the atrial and ductal level [1], [2], [4].
In the normal fetal circulation, more highly oxygenated blood from the placenta passes the PFO, and the oxygen saturation in the left ventricle is higher than that in the right ventricle. However, in fetuses with TGA, this more highly oxygenated blood in the left ventricle flows through the pulmonary artery. In addition, this blood returns to the pulmonary veins and may lead to even higher oxygen saturation in the left ventricle than in the normal heart, suggesting that the increased oxygen content in the pulmonary artery may lead to ductal constriction and decreased pulmonary vascular resistance [7].
Aziz reported the importance of bronchial artery–pulmonary artery anastomosis as a mechanism by which pulmonary artery lesions develop soon after birth [8]. Bronchial artery–pulmonary artery anastomosis, which is commonly present in normal fetal stages, remains high after birth in TGA. The influence of aortic blood with low oxygen partial pressure decreases the partial pressure of oxygen in the peripheral pulmonary artery. The bronchial artery perfuses the pulmonary artery wall; however, when the systemic circulation has low oxygen partial pressure, the pulmonary artery wall also becomes hypoxic. As a result, local hypoxia in the pulmonary vascular bed is induced and vasoconstriction occurs. In addition, shear stress may be increased due to elevation of hematocrit in association with hypoxemia, and vascular endothelial dysfunction progresses. In TGA, the central part of the pulmonary artery has high oxygen partial pressure; however, since hypoxic blood flows distally, away from the anastomosis, vasoconstriction proceeds and pH worsens. Furthermore, hypoxia in the artery may be involved in stimulating chemoreceptors and causing a persistent pulmonary vasoconstrictive reflex.
The combination of a restrictive PFO and PDA constriction, which can also be identified prenatally, may be associated with very early neonatal death despite aggressive resuscitation [7]. Comprehensive prenatal echocardiographic findings of restrictive interatrial communication and ductal constriction in the fetus should alert care providers at the time of delivery to prepare and manage potential PPHN. A recent study found that the use of the criteria of a tethered or bowing septum primum in TGA fetuses did not predict postnatal compromise and the need for emergent intervention with an acceptable sensitivity or specificity [7].
Chest X-ray, blood gas analysis, and the histology, in which the abnormality in the distal airway is more prominent than the abnormality in the pulmonary vasculature, suggest that problem in respiration, such as respiratory distress syndrome or transient tachypnea of the newborn, may also contribute, at least in part, to this early death of a patient with TGA. The natural progression of pulmonary vascular disease is unpredictable; therefore, standby for catheterization and BAS should be instituted.
The patient was delivered at 37 weeks with elective cesarean. According to the statement from the American Heart Association, elective induction for fetuses with CHD before 39 weeks is not recommended if there are no patient-specific obstetric or logistic problems or fetus-specific concerns about well-being, because the delivery before 39-week gestation is associated with increased mortality [7]. Cesarean section at 37-week gestation, particularly in the case without labor such as in this patient, may increase the risk of respiratory insufficiency in neonates [9]. TGA patients with abnormal pulmonary hemodynamics may be more susceptible to respiratory problems.
The etiology and pathophysiology of PPHN in a patient with TGA/IVS is better understood through examination of histological sections of post-mortem lung specimens. Intimal proliferation leads to luminal occlusion, as seen in a case of PPHN [10]. The reported histological changes in this case were not similar to those seen in our case. We did not identify a closed PDA and restrictive PFO on fetal echocardiography. The major determinant of pulmonary vascular resistance was suggested by the state of the pulmonary vascular bed. In our case, the most important factor was the presence of hemosiderin deposition. Findings characteristic of pulmonary vein obstruction, such as intimal hyperplasia and media thickening, were not observed. The reason for such early appearance of these histological abnormalities is unclear. Hemosiderin deposition was already observed in lung specimens, suggesting chronic prenatal congestion or bleeding. Severe PPHN may occur because of pulmonary arterial spasm accompanying pulmonary congestion in the fetal stage.
The progression of pulmonary vascular disease in TGA/IVS is unpredictable; therefore, the delivery of a fetus diagnosed with TGA/IVS should be performed at an institution where emergent catheterization and BAS can be performed.
Conflict of interest
The authors declare that they have no conflict of interests.
Acknowledgments
The authors have no industry affiliation, funding sources, or conflict of interest directly relevant to the content of this article. We are grateful to Dr Naoki Masaki and Yoshikatsu Saiki for verifying lung histology findings and engaging in helpful discussion.
References
- 1.Luciani G.B., Chang A.C., Starnes V.A. Surgical repair of transposition of the great arteries in neonates with persistent pulmonary hypertension. Ann Thorac Surg. 1996;61:800–805. doi: 10.1016/0003-4975(95)01089-0. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A., Taylor G.P., Sandor G.G., Patterson M.W. Pulmonary vascular disease in neonates with transposition of the great arteries and intact ventricular septum. Br Heart J. 1993;69:442–445. doi: 10.1136/hrt.69.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newfeld E.A., Paul M.M., Muster A.J., Idriss F.S. Pulmonary vascular disease in complete transposition of the great arteries: a study of 200 patients. Am J Cardiol. 1974;34:75–82. doi: 10.1016/0002-9149(74)90096-4. [DOI] [PubMed] [Google Scholar]
- 4.Roofthooft M.T., Bergman K.A., Waterbolk T.W., Ebels T., Bartelds B., Berger R.M. Persistent pulmonary hypertension of the newborn with transposition of the great arteries. Ann Thorac Surg. 2007;83:1446–1450. doi: 10.1016/j.athoracsur.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Dick M., 2nd, Heidelberger K., Crowley D., Rosenthal A., Hees P. Quantitative morphometric analysis of the pulmonary arteries in two patients with D-transposition of the great arteries and persistence of the fetal circulation. Pediatr Res. 1981;15:1397–1401. doi: 10.1203/00006450-198111000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Maeno Y.V., Kamenir S.A., Sinclair B., van der Velde M.E., Smallhorn J.F., Hornberger L.K. Prenatal features of ductus arteriosus constriction and restrictive foramen ovale in d-transposition of the great arteries. Circulation. 1999;99:1209–1214. doi: 10.1161/01.cir.99.9.1209. [DOI] [PubMed] [Google Scholar]
- 7.Donofrio M.T., Moon-Grady A.J., Hornberger L.K., Copel J.A., Sklansky M.S., Abuhamad A., Cuneo B.F., Huhta J.C., Jonas R.A., Krishnan A., Lacey S., Lee W., Michelfelder E.C., Sr., Rempel G.R., Silverman N.H. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 8.Aziz K.U., Paul M.H., Rowe R.D. Bronchopulmonary circulation in d-transposition of the great arteries: possible role in genesis of accelerated pulmonary vascular disease. Am J Cardiol. 1977;39:432–438. doi: 10.1016/s0002-9149(77)80101-x. [DOI] [PubMed] [Google Scholar]
- 9.Tita A.T., Landon M.B., Spong C.Y., Lai Y., Leveno K.J., Varner M.W., Moawad A.H., Caritis S.N., Meis P.J., Wapner R.J., Sorokin Y., Miodovnik M., Carpenter M., Peaceman A.M., O'Sullivan M.J. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360:111–120. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenzie S., Haworth S.G. Occlusion of peripheral pulmonary vascular bed in a baby with idiopathic persistent fetal circulation. Br Heart J. 1981;46:675–678. doi: 10.1136/hrt.46.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]