Abstract
Peripartum cardiomyopathy (PPCM) is an idiopathic left ventricular dysfunction in women who are in late pregnancy or the postpartum period. PPCM is a rare but sometimes fatal disease, and mechanical circulatory support is required if heart failure is refractory to conventional therapy. A 28-year-old woman in late pregnancy was admitted to our hospital due to congestive heart failure with cardiogenic shock. Her heart rate was 200 beats per minute (sinus tachycardia), and left ventricular ejection fraction (LVEF) was 10%. Additionally, fetal heart rate decreased to 80 beats per minute. It was extremely difficult to continue her pregnancy because of decompensated heart failure and fetal asphyxia; therefore, we delivered her baby via caesarean section after initiating mechanical circulatory support. With optimal medical therapy, including bromocriptine, we were able to remove mechanical circulatory support. Additionally, LVEF improved to 42%, and she was discharged with her baby who had no growth failure. This case highlights the safety and risk of caesarean section under mechanical circulatory support, and the effectiveness of bromocriptine.
<Learning objective: Mechanical circulatory support is occasionally required if heart failure is refractory to conventional medical therapy in peripartum cardiomyopathy (PPCM). Mechanical circulatory support, particularly extracorporeal membrane oxygenation needing sufficient anticoagulation, should be initiated following delivery via caesarean section because of bleeding from the uterus. Moreover, bromocriptine may be effective in improving PPCM and should be the first-line treatment in clinical practice.>
Keywords: Peripartum cardiomyopathy, Caesarean section, Extracorporeal membrane oxygenation, Intra-aortic balloon counterpulsation, Bromocriptine
Introduction
Peripartum cardiomyopathy (PPCM) is an idiopathic left ventricular dysfunction, similar to dilated cardiomyopathy, in women who are in late pregnancy or the postpartum period. It is reported that PPCM is a rare but sometimes fatal disease [1], particularly during subsequent pregnancy in women with a history of PPCM who had preserved left ventricular dysfunction [2]. There is no effective and specific therapy for PPCM, and mechanical circulatory support and heart transplantation are required if heart failure is refractory to conventional therapy. In fact, because PPCM is a life-threatening condition, rapid therapeutic interventions are necessary, and are typically performed and managed after delivery or abortion. To the best of our knowledge, there are only a few case reports demonstrating caesarean section under mechanical circulatory support before delivery. In addition, a small randomized pilot study and several observational reports have suggested the usefulness of bromocriptine for PPCM [3], [4], [5], [6]. We report our experience with a patient who developed PPCM that required emergent caesarean section under mechanical circulatory support, and who was prescribed bromocriptine.
Case report
We present a 28-year-old woman, gravida 3 and para 2, at 35 weeks and 6–7 days of gestation. Her first delivery was at 24 years and her second was at 26 years. During these pregnancies, she was asymptomatic, but sinus tachycardia was indicated. At 28 years, she became pregnant a third time. Her heart rate was approximately 140 beats per minute at 10 weeks of gestation, and then she experienced feebleness. At 34 weeks of gestation, her heart rate increased to 170 beats per minute. The patient presented with sinus tachycardia throughout her pregnancy but without hypertension and preeclampsia. At 35 weeks and 6–7 days of gestation, she was admitted into a local clinic due to premature labor. Obstetricians administered ritodrine hydrochloride, and her heart rate continued to rise, up to 200 beats per minute, and she experienced progressive dyspnea. Her heart rate did not decrease once the medicine was discontinued; therefore, she was transferred to our hospital. Upon examination, her systolic blood pressure was 70 mmHg and oxygen saturation was 94% on 2 L/min via oxygen cannula. Respiratory sound was coarse crackle, and her chest X-ray showed pulmonary edema (Fig. 1). Her brain natriuretic peptide (BNP) level was 1000 pg/mL, but troponin I was not elevated. Hemoglobin level was 9.4 g/dL and thyroid function was as follows: thyroid stimulation hormone, 1.120 μIU/mL; free T4, 1.03 ng/dL. Her electrocardiogram demonstrated a heart rate at 203 beats per minute (sinus tachycardia) (Fig. 2), and her left ventricular ejection fraction (LVEF) was 10% and left ventricular diastolic dimension was 45 mm based on echocardiogram.
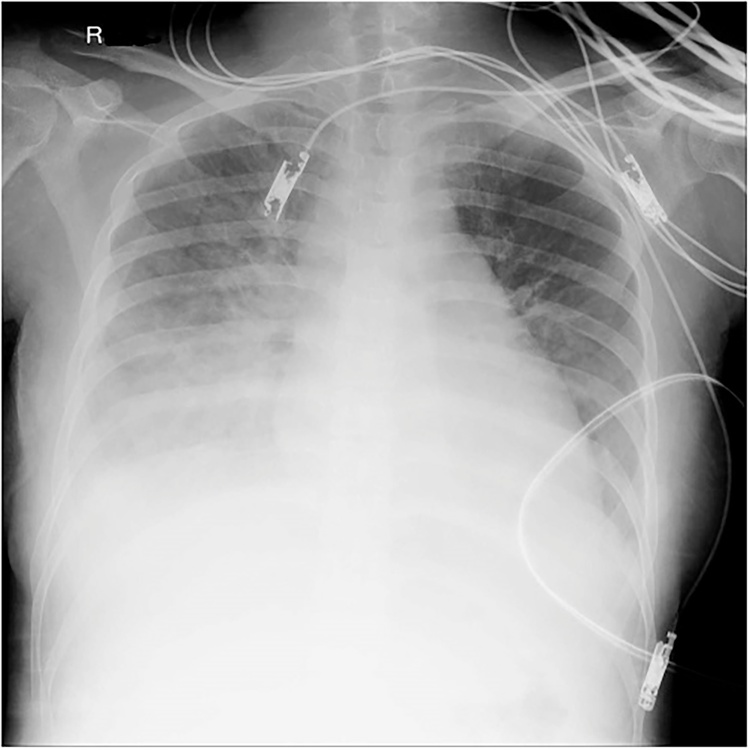
Fig. 1.
Chest X-ray upon admission showed pulmonary edema.
Fig. 2.
Electrocardiogram upon admission demonstrated a heart rate of 203 beats per minute (sinus tachycardia).
Her respiratory condition rapidly deteriorated, as her arterial blood gas showed severe respiratory acidosis: pH, 6.765; PaCO2, 94 mmHg; PaO2, 26 mmHg. Fetal heart rate also decreased to 80 beats per minute. Because of these factors, it was extremely difficult to continue her pregnancy. We needed to deliver her baby immediately, but vaginal delivery and caesarean section were also considered dangerous due to decompensated heart failure. Therefore, we initiated mechanical circulatory support, which included intra-aortic balloon pump (IABP) counterpulsation and peripheral veno-arterial extracorporeal membrane oxygenation (ECMO), prior to delivery. Blood flow rate was maintained at approximately 3.0 L/min through ECMO. After these treatments, caesarean section was performed under general anesthesia by obstetricians. Coronary angiography, which was performed after the caesarean section, showed no stenosis, and histological examination of cardiac tissue obtained via endomyocardial biopsy during cardiac catheterization found no characteristic findings (Fig. 3). As a result, we diagnosed her with PPCM due to the absence of another identifiable cause for heart failure, and we prescribed bromocriptine (2.5 mg twice daily) with noradrenaline (up to 0.015 μg/kg/min) to increase blood pressure and landiolol (up to 80 μg/kg/min) to decrease heart rate. Following these medicinal treatments, her blood pressure gradually increased and her heart rate gradually decreased. We then tapered her off landiolol and changed her prescription to bisoprolol at a dose of 0.625 mg daily on the third day after admission. During this period, we intravenously injected furosemide (5 mg/h) to treat her pulmonary congestion. She also experienced sustained bleeding from her uterus because of administered unfractionated heparin, which kept activated clotting time from 180 to 200 s in order to maintain mechanical circulatory support, and she was in dire need of a blood transfusion. Since the insertion of a Bakri balloon (Cook Women’s Health, Spencer, IN, USA) and a transcatheter arterial embolization were not effective, we performed a hysterectomy on the fifth day after admission. Her left ventricular dysfunction gradually improved, indicating that we could remove the mechanical circulatory support on the eighth day and the ventilator on the twelfth day after admission. One month later, her LVEF improved to 42% and BNP level decreased to 43.9 pg/mL. She was then discharged on the forty-ninth day after admission without any symptoms. We prescribed bisoprolol at a dose of 2.5 mg daily at discharge.
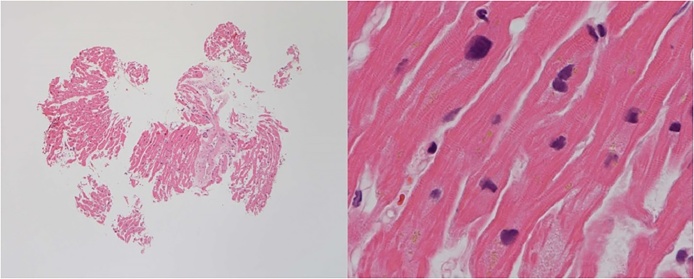
Fig. 3.
Endomyocardial biopsy obtained during cardiac catheterization following caesarian section showed anisonucleosis and slight fibrosis. There was no infiltration of inflammatory cells.
In addition, her female child, weighing 2602 g, had neonatal asphyxia, indicated by an Apgar score of 2 points at one minute and 3 points at five and ten minutes, and the umbilical artery blood gas showed severe metabolic acidosis: pH, 6.8; PaCO2, 47 mmHg; PaO2, 59 mmHg; Lac, 18.90 mmol/L. This condition was attributed to insufficient circulation because her baby’s heart rate was approximately 80 beats per minute before delivery. However, with administration of appropriate treatment, which included catecholamines and therapeutic hypothermia, her baby had no growth failure at discharge.
Discussion
This case showed that we successfully treated both maternal and neonatal life-threatening conditions via emergent caesarean section under mechanical circulatory support, such as IABP and ECMO, for acute severe PPCM, as well as bromocriptine, suggesting that the medication was effective in improving LVEF. To the best of our knowledge, this is a rare case where emergent caesarean section under mechanical circulatory support was performed for PPCM. This patient had sinus tachycardia in the early pregnancy period, as well as during her previous pregnancy. There was no obvious cause of sinus tachycardia, such as anemia and hyperthyroidism. We considered that she might have contracted PPCM during her prior pregnancy, and the third pregnancy worsened her cardiac dysfunction. Subsequent pregnancy in women with a history of PPCM who have preserved left ventricular dysfunction is dangerous, because pregnancy is associated with significant decreases in left ventricular function, and can result in clinical deterioration and even death [2].
The clinical course of this patient suggested two important clinical issues.
First, mechanical circulatory support, particularly ECMO needing sufficient anticoagulation, should be initiated following delivery via caesarean section because of bleeding from the uterus. Treatment of acute PPCM is similar to treatment for other types of heart failure, which includes supplemental oxygen therapy and pharmacologic therapy (such as diuretics and vasodilators). When heart failure is refractory to these optimal conventional treatments, mechanical circulatory support and cardiogenic transplantation are required. Mechanical circulatory support, such as IABP, ECMO, and left ventricular assist device, are effective for severe PPCM as a bridge to recovery or cardiogenic transplantation [7], [8], [9]. All of ECMO have been conducted and managed after delivery in previous reports; however, in the present study, insertion of an IABP and peripheral veno-arterial ECMO were indicated before delivery because of cardiac shock and fetal asphyxia. We also considered the possibility of cardiopulmonary arrest after anesthesia without veno-arterial ECMO. This case indicates that mechanical circulatory support, particularly ECMO needing sufficient anticoagulation, before delivery may lead to bad outcomes. It is difficult to stop bleeding from the uterus because of anticoagulation in an effort to maintain mechanical circulatory support. Therefore, hysterectomy may be necessary at the same time as caesarean section.
Second, bromocriptine use may be significantly effective in PPCM. In this case, LVEF improved within one month, regardless of severe left ventricular dysfunction, indicating that early administration and continuation of bromocriptine might be effective. A small randomized pilot study and several observational studies have suggested a beneficial response to bromocriptine therapy in patients with PPCM [3], [4], [5], [6], but there is insufficient evidence to recommend routine use of bromocriptine treatment for PPCM. Currently, the efficacy of bromocriptine is being investigated in a larger randomized multicenter trial in Germany [10]; the results of this trial are highly anticipated.
Clinicians must be aware that mechanical circulatory support should begin after caesarean section and bromocriptine should be considered as the first-line of treatment in PPCM.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Elkayam U., Akhter M.W., Singh H., Khan S., Bitar F., Hameed A. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111:2050. doi: 10.1161/01.CIR.0000162478.36652.7E. [DOI] [PubMed] [Google Scholar]
- 2.Elkayam U., Tummala P.P., Rao K., Akhter M.W., Karaalp I.S., Wani O.R. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344:1567. doi: 10.1056/NEJM200105243442101. [DOI] [PubMed] [Google Scholar]
- 3.Sliwa K., Blauwet L., Tibazarwa K., Libhaber E., Smedema J.P., Becker A. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121:1465. doi: 10.1161/CIRCULATIONAHA.109.901496. [DOI] [PubMed] [Google Scholar]
- 4.Hilfiker-Kleiner D., Meyer G.P., Schieffer E., Goldmann B., Podewski E., Struman I. Recovery from postpartum cardiomyopathy in 2 patients by blocking prolactin release with bromocriptine. J Am Coll Cardiol. 2007;50:2354. doi: 10.1016/j.jacc.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Habedank D., Kühnle Y., Elgeti T., Dudenhausen J.W., Haverkamp W., Dietz R. Recovery from peripartum cardiomyopathy after treatment with bromocriptine. Eur J Heart Fail. 2008;10:1149. doi: 10.1016/j.ejheart.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Haghikia A., Podewski E., Libhaber E., Labidi S., Fischer D., Roentgen P. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108:366. doi: 10.1007/s00395-013-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouabdallaoui N., Demondion P., Leprince P., Lebreton G. Short-term mechanical circulatory support for cardiogenic shock in severe peripartum cardiomyopathy: La Pitié-Salpêtrière experience. Interact Cardiovasc Thorac Surg. 2017;25:52–56. doi: 10.1093/icvts/ivx106. [DOI] [PubMed] [Google Scholar]
- 8.Lueck S., Sindermann J., Martens S., Scherer M. Mechanical circulatory support for patients with peripartum cardiomyopathy. J Artif Organs. 2016;19:305–309. doi: 10.1007/s10047-016-0891-z. [DOI] [PubMed] [Google Scholar]
- 9.Gevaert S., Van Belleghem Y., Bouchez S., Herck I., De Somer F., De Block Y. Acute and critically ill peripartum cardiomyopathy and ‘bridge to’ therapeutic options: a single center experience with intra-aortic balloon pump, extra corporeal membrane oxygenation and continuous-flow left ventricular assist devices. Crit Care. 2011;15:R93. doi: 10.1186/cc10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haghikia A., Podewski E., Berliner D., Sonnenschein K., Fischer D., Angermann C.E. Rationale and design of a randomized, controlled multicentre clinical trial to evaluate the effect of bromocriptine on left ventricular function in women with peripartum cardiomyopathy. Clin Res Cardiol. 2015;104:911–917. doi: 10.1007/s00392-015-0869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]