Abstract
Radiation-induced heart disease (RIHD) is a serious side effect of thoracic radiation therapy (RT) and is associated with significant morbidity and mortality. Radiation-induced cardiomyopathy (RICM) is one of the manifestations of RIHD, which represents with left ventricular (LV) systolic and diastolic dysfunction due to myocardial fibrosis. Although the diagnosis of RIHD is challenging and is generally an exclusion diagnosis, multimodality imaging including echocardiography, cardiac computed tomography and cardiac magnetic resonance (CMR) imaging could help the diagnosis. Herein, we report a case of 70-years-old male, who had been treated with chemo-radiation therapy for early esophageal cancer, was suffered from medically refractory heart failure due to severely reduced LV systolic function and constrictive pericarditis 8 years after chemo-radiation therapy. Although no gadolinium-enhancement (LGE) was detected on CMR, T1 mapping depicted increased extracellular matrix volumes of 45%, which suggested global myocardial fibrosis. Histopathological analysis by endomyocardial biopsy (EBM) revealed marked degeneration of myocytes and interstitial fibrosis, while vacuolation in myocytes which is characteristics of chemotherapy induced cardiomyopathy was not specific by electron microscopy. Therefore, we diagnosed that the present case was likely to the RICM.
<Learning objective: RICM is characterized by inflammation followed by the development of a diffuse, patchy interstitial fibrosis of the myocardium, which is usually obtained either by EBM or at autopsy. Native and post-contrast T1-mapping by CMR enables to estimate extracellular volume (ECV), which is believed to be increased as a result of diffuse myocardial fibrosis. The assessment of myocardial fibrosis using ECV should be useful for early detection of myocardial damage due to RT, and which probably taking place of EBM.>
Keywords: Radiation-induced heart disease, Myocardial fibrosis, Onco-cardiology
Introduction
With the recent improvement in survival rate of thoracic malignancies with chemo-radiation therapy, radiation-induced heart disease (RIHD) has come to be recognized. Radiation-induced cardiomyopathy (RICM) is one of the manifestations of RIHD, which presents with left ventricular (LV) systolic or diastolic dysfunction due to myocardial fibrosis. We experienced medically refractory heart failure (HF) due to severely reduced LV systolic function suggesting RICM, and T1 mapping by cardiac magnetic resonance (CMR) image was useful to depict myocardial properties.
Case presentation
A 70-year-old man was admitted to Tottori University Hospital due to exertional dyspnea and bilateral leg edema. He had been treated with chemo-radiation therapy for early esophageal cancer (Stage 1; cT1N0M0) 8 years earlier. He underwent mediastinal external beam radiation with a total dose of 60 Gy/30 fr, with 48 Gy and 12 Gy using anterior–posterior fields and parallel–oblique fields, respectively. Docetaxel and 5-fluorouracil (5-FU) were administered and continued as an adjuvant chemotherapy for two years, then he achieved remission. He had no previous medical history of any cardiac disease. However, left pleural effusion was increased 4 years after chemo-radiation therapy suggesting radiation pleuritis. Despite taking diuretics, exertional dyspnea and bilateral leg edema developed and gradually worsened. He was referred to our cardiology department for the first time because he was suspected of HF. On admission, blood pressure was 141/69 mmHg and heart rate was 129 beats/min. He had hypoxemia of 95% of SpO2 (room air) and jugular vein was distended. Blood examination revealed mild renal dysfunction (blood urea nitrogen of 27.3 mg/dl, creatinine, 1.07 mg/dl, estimated glomerular filtration rate, 51.6 ml/min/1.73m2) and mildly elevated brain natriuretic peptide of 105 pg/ml. A 12-lead electrocardiogram (ECG) showed low voltage in the extremity leads and poor R progression in the chest leads, whereas normal ECG before chemo-radiation therapy was identified. Cardiomegaly and bilateral pleural effusion were revealed in the chest X-ray (Fig. 1). Echocardiography revealed severely reduced LV systolic function without LV dilatation, and ejection fraction (EF) of 23%, and normal valvular function. In addition, small pericardial effusion and thickened pericardium with increased brightness existed. CMR imaging was performed with a 3.0T scanner (Magnetom Skyra, Siemens Healthcare, Erlangen, Germany) to investigate myocardial properties. The gadolinium-based contrast medium was injected intravenously at 0.1 mmol/kg. Ten minutes after injection on the contrast medium, late gadolinium-enhancement (LGE)-magnetic resonance images were obtained using a three-dimensional phase-sensitive inversion recovery sequence after the acquisition of a T1-weighted scout image. Quantitative native T1 mapping with a modified lock-locker inversion recovery was performed and post-T1 mapping was also performed 10 min after contrast injection. Although no LGE was detected on CMR, T1 mapping depicted increased extracellular volume (ECV) of 45% despite upper-limit of the reference range of native T1 value of 1303 ms (Fig. 2). Coronary angiogram showed no evidence of obstructive lesion, however, the mean pulmonary capillary wedge pressure was elevated (17 mmHg) and cardiac index was significantly decreased to 1.23 l/min/m2. There were also typical features of constrictive pericarditis including pulsus paradoxus, elevated right heart chamber pressure, and LV and right ventricular interdependence. Histopathological and ultrastructural analysis of the endomyocardial biopsy (EMB) revealed marked degeneration of myocytes such as myofibrillary loss, vacuolations, and interstitial fibrosis (Fig. 3). He was finally diagnosed with HF due to radiation-induced cardiomyopathy (RICM) and constrictive pericarditis. Because his symptoms were medically refractory and his cardiac function was catecholamine-dependent, he died 6 months after admission.
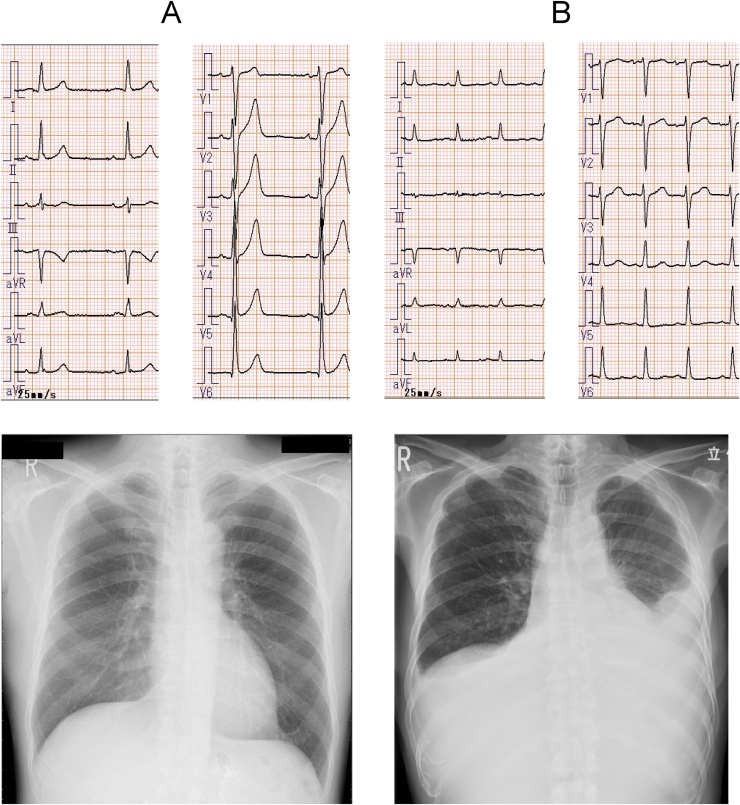
Fig. 1.
(Upper) Electrocardiogram (ECG) and (Lower) chest X-ray before and after chemo-radiation therapy. Before chemo-radiation therapy (A), both ECG and chest X-ray showed no definite abnormality. On this admission (B), ECG showed low voltage in the extremity leads and poor R progression in the chest leads. Cardiomegaly and bilateral pleural effusion (left dominant) were revealed in the chest X-ray.
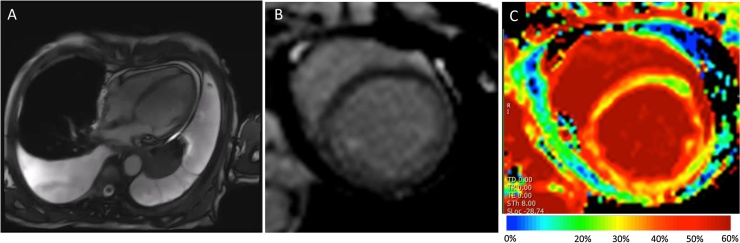
Fig. 2.
Cardiac magnetic resonance (CMR) imaging. Cine CMR imaging (A) showed severely reduced left ventricular (LV) systolic function without LV dilatation (end-diastolic volume index, 66 ml/m2, end-systolic volume index, 53 ml/m2), and ejection fraction of 19%. In addition, the pericardium thickness was up to 3–4 mm (normal range <2–3 mm), and small pericardial and pleural effusion existed. T2 weighted imaging showed no high-intensity lesion which suggests myocardial edema. Late gadolinium enhancement (LGE) images in short-axis view did not show definite enhancement in the LV wall (B). However, extracellular volume (ECV) maps with T1 mapping (C) showed increased ECV of 45% (reference value: 20–30%), in the whole myocardium, which suggested global myocardial fibrosis.
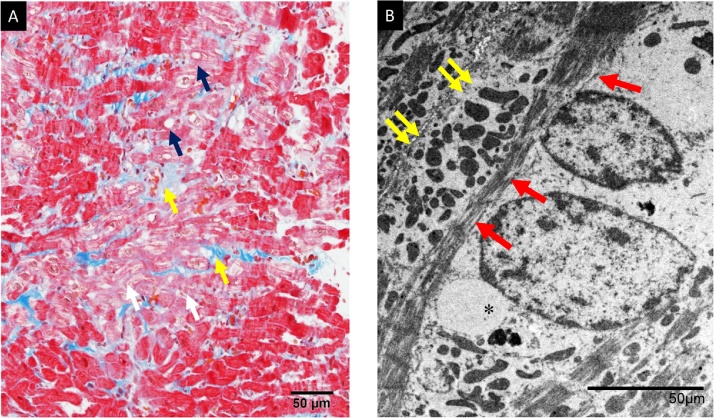
Fig. 3.
Histopathological analysis of the resected myocardium. (A) Histopathologically, interstitial fibrosis (yellow arrow) and myocardial degeneration such as irregular arrangement (white arrow) or vacuolar changes (blue arrow) are seen. Masson trichrome stain ×200. (B) Myofibrillary loss (red arrow), dilatation of sarcoplasmic reticulum (asterisks), and proliferation of small sized mitochondria (yellow arrows) are recognized by electron microscopy ×500.
Discussion
In recent years, as long-term survival of cancer patients has improved, it has been recognized that various heart diseases develop as late complications of radiation therapy (RT). Radiation exposure to the thorax leads to microvascular insufficiency and coronary artery disease, increases risks of pericardial and valvular heart disease, systolic and diastolic dysfunction due to cardiomyopathy, and conduction abnormalities. These medical conditions are called RIHD. RICM, which is one of the manifestations of RIHD, could represent both LV systolic and diastolic dysfunction, the latter is more common, which presents as a restrictive cardiomyopathy [1]. LV systolic dysfunction is commonly seen when anthracyclines are used in combination. However, myocardial damage associated with 5-FU, which was used in the present case, is extremely rare, and that of docetaxel also has been reported from 3% to 8% [2], but in all those cases anthracyclines have been used in combination. Pathologically, RICM is characterized by inflammation followed by the development of a diffuse, patchy interstitial fibrosis of the myocardium, and effacement of the peri-myocyte endothelium. In our case, pathological findings by EMB showed marked degeneration of myocytes and interstitial fibrosis, while vacuolation in myocytes which is characteristic of chemotherapy-induced cardiomyopathy was not specific by electron microscopy. Therefore, we considered the present case was likely to be RICM rather than the effect of chemotherapy.
In order to detect the onset of RIHD at an early stage, the European Association of Cardiovascular Imaging and the American Society of Echocardiography recommend a yearly history and physical examination and even though asymptomatic, a screening echocardiography should be performed 5 years after exposure in high-risk patients and 10 years after exposure in the others [3]. Indeed, echocardiography plays an important role in the detection of various complications due to RT, however, conventional measurement including LVEF may not be sensitive enough to reveal subclinical myocardial damage at an early stage. On the other hand, CMR is a highly sensitive modality that provides detailed information beyond the capability of echocardiography, particularly with regard to myocardial tissue characterization. CMR with LGE is the gold standard for non-invasive detection of myocardial fibrosis [4], and has been detected in half of patients who received RT f or esophageal cancer [5]. However, LGE likely lacks sensitivity and leads to underestimation when myocardial fibrosis is widely expanded as in the present case. Native and post-contrast T1 mapping enables to estimate the ECV with an excellent reproducibility, as derived from changes in T1 relaxation rate before and after contrast application in tissue and blood [6]. Although ECV is believed to be increased as a result of diffuse myocardial fibrosis, it can also increase by pathologies other than fibrosis such as enhanced intra-capillary plasma volume, interstitial infiltration, or extracellular edema [7]. However, several recent studies have demonstrated a significant correlation between ECV and myocardial fibrosis as quantified in EMB [8], [9]. Sado et al. have shown that ECV is mildly elevated in both hypertrophic cardiomyopathy at 29.1 ± 0.5% and dilated cardiomyopathy at 28 ± 0.4% [10]. Diffuse fibrosis, however, rarely increases beyond 40%. Therefore, ECV of 45% in the present case suggested substantial myocardial damage. The assessment of myocardial fibrosis using ECV should be useful for early detection of myocardial damage due to RT, and possibly guide the proper timing of initiation of anti-fibrotic agents such as angiotensin-converting enzyme inhibitors and aldosterone inhibitors. Due to the improvement in treatment outcomes for thoracic malignancies with RT, the frequency of RIHD is expected to increase more and more in the future. All patients undergoing thoracic RT should be followed up periodically using echocardiography, however detecting early changes in myocardial properties due to RT, T1 mapping and ECV measurement on CMR should be considered particularly in high-risk patients.
Disclosures
None.
References
- 1.Lipshultz S.E., Adams M.J. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010;28:1276–1281. doi: 10.1200/JCO.2009.26.5751. [DOI] [PubMed] [Google Scholar]
- 2.Yeh E.T., Bickford C.L. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 3.Lancellotti P., Nkomo V.T., Badano L.P., Bergler-Klein J., Bogaert J., Davin L. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:721–740. doi: 10.1093/ehjci/jet123. [DOI] [PubMed] [Google Scholar]
- 4.Kim R.J., Wu E., Rafael A., Chen E.L., Parker M.A., Simonetti O. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 5.Umezawa R., Ota H., Takanami K., Ichinose A., Matsushita H., Saito H. MRI findings of radiation-induced myocardial damage in patients with oesophageal cancer. Clin Radiol. 2014;69:1273–1279. doi: 10.1016/j.crad.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Taylor A.J., Salerno M., Dharmakumar R., Jerosch-Herold M. T1 mapping: basic techniques and clinical applications. JACC Cardiovasc Imaging. 2016;9:67–81. doi: 10.1016/j.jcmg.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Lurz J.A., Luecke C., Lang D., Besler C., Rommel K.P., Klingel K. CMR-derived extracellular volume fraction as a marker for myocardial fibrosis: the importance of coexisting myocardial inflammation. JACC Cardiovasc Imaging. 2018;11:38–45. doi: 10.1016/j.jcmg.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 8.aus dem Siepen F., Buss S.J., Messroghli D., Andre F., Lossnitzer D., Seitz S. T1 mapping in dilated cardiomyopathy with cardiac magnetic resonance: quantification of diffuse myocardial fibrosis and comparison with endomyocardial biopsy. Eur Heart J Cardiovasc Imaging. 2015;16:210–216. doi: 10.1093/ehjci/jeu183. [DOI] [PubMed] [Google Scholar]
- 9.Nakamori S., Dohi K., Ishida M., Goto Y., Imanaka-Yoshida K., Omori T. Native T1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc Imaging. 2018;11:48–59. doi: 10.1016/j.jcmg.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Sado D.M., Flett A.S., Banypersad S.M., White S.K., Maestrini V., Quarta G. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2012;98:1436–1441. doi: 10.1136/heartjnl-2012-302346. [DOI] [PubMed] [Google Scholar]